www.elsevier.com / locate / bres
Research report
Neuroglial interactions in a model of
para-chlorophenylalanine-induced serotonin depletion
´
´
Alberto Javier Ramos, Patricia Tagliaferro, Ester Marıa Lopez, Jorge Pecci Saavedra,
*
Alicia Brusco
´
Instituto de Biologıa Celular y Neurociencia‘‘Prof. Eduardo De Robertis’’, Facultad de Medicina, Universidad de Buenos Aires,
Paraguay2155 (1121), Buenos Aires, Argentina Accepted 15 August 2000
Abstract
Serotonin (5HT) is involved in the development and plasticity of the CNS through the release of S-100b, a glial trophic factor which stabilizes synapses and neuronal cytoskeleton and promotes neuronal development. S-100bis released from glial cells after activation of glial 5HT1Areceptors. We present in this paper the effects upon neurons and glia of a 5HT depletion induced by 14 days of treatment with
para-chlorophenylalanine (PCPA) in adult rats. S-100b, 5HT, 5HT-transporter (5HT-T) and neurofilaments (Nf-200 and Nf-68) expressions were studied by immunohistochemistry and image analysis in striatum, hippocampus, parietal and frontal cortex. Immediately after ending PCPA treatment we found increased intracellular S-100b immunoreactivity in glial cells, reduced 5HT immunolabelling, reduced density of 5HT-T, Nf-200 and Nf-68 fibers and morphological alterations in neuronal cytoskeleton. One week after PCPA treatment S-100bimmunoreactivity decreased towards control levels, 5HT was normalized in dorsal raphe nucleus, but not in innervation areas; 5HT-T, Nf-200 and Nf-68 fiber densities increased but some neuronal cytoskeletal alterations were still present in striatum. Two weeks after PCPA treatment S-100b had returned to control levels in most studied regions; 5HT immunoreactivity was normalized, meanwhile 5HT-T, Nf-200 and Nf-68 fiber densities increased reaching values over the control level. We propose that S-100bcould be accumulated in glial cells during the 5HT depletion period, to be released once 5HT levels have recovered. Neuronal cytoskeletal alterations and reduced fiber density may be the expression of decreased extracellular availability of S-100b. Conversely, increased 5HT-T, Nf-200 and Nf-68 expressions, once S-100bis normalized, may be the biological response to the growth factor release. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Neurotrophic factors: biological effects
Keywords: Serotonin; Parachlorophenylalanine; Neurotrophic factor; S-100b; Neurofilament; Serotonin transporter
1. Introduction 5HT role as a differentiation signal in the development of
the central nervous system (CNS). This hypothesis is Serotoninergic neurons located in the brainstem project supported by several facts: (i) 5HT is the classical to cortical and subcortical brain regions through a network neurotransmitter expressed in the early brain development, of fibers with dense branching. Terminal fibers containing prior to finishing synaptogenesis and before reaching the serotonin (5HT) present a broad distribution at synaptic as complete stabilization of neurotransmission systems and it well as non-synaptic sites, raising the hypothesis of seems to promote neuronal growth [26,28]; (ii) neuronal another role for this amine, besides neurotransmission. differentiation in specific regions correlates with the arrival The early expression of 5HT in the brain development of 5HT axons [27]; (iii) a depletion of 5HT in pregnant was the starting evidence [26,27] for the hypothesis of the rats [26] or during the synaptogenesis [34] leads to
decreased brain development.
In the adult brain, 5HT plays a role in neuronal and glial *Corresponding author. Correspondence address: Rivera Indarte 132
plasticity. It has been reported that a 5HT depletion 1ro ‘‘A’’, (1406), Buenos Aires, Argentina. Fax:154-11-4941-5618.
E-mail address: [email protected] (A. Brusco). produces significant glial reaction [11,47], increases nitric 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
oxide synthase (NOS) activity [48] and reduces MAP-2 neurite growth (see [20,23] for review) and 200-kDa and synaptophysin immunoreactivities [5]. In addition, neurofilaments (Nf-200) which are characteristic of mature 5HT is involved in the regulation of hippocampal and axons (see [40] for review). Specific morphology of 5HT subventricular neurogenesis in the adult brain [6]. 5HT’s fibers has been studied by 5HT transporter (5HT-T) role in the development and plasticity of CNS seems to be immunostaining. Recovery of 5HT has been followed by mediated by the stimulation of glial 5HT1A receptors that 5HT immunoreactivity (5HT-IR) in the dorsal raphe cause S-100b protein release [51]. S-100b is a growth nucleus (DRN) and innervation areas.
factor that derives from glial cells. S-100b has important effects on axonal growth of 5HT neurons [2,3,33],
partici-pates in neurite extension in 5HT innervated areas [24,39], 2. Materials and methods
stabilizes neuronal cytoskeleton [18] and regulates GAP-43
phosphorylation [32]. PCPA methyl ester, mouse monoclonal anti-S-100b
Neurofilaments are the major cytoskeletal constituents of protein, mouse monoclonal anti-Nf-200, mouse monoclo-neurons. They are intrinsic determinants of axonal caliber nal anti-Nf-68, secondary biotinylated antibodies and [19] and their dynamic remodeling is necessary for axonal streptavidin complex used for immunohistochemistry growth and guidance. By regulating axonal caliber, neuro- studies were purchased from Sigma. Monoclonal anti 5HT-filaments proteins can affect both axonal transport and T serum was purchased from Chemicon. Polyclonal rabbit neuronal function [40]. Down-regulation of neurofilaments antiserum against 5HT was produced and characterized in expression and the presence of altered neurofilaments are our laboratory [7]. All chemical substances were of common in several human neurodegenerative diseases (see analytical grade.
for review [23]). Recent evidence coming from transgenic
mouse shows that disorganized neurofilaments can lead to 2.1. Treatment neuronal degeneration and death [23]. Conversely,
trans-genic mice over expressing S-100b show significant Thirty adult male Wistar rats weighing 250–300 g were alterations in neuronal cytoskeleton [53]. used. The model of PCPA treatment was modified from a There is evidence of defects in the 5HT system in previous report [47]. Fifteen rats were treated with PCPA Alzheimer’s disease [8,17,55]. Brains from patients with and another fifteen rats with saline. The sterile solution Alzheimer’s disease present increased level of S-100b containing PCPA was injected intraperitoneally (i.p.) for 14 protein [14], altered neurofilament expression [23], glial days. Individual PCPA doses were 100 mg / kg / day, in-reaction and increased NOS activity [42]. Moreover, a high jection volumes ranged from 0.2 to 0.3 ml. All doses were S-100b level seems to be involved in the pathogenesis of injected in the morning between 9:00 and 10:00 a.m. The Alzheimer’s disease [15,31,46]. Considering our previous amount of PCPA injected was one-third of that which findings of a glial reaction [43,47] and increased NOS inhibits 33% of the brain protein synthesis when it is activity [48] after 5HT depletion, as well as the relation- administered intravenously [30]. Animals were divided ship between 5HT levels and S-100b, it is interesting to into three experimental groups (T0, T1, T2) and three study the consequences of an experimental lack of 5HT on control groups (C0, C1, C2) of five animals each. Ex-neuronal cytoskeleton and glial S-100b level in the adult perimental groups were treated with PCPA, control groups brain. received the same volume of sterile saline solution and In spite of the wide range of pharmacological models were kept in the same environment as those treated with available to reach an effective 5HT depletion, we have PCPA (12 h light–dark cycle, controlled humidity and chosen to treat with an inhibitor of tryptophan hydroxy- temperature, free access to standard rat food and water). lase, parachlorophenylalanine (PCPA) [25] that produce a The animal care for this experimental protocol was in significant depletion of brain 5HT [1,25,44,49] leaving the accordance with the NIH guidelines for the Care and Use 5HT innervation intact [10]. Other common serotoninergic of Laboratory Animals and the principles presented in the drugs (i.e. substituted amphetamines, dihydroxytryp- Guidelines for the Use of Animals in Neuroscience Re-tamines) also produce significant 5HT depletion, but they search by the Society for Neuroscience.
are potent neurotoxins for 5HT neurons [10,37,38,45].
In this work we report the study of the neuronal and 2.2. Fixation glial response after a PCPA induced 5HT depletion in adult
paraformaldehyde and 0.25% v / v glutaraldehyde in 0.1 M values into ROD by using the formula: ROD5log (256 / phosphate buffer, pH 7.4. Brains were removed and kept in mean gray). The ROD value was chosen to evaluate the the same cold fixative solution for 2–4 h. After that, brains intensity of S-100b and 5HT immunoreactivities. A back-were washed three times in cold 0.1 M phosphate buffer, ground parameter was obtained from each section out of pH 7.4, containing 5% w / v sucrose, and left in this the immunolabelled structures, and subtracted from each washing solution for 18 h at 48C. Coronal and saggital cell ROD before statistically processing values. For the 40-mm thick brain sections were cut using a vibratome. evaluation of 5HT-T, Nf-68 and Nf-200 positive fibers, the The sections were stored at 2208C in 0.1 M phosphate total area of immunolabelled fibers was related to the total buffer, pH 7.4, with 25% w / v sucrose added as a area of the evaluated field, giving a fiber density
parame-cryoprotector. ter.
2.3. Immunohistochemistry 2.5. Statistics
Brain sections of both PCPA-treated and control groups Four to ten separate immunohistochemical experiments were simultaneously processed in the free floating state. In were run for each primary antibody. Individual experi-order to inhibit endogenous peroxidase activity, tissue ments were composed of six to ten tissue sections of each sections were previously dehydrated, treated with 0.5% animal from each group. Seven to ten fields were measured v / v H O in methanol for 30 min at room temperature and2 2 for each brain area in each section of each animal. Inter-rehydrated. Free-floating brain sections were blocked for 1 animal differences in each group were not significant. h with 3% v / v normal goat serum in phosphate buffer Values represent the means of experiments performed for saline (PBS). After two rinses in PBS, the sections were each marker, time and brain area. Differences among the incubated for 48 h at 48C with one of the following means were statistically analyzed using one-way ANOVA primary antibodies against 5HT, S-100b, Nf-200, Nf-68 or and Student–Newman–Keuls post test. Statistical signifi-5HT-T diluted 1:8000; 1:500; 1:3000; 1:3000 or 1:1000 cance was set at P,0.05.
(v / v), respectively. Following five rinses in PBS, sections Differences among the means of control groups (C0, C1, were incubated for 1 h at room temperature with C2) were not significant, and one mean for control group is biotinylated secondary antibodies diluted 1:100. After shown in the figures.
further washing in PBS, sections were incubated for 1 h with streptavidin–peroxidase complex solution diluted
1:200. After washing again five times in PBS and twice in 3. Results
0.1 M acetate buffer, pH 6 (AcB), development of
peroxidase activity was carried out with 0.035% w / v 3.1. Serotonin 3,39-diaminobenzidine plus 2.5% w / v nickel ammonium
sulfate and 0.1% v / v H O dissolved in AcB. Following2 2 The evaluation of 5HT-IR in the somata of serotoniner-the enzymatic incubation step, sections were washed in gic neurons from the mesencephalic DRN is useful to test AcB three times and once in distilled water. Sections were the effectiveness of PCPA treatment in reducing 5HT mounted on gelatin-coated slides, dehydrated and cover- levels, based on the sensitivity of this nucleus to PCPA slipped using Permount for light microscopic observation. [47]. In the T0 group, 5HT-IR was significantly reduced in All antibodies, as well as the streptavidin complex, were the DRN dropping to 40% of control values (P,0.001). In dissolved in PBS containing 1% v / v normal goat serum the T1 group immunoreactivity was partially recovered and 0.3% v / v Triton X-100, pH 7.4. (88% of control) and it continued to increase to give the largest value in the T2 group (172% of control; P,0.001) 2.4. Morphometric measurement (Fig. 1). PCPA treatment also reduced 5HT-IR in serotoninergic projecting fibers. Varicose fibers with dense All measurements were performed on coded slides to branching were detected by anti-5HT antibodies in ensure objectivity. Mean gray of immunostained glial cells striatum, hippocampus, parietal and frontal cortex in the and total area of fibers were measured in an Axiophot control groups. Meanwhile 5HT-IR fibers disappeared in Zeiss light microscope equipped with a video camera on the T0 and T1 groups. 5HT-IR fibers from these regions line with a Zeiss-Kontron VIDAS image analyzer. Images were detected again in the T2 group (data not shown). obtained with the light microscope were transferred to a
video camera attached and connected to an interactive 3.2. S-100b immunostaining
image analysis system on line. The images were digitized
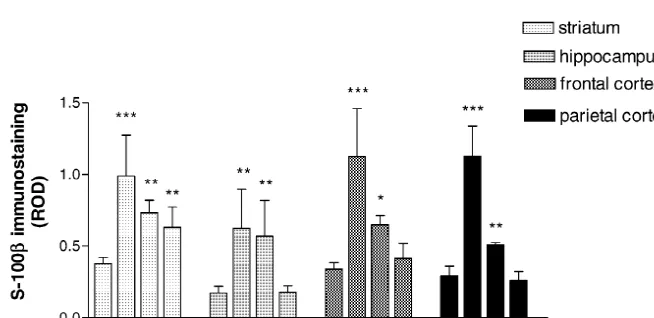
increased in the T0 and T1 animals (363%, P,0.01 and 331% of controls, P,0.01; respectively). In the T2 group, S-100b immunoreactivity decreased drastically and re-turned to the control level (Figs. 2 and 3A–D).
In the frontal cortex, astrocytes showed an increase of intracellular S-100b immunoreactivity in the T0 group (331% of control; P,0.001). In the T1 group, S-100b
immunoreactivity was lower, but it was still significantly increased over the control values (191% of control; P,
0.05). In the T2 group, the intensity of S-100b immuno-staining was not significantly different from the control animals (Figs. 2 and 4A–D).
Astrocytes from the parietal cortex presented a similar Fig. 1. Optical density of 5HT-IR neurons in the DRN after PCPA
profile, compared with frontal cortex, showing the maxi-treatment. Data are expressed in ROD units and represent the means of
mal S-100b intracellular immunoreactivity in the T0 four to seven experiments. Error bars represent the standard deviation of
the means. Optical density of 5HT-IR neurons in the DRN is significantly animals (385% of control; P,0.001) followed by a reduced in the T0 group, whereas it is increased significantly in the T2 recovering towards control level, being 174% of control group. ***, P,0.001 vs. control group, after one-way ANOVA and
(P,0.01) in the T1 group and not different from control Student–Newman–Keuls post-test.
levels in the T2 group (Fig. 2).
all studied regions, but area-dependent differences in the 3.3. Neurofilaments (200 and 68 kDa) response were observed during the recovering (T1 and T2
groups). In the striatum, we found important alterations in Nf-In the striatum, astroglial intracellular S-100bimmuno- 200 expression pattern. In the T0 and T1 animals, the reactivity was significantly increased in the T0 group neurofilaments inside striatal patches (striosomes) were (261% of control; P,0.001). Although, values obtained observed with abnormal, enlarged and fragmented struc-for T1 and T2 groups were lower showing a trend to ture. The maximal disruption was observed in the T0 and recover, they were significantly different from controls T1 groups. In the T2 group alterations were lower, and the (193% of control; P,0.01 and 166% of control; P,0.01, total number of Nf-200 labelled fibers were increased (Fig. respectively). Thus, S-100b immunostaining in the 5A–H). Image analysis for this region, performed inside striatum did not return to control level 2 weeks after the patches, was expressed as area of immunostained ending PCPA treatment (Figs. 2 and 3E–H). neurofilaments per unit area of patches. Thus, these data The analysis of hippocampal astrocytes was focused on imply an abundance of neurofilaments expression in the the stratum radiatum of CA-1, an area containing basal striosomes. Data obtained in this way showed a progres-dendrites of the pyramidal neurons. After PCPA treatment, sive reduction from T0 (75.1% of control) to T1 (59.4% of the intracellular S-100b immunostaining was significantly control, P,0.05). In the T2 group, a significant recovery
Fig. 2. Optical density of S-100bimmunostained glial cells after PCPA treatment. Data are expressed in ROD units and represent the means of four to ten experiments for each region. Error bars represent the standard deviation of the means. The significant increase in the optical density of S-100b
Fig. 3. Above: photographs show S-100bimmunostaining in the hippocampus, CA-1 area of stratum radiatum. (A) control; (B) 1 day after ending PCPA treatment (T0); (C) 7 days after ending PCPA treatment (T1); (D) 14 days after PCPA treatment (T2). Bar523.5mm. Below: S-100bimmunostaining in the striatum. (E) control; (F) 1 day after ending PCPA treatment (T0); (G) 7 days after ending PCPA treatment (T1); (H) 14 days after PCPA treatment (T2). Bar523.5mm.
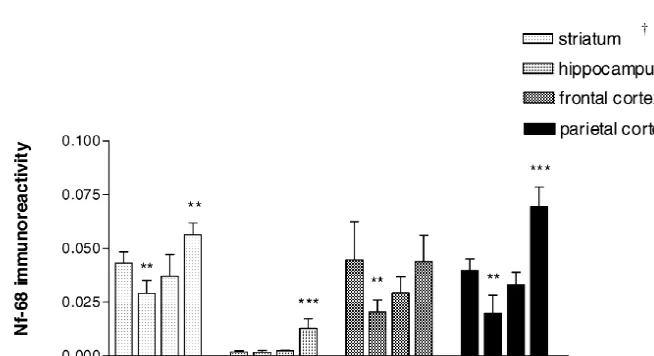
was observed (96.8% of control), being significantly control level (91.5% of control) being significantly differ-different from T1 (P,0.01 vs. T1) (Fig. 6). The Nf-68 ent from the T0 group (P,0.001 vs. T0). In the T2 group, expression in the striatal patches also showed a decrease in the number of Nf-200 positive dendrites was greater than the T0 group (67.5% of control, P,0.01), followed by an in the T1 group, but they were abnormal compared with increase in the T1 group (85.5% of control); and a further the paired controls (Fig. 8A–D). Image analysis confirmed increase to reach a value of 130.7% of control (P,0.01) in the increased number of Nf-200 labelled fibers showing a the T2 group (Figs. 7 and 9E–H). value significantly higher than control (137.1% of control, In the hippocampus, Nf-200 expression was decreased P,0.05) (Fig. 6). The profile of Nf-68 expression in the in the stratum radiatum of treated animals. In the T0 group, hippocampal areas was altered after the PCPA treatment, the basal dendrites of pyramidal neurons, as seen by being reduced in the T0 animals, but showing a continuous Nf-200 immunoreactivity, were shorter and thicker than increase from the T1 to T2 groups. Moreover, in the T2 those from control groups. Also a reduction in the total group Nf-68 expression was increased in CA-1, where it number of Nf-200 labelled structures was observed (Fig. was normally very low. Image analysis focused on the 8A–D). Image analysis, focused in the stratum radiatum of stratum radiatum of CA-1 clearly showed the increased CA-1 showed a significant reduction in Nf-200 labelled expression of Nf-68 in the T2 group (745.3% of control, structures (47.0% of control P,0.001). In the T1 group, P,0.001) (Fig. 7).
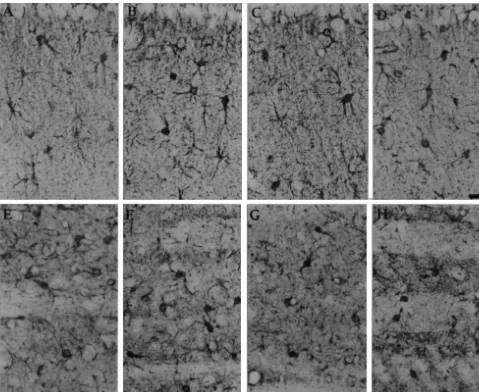
Fig. 4. S-100bimmunostaining in the frontal cortex. (A) control; (B) 1 day after ending PCPA treatment (T0); (C) 7 days after ending PCPA treatment (T1); (D) 14 days after PCPA treatment (T2). Arrows show typical images of S-100bimmunolabelled astrocytes. Bar522.2mm.
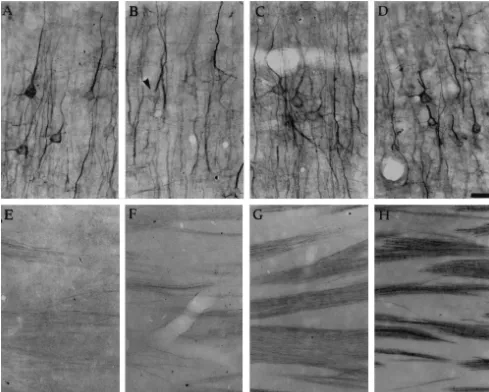
Fig. 5. Above: immunostaining for 200 KDa neurofilaments (Nf-200) in the striatum. (A) control; (B) 1 day after ending PCPA treatment (T0); (C) 7 days after ending PCPA treatment (T1); (D) 14 days after PCPA treatment (T2). Note the Nf-200 alterations in these low magnification photographs. Bar547
2
Fig. 6. Area of Nf-200 immunoreactive fibers after PCPA treatment. Data are expressed as area of Nf-200 immunolabelled fibers permm of tissue and represent the means of six to seven experiments for each region. Error bars represent the standard deviation of the means. The significant decrease in the striatal (for T1 group), hippocampal and cortical (for T0 group) Nf-200 immunolabelled fibers is followed by a significant increase in the T2 group. *,
P,0.05, **, P,0.01 and ***, P,0.001 vs. control, after one-way ANOVA and Student–Newman–Keuls post-test. †, For the striatum, values are 2
expressed as area permm of patches (striosomes) and multiplied by 0.1 to show them in the same scale as the other brain regions.
showed alterations over time. The total quantity of im- Nf-68 labelled fibers in the T0 group (45.7% of control, munocytochemically stained Nf-200 was reduced in the T0 P,0.01). In the T1 group, Nf-68 immunoreactivity began group. The reduction was observed in the thin network of to increase towards the control level (65.7% of control) to cortical dendrites from pyramidal neurons in cortical layers reach control values in the T2 group (98.4% of control), I, II and III. Image analysis, focused in the cortical layer being significantly different from T0 group (P,0.01) (Fig. III showed a reduction in Nf-200 immunostaining (85% of 7).
control), but it was not statistically significant. In the T1 Nf-200 immunolabelling in the parietal cortex showed a and T2 groups, Nf-200 labelled structures in the cortical pattern that shares some observations with the frontal layer III were significantly increased (139 and 152%, cortex. In the T0 group the Nf-200 density was sig-respectively, of control, P,0.01 for both) in the T2 group nificantly reduced in the network of dentrites from pyrami-(Fig. 6). In the T0 group Nf-68 immunolabelling was dal neurons. Image analysis, focused in the cortical layer reduced in dendrites and soma of pyramidal neurons from III confirmed this observation showing a value of 62.4% of cortical layers III and V. Image analysis, again focused on control (P,0.001), being a more important reduction than the cortical layer III showed a significant reduction of in frontal cortex. In the T1 group, an increased number of
2
Fig. 7. Area of Nf-68 immunoreactive fibers after PCPA treatment. Data are expressed as area of Nf-68 immunolabelled fibers permm of tissue and represent the means of five to eight experiments for each brain region. Error bars represent the standard deviation of the means. The significant decrease in the striatal and cortical Nf-200 immunolabelled fibers in the T0 group is followed by a significant increase in the T2 group. Hippocampal Nf-68 immunolabelled fibers in the stratum radiatum of CA-1 also increases in the T2 group. **, P,0.01, ***, P,0.001 vs. control, after one-way ANOVA and
2
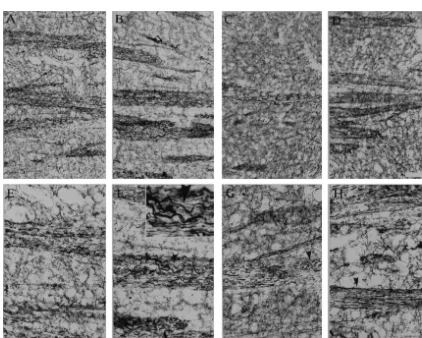
Fig. 8. Immunostaining for 200 kDa neurofilaments (Nf-200) in the hippocampus, CA-1 area of stratum radiatum. (A) control; (B) 1 day after ending PCPA treatment (T0); (C) 7 days after ending PCPA treatment (T1); (D) 14 days after PCPA treatment (T2). Note the decreased number of Nf-200 positive fibers in the T0 group, and the increase in the T2 group. Bar552.2mm.
Nf-200 immunolabelled dendrites was observed (91.8% of 5HT-T fibers were increased presenting a value similar to control), being significantly different from T0 (P,0.05 vs. control groups (96.5% of control). The area of 5HT-T T0 group). In the T2 group, Nf-200 immunoreactivity labelled fibers in the T2 group was not significantly continued increasing to reach a value of 106% of control, different from control groups (101.0% of control) (Fig. giving a value significantly different from T0 group (P, 10).
0.001) (Fig. 6). Nf-68 expression was significantly reduced In the T0 group 5HT-T positive fibers from the frontal in the dendrites and somata of pyramidal neurons in the T0 cortex were not different from those of control groups group (49.8% of control, P,0.01). In the T1 group the (99.7% of control). However, a significant increase in the density of Nf-68 immunolabelled structures began to 5HT-T positive fibers was demonstrated in the T1 (146.8% recover towards the control level reaching 83.3% of of control; P,0.001) and T2 groups (131.2% of control; control groups, being significantly different from T0 (P, P,0.001) (Figs. 10 and 11E–H).
0.01 vs. T0). Nf-68 immunoreactivity continued increasing The area covered by 5HT-T labelled fibers in the in the T2 group reaching a value of 175.1% of control parietal cortex was lower compared with the frontal cortex. (P,0.001) (Figs. 7 and 9A–D). Quantification of 5HT-T immunostained fibers showed an increase in the T0 and T1 groups (144.4 and 216.2%, 3.4. 5HT-transporter (5HT-T) respectively); only in the T1 group did it prove to be significantly different from controls (P,0.001). In the T2 5HT-T is expressed in every 5HT fiber, it is useful to group, the area of 5HT-T labelled fibers dropped to a value follow 5HT fibers when they are depleted of neurotrans- not significantly different from the controls (Fig. 10). mitter, because they are not detected by 5HT
anti-bodies. 5HT-T expression was observed in all studied
areas as thin fibers with a dense branching. Quantitative 4. Discussion
studies allowed the evaluation of the fiber density,
ex-pressed as the area of 5HT-T immunoreactive fibers per PCPA treatment is very effective at producing important unit area of tissue. 5HT depletion in brain 5HT innervation areas 5HT-T positive fibers in the striatum were observed in [1,13,25,47,49]. Some side effects in brain protein syn-the matrix, sparing syn-the patches. The image analysis was thesis have been described for high endovenous PCPA focused on the matrix where dense 5HT innervation is doses [29,30]. Considering this, we used a lower dose by present. The area of striatal fibers expressing 5HT-T was the i.p. route for our model that does not produce the same significantly reduced in the T0 group (66.4% of control; plasmatic PCPA level [12] that could saturate brain
aro-P,0.001) followed by a sharp increase in the T1 (131.3% matic amino acid carriers. We have chosen PCPA because of control, P,0.01) and T2 groups (169.7% of control; it produces an important 5HT depletion without being
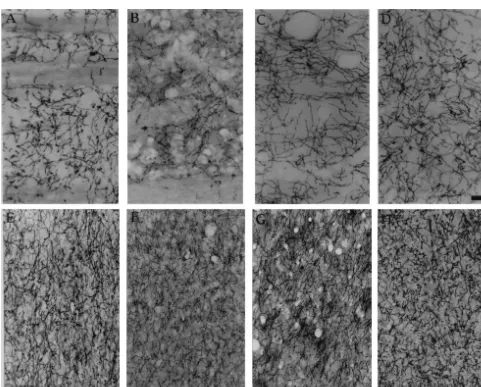
Fig. 9. Above: immunostaining for 68 kDa neurofilaments (Nf-68) in the parietal cortex. (A) control; (B) 1 day after ending PCPA treatment (T0); (C) 7 days after ending PCPA treatment (T1); (D) 14 days after PCPA treatment (T2). Note that Nf-68 immunostaining decreased significantly in the dendrites and soma (arrow) of cortical pyramidal neurons in the T0 group. Bar522.2mm. Below: immunostaining for Nf-68 in the striatum. (E) control; (F) 1 day after ending PCPA treatment (T0); (G) 7 days after ending PCPA treatment (T1); (H) 14 days after PCPA treatment (T2). Decreased Nf-68 immunostaining in the T0 group is followed by an increase in the T1 and T2 groups. Bar541.2mm.
treatment [47]. Astroglial reaction detected in this model groups, they were observed again in the T2 group. The of PCPA induced 5HT depletion reaches a peak 1 week recovery delay observed in 5HT-IR fibers is in accordance after the PCPA treatment, and seems to be completely with previous reports [1,49]. Biochemical reports have reversible [43]. indicated that the 5HT level is recovered in 12–15 days In this paper we studied the time-course events of glial [25] after PCPA treatment, probably involving an up-and neuronal response after PCPA treatment. The treatment regulation of TrpOH gene expression [41]. In spite of the effectiveness was analyzed by the quantification of 5HT-IR absence of 5HT-IR fibers in innervation areas in the T0 in the DRN and the observation of 5HT-IR fibers in group, 5HT-T immunoreactivity has demonstrated that innervation areas. The 5HT-IR in the somata of DRN serotoninergic fibers are present, but depleted of 5HT. This neurons was significantly reduced by PCPA treatment. evidence confirms previous results which demonstrated Once the treatment ended, 5HT-IR rapidly recovered that PCPA is not neurotoxic for serotoninergic projections towards the control level. In the T2 group, 5HT-IR was [10].
2
Fig. 10. Area of 5HT-T immunolabelled fibers after PCPA treatment. Data are expressed as area of 5HT-T immunolabelled fibers permm of tissue and represent the means of five to nine experiments for each region. Error bars represent the standard deviation of the means. The significant decrease in the striatal and hippocampal 5HT-T immunolabelled fibers is followed by a significant increase in the T1 and T2 groups. Parietal and frontal cortex also showed a significant increase in the 5HT-T fibers in the T1 (parietal and frontal cortex) and T2 groups (only frontal cortex). *, P,0.05, **, P,0.01, ***,
P,0.001 vs. control, after one-way ANOVA and Student–Newman–Keuls post-test.
S-100b protein level in astroglial cells. Intracellular S- creased caliber, fragmentation and loss striosomal organi-100b immunostaining is increased after PCPA treatment. zation. These alterations are important in the T0 and T1 In fact, this result is not totally in accordance with those groups, but are minimal in the T2 group. The decreased found in other 5HT-depletion models [16]. Considering Nf-200 positive fibers in hippocampal CA-1 area, where that the activation of glial 5HT1A receptors (by 5HT or basal dentrites from pyramidal neurons are located, have another agonist) leads to the release of S-100b from been also described by other authors who found decreased astrocytes [51,52,54], we propose that low 5HT levels immunostaining for the dendritic marker MAP-2 in another could reduce such a release giving an accumulation of model of 5HT depletion [5]. Our results showed that synthesized S-100b within the astrocytes. However, it neurofilaments expression is reduced and Nf-200 mor-cannot be discounted that up-regulation of S-100b protein phological alterations are important when intracellular S-synthesis might contribute to the observed increase of the 100b immunostaining is maximal, probably reflecting the intracellular S-100b immunostaining. The most important reduced extracellular availability of this growth factor. intracellular S-100b accumulation in astroglial cells was Interestingly, in brains of patients with Alzheimer’s dis-observed when 5HT-IR was minimal, in the T0 group. ease, neurofilament alterations are found together with During the recovery of the 5HT levels, S-100b immuno- increased S-100b immunostaining in astrocytes [14,15]. reactivity decreased dramatically. Two weeks after ending The increased Nf-68 and Nf-200 expressions in some the treatment (T2 group) both cortex and hippocampus regions in the T2 group might indicate a sprouting have recovered the normal S-100b immunostaining, phenomena after S-100b level are normalized. The ob-whereas the striatum still shows more intense S-100b served sprouting could be an in vivo effect of released immunoreactivity. Considering the recovery of the 5HT S-100b.
level after PCPA treatment, it could be hypothesized that The study of 5HT fibers was performed following the accumulated S-100b is released from glial cells when the 5HT-T immunoreactivity. The reduction in striatal and 5HT level recovers. The expression of this event may be hippocampal 5HT-T positive fibers in the T0 group was an the reduction of the intracellular S-100b immunostaining. unexpected result, because PCPA is not neurotoxic for Interestingly, maximal S-100b immunostaining, which 5HT projections [10]. Considering our hypothesis of occurred 1 day after the treatment, did not seem to be reduced extracellular availability of S-100bdue to the lack coincident with the maximal astroglial reaction, that we of 5HT1A stimulation, we propose that the reduction in the demonstrated by glial fibrillary acidic protein (GFAP) 5HT-T fibers might be secondary to the reduced extracellu-immunostaining 1 week after the PCPA treatment [43]. lar level of the growth factor. It has been proposed that the Moreover, glial morphology was not totally recovered until release of glial S-100bcould cause regeneration or sprout-35 days post-treatment [unpublished observations]. ing of neuronal terminals [4,52]. This hypothesis may be It is well known that serotoninergic raphe nuclei have supported by the similar profile of neurofilament expres-different sensitivities to PCPA and other serotoninergic sion and the increase in 5HT-T fibers found in the T1 and toxins, being the DRN most affected by PCPA and other T2 groups. Moreover, considering that S-100b stabilizes serotoninergic toxins, whereas the median raphe is less the neuronal cytoskeleton [18], we may speculate that sensitive [35,36,47,49]. Another interesting result could be neurofilament alterations observed immediately after PCPA gained by taking into account the different sources of 5HT treatment, during S-100bintracellular accumulation, could innervation: hippocampus receives 5HT innervation from be due to the reduction of extracellular S-100b. The median raphe predominantly, cortex receives innervation complete idea is summarized in Fig. 12.
from dorsal and median raphe [9,50]. Both cortex and Obviously our speculations should be verified by a hippocampus have recovered the control levels of S-100b biochemical quantification of extracellular S-100b, during immunostaining in the T2 group showing a very similar these events. We believe that our in vivo model with a profile. On the other hand striatum, predominantly inner- morphometric semiquantitative approach allows a fine vated by fibers from the DRN [9,50], does not recover anatomical resolution. We are currently performing some S-100b control levels in the T2 group. experiments to confirm the S-100b release by studying in The neuronal cytoskeleton presents a triplet of neurofila- vivo other biological effects described in vitro [21,22]. The ment proteins that are the main determinants of axonal presented 5HT-depletion model that implies a S-100b
caliber [19]. Altered neurofilament expression may lead to intracellular accumulation, with a consequent reduction in neurodegeneration [23]. We analyzed the expression of two the availability of the growth factor can be interesting to neurofilaments: Nf-200 which is present in mature axons study in vivo biological effects of S-100b. It would be and Nf-68 which increases during neurite outgrowth and useful to complement findings already described in vivo in maturation. After PCPA treatment we found reduced Nf- transgenic mouse [53] and in vitro [21,22]. The S-100b
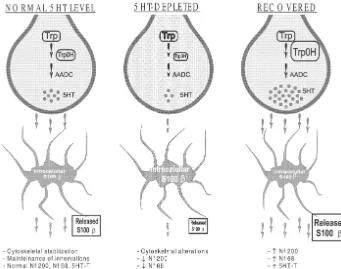
re-Fig. 12. Proposed sequence of events in the recovering after PCPA-induced 5HT depletion. Normal: in the normal brain tryptophan (Trp) is hydroxylated by the TrpOH (the rate limiting enzyme in the 5HT synthesis) and decarboxylated by the aromatic amino acid decarboxylase (AADC) to give 5HT. Released 5HT interacts with 5HT1Areceptor in glial cells. By an unknown mechanism S-100bis released from glial cells. Extracellular S-100bstabilizes cytoskeleton and maintains innervation. Depleted: during 5HT depletion the activity of TrpOH is reduced, giving a lower 5HT release. There is lower activation of 5HT1Areceptors and minor S-100b release, S-100bis accumulated inside glial cells giving increased S-100b immunostaining. The low availability of extracellular S-100bproduces alterations in the neuronal cytoskeleton and the reduction of Nf-68, Nf-200 and 5HT-T positive fibers. Recovering: an important pool of 5HT is synthesized because of the increased TrpOH expression [41]. 5HT produces activation of 5HT1Areceptors and the S-100b release. A reduction of intracellular S-100b immunostaining is observed. The effect of the S-100b release could be the normalization of cytoskeleton and the sprouting of fibers.
giant: anatomy and plasticity of the brain serotonergic system, J. action, S-100b accumulation and neuronal morphological
Clin. Psychiatry 52 (Suppl.) (1991) 4–16. and functional alterations.
[5] E.C. Azmitia, V.J. Rubinstein, J.A. Strafaci, J.C. Rios, P.M. Whitaker-Azmitia, 5-HT1A agonist and dexamethasone reversal of
p-chloroamphetamine induced loss of MAP-2 and synaptophysin
immunoreactivity in adult rat brain, Brain Res. 677 (1995) 181–192. [6] J.M. Brezun, A. Daszuta, Depletion in serotonin decreases
neuro-Acknowledgements
genesis in the dentate gyrus and the subventricular zone of adult rats, Neuroscience 89 (1999) 999–1002.
We would like to thank Emerita Vilela de Bianchieri for [7] A. Brusco, S. Peressini, J. Pecci Saavedra, Serotonin-like immuno-her technical assistance. Work supported by grants reactivity and anti 5-hydroxytryptamine (5HT) antibodies: ultra-UBACYT TM-05 and CONICET PIP4455. structural application in the central nervous system, J. Histochem.
Cytochem. 31 (1983) 524–530.
[8] W.J. Burke, D.H. Park, H.D. Chung, G.L. Marshall, J.H. Haring, T.H. Joh, Evidence for decreased transport of tryptophan hydroxy-lase in Alzheimer’s disease, Brain Res. 537 (1990) 83–87.
References [9] A. Consolazione, A.C. Cuello, CNS serotonin pathways, in: N.N.
Osborne (Ed.), Biology of Serotoninergic Transmission, Wiley, New [1] C.K. Aghajanian, M.J. Kuhar, R.H. Roth, Serotonin-containing York, 1982, pp. 29–61.
3 neuronal perikarya and terminals: Differential effects of p-chloro- [10] K.M. Dewar, L. Grondin, M. Carli, L. Lima, T.A. Reader, [ H] phenylalanine, Brain Res. 54 (1973) 85–101. Paroxetine binding and serotonin content of rat cortical areas, [2] E.C. Azmitia, K. Dolan, P.M. Whitaker-Azmitia, S-100B but not hippocampus, neostriatum, ventral mesencephalic tegmentum and NGF, EGF, insulin or calmodulin is a CNS serotonergic growth midbrain raphe nuclei region following p-chlorophenylalanine and factor, Brain Res. 516 (1990) 354–356. p-chloroamphetamine treatment, J. Neurochem. 58 (1992) 250–257. [3] E.C. Azmitia, D.R. Marshak, P.M. Whitaker-Azmitia, Functional [11] C. Fages, G. Le Prince, M. Didier-Bazes, B. Rolland, H. Hardin, M. interactions between glial S-100bprotein and CNS 5HT neurons, J. Tardy, Long-term astroglial reaction to serotonergic fiber degenera-Cell. Biochem. 14F (1990) 8. tion, Brain Res. 639 (1994) 161–166.
14
p-chloro-phenylalanine as an inhibitor of tryptophan-5-hydroxylase, J. Neuro- [33] J.P. Liu, J.M. Lauder, S-100b and insulin-like growth factor-II differentially regulate growth of developing serotonin and dopamine chem. 17 (1970) 1221–1235.
neurons in vitro, J. Neurosci. Res. 33 (1992) 248–256. [13] G. Griebel, 5-Hydroxytryptamine-interacting drugs in animal models
[34] C. Mazer, J. Muneyyirci, K. Taheny, N. Raio, A. Borella, P. of anxiety disorders: more than 30 years of research, Pharmacol.
Whitaker-Azmitia, Serotonin depletion during synaptogenesis leads Ther. 65 (1995) 319–395.
to decreased synaptic density and learning deficits in the adult rat: a [14] W.S. Griffin, L.C. Stanley, C. Ling, L. White, V. MacLeod, L.J.
possible model of neurodevelopmental disorders with cognitive Perrot, C.L. White III, C. Araoz, Brain interleukin 1 and S-100
deficits, Brain Res. 760 (1997) 68–73. immunoreactivity are elevated in Down syndrome and Alzheimer
[35] L.A. Mamounas, M.E. Molliver, Evidence for dual serotonergic disease, Proc. Natl. Acad. Sci. USA 86 (1989) 7611–7615.
projections to neocortex: axons from the dorsal and median raphe [15] W.S. Griffin, J.G. Sheng, J.E. McKenzie, M.C. Royston, S.M.
nuclei are differentially vulnerable to the neurotoxin p-chloro-Gentleman, R.A. Brumback, L.C. Cork, M.R. Del Bigio, G.W.
amphetamine (PCA), Exp. Neurol. 102 (1988) 23–36. Roberts, R.E. Mrak, Life-long overexpression of S100bin Down’s
[36] L.A. Mamounas, C.A. Mullen, E. O’Hearn, M.E. Molliver, Dual syndrome: implications for Alzheimer pathogenesis, Neurobiol.
serotoninergic projections to forebrain in the rat: morphologically Aging 19 (1998) 401–405.
distinct 5-HT axon terminals exhibit differential vulnerability to [16] J.H. Haring, A. Hagan, J. Olson, B. Rodgers, Hippocampal serotonin
neurotoxic amphetamine derivatives, J. Comp. Neurol. 314 (1991) levels influence the expression of S100 b detected by
immuno-558–586. cytochemistry, Brain Res. 631 (1993) 119–123.
[37] D.C. Molliver, M.E. Molliver, Anatomic evidence for a neurotoxic [17] G.R. Heninger, Indoleamines: the role of serotonin in clinical effect of (6)-fenfluramine upon serotonergic projections in the rat,
disorders, in: Psychopharmacology: The Fourth Generation of Brain Res. 511 (1990) 165–168.
Progress, Raven Press, New York, 1995, pp. 471–482. [38] M.E. Molliver, U.V. Berger, L.A. Mamounas, D.C. Molliver, E. [18] J. Hesketh, J. Baudier, Evidence that S100 proteins regulate O’Hearn, M.A. Wilson, Neurotoxicity of MDMA and related microtubule assembly and stability in rat brain extracts, Int. J. compounds: anatomic studies, Ann. NY Acad. Sci. 600 (1990)
Biochem. 18 (1986) 691–695. 649–664.
[19] P.N. Hoffman, D.W. Cleveland, J.W. Griffin, P.W. Landes, N.J. [39] C.M. Muller, A.C. Akhavan, M. Bette, Possible role of S-100 in Cowan, D.L. Price, Neurofilament gene expression: a major deter- glia-neuronal signalling involved in activity-dependent plasticity in minant of axonal caliber, Proc. Natl. Acad. Sci. USA 84 (1987) the developing mammalian cortex, J. Chem. Neuroanat. 6 (1993)
3472–3476. 215–227.
[20] P.N. Hoffman, D.W. Cleveland, Neurofilament and tubulin expres- [40] R.A. Nixon, R.K. Sihag, Neurofilament phosphorylation: a new look sion recapitulates the developmental program during axonal regene- at regulation and function, Trends Neurosci. 14 (1991) 501–506. ration: induction of a specificb-tubulin isotype, Proc. Natl. Acad. [41] D.H. Park, D.M. Stone, H. Baker, K.S. Kim, T.H. Joh, Early Sci. USA 85 (1988) 4530–4533. induction of rat brain tryptophan hydroxylase (TPH) mRNA follow-[21] J. Hu, F. Castets, J.L. Guevara, L.J. Van Eldik, S100b stimulates ing p-chlorophenylalanine (PCPA) treatment, Molec. Brain Res. 22
inducible nitric oxide synthase activity and mRNA levels in rat (1994) 20–28.
cortical astrocytes, J. Biol. Chem. 271 (1996) 2543–2547. [42] T.V. Petrova, J. Hu, L.J. Van Eldik, Modulation of glial activation by [22] J. Hu, A. Ferreira, L.J. Van Eldik, S100binduces neuronal cell death astrocyte-derived protein S100B: differential responses of astrocyte
through nitric oxide release from astrocytes, J. Neurochem. 69 and microglial cultures, Brain Res. 853 (2000) 74–80.
(1997) 2294–2301. [43] A.J. Ramos, P. Tagliaferro, E.M. Lopez, J. Pecci-Saavedra, A. [23] J.P. Julien, W.E. Mushynski, Neurofilaments in health and disease, Brusco, Neuronal and glial recovering after a parachloro-Prog. Nucleic Acid Res. Mol. Biol. 61 (1998) 1–23. phenylalanine-induced serotonin depletion, J. Neurochem. 72 (1999) [24] D. Kligman, D.R. Marshak, Purification and characterization of a S54C.
neurite extension factor from bovine brain, Proc. Natl. Acad. Sci. [44] F. Richard, J.L. Sanne, O. Bourde, D. Weissman, M. Ehret, C. Cash, USA 82 (1985) 7136–7139. M. Maitre, J.F. Pujol, Variation of tryptophan-5-hydroxylase con-[25] B.K. Koe, A. Weissman, p-Chlorophenylalanine: A specific depletor centration in the rat raphe dorsalis nucleus after p-chloro-of brain serotonin, J. Pharm. Exp. Ther. 154 (1966) 499–516. phenylalanine administration. I. A model to study the turnover of the [26] J.M. Lauder, H. Krebs, Serotonin as a differentiation signal in early enzymatic protein, Brain Res. 536 (1990) 41–45.
neurogenesis, Dev. Neurosci. 1 (1978) 15–30. [45] H.G. Series, M.E. Molliver, Immunocytochemical evidence for [27] J.M. Lauder, J.A. Wallace, M.B. Wilkie, A. DiNome, H. Krebs, serotoninergic neurotoxicity of
N-ethyl-methylenedioxyam-Roles for serotonin in neurogenesis, Monogr. Neural Sci. 9 (1983) phetamine (MDE), Exp. Neurol. 128 (1994) 50–58.
3–10. [46] J.G. Sheng, R.E. Mrak, K.R. Bales, B. Cordell, S.M. Paul, R.A. [28] J.M. Lauder, Ontogeny of the serotonergic system in the rat: Jones, S. Woodward, X.Q. Zhou, J.M. McGinness, W.S. Griffin, serotonin as a developmental signal, Ann. NY Acad. Sci. 600 (1990) Overexpression of the neuritotrophic cytokine S100bprecedes the
297–314. appearance of neuritic b-amyloid plaques in APPV717F mice, J.
[29] P. Lepetit, M. Touret, E. Grange, N. Gay, P. Bobillier, Inhibition of Neurochem. 74 (2000) 295–301.
methionine incorporation into brain proteins after the systemic [47] P. Tagliaferro, A.J. Ramos, E.M. Lopez, J. Pecci Saavedra, A. administration of p-chlorophenylalanine andL-5-hydroxytryptophan, Brusco, Neural and glial effects of a chronic parachloro-Eur. J. Pharmacol. 209 (1991) 207–212. phenylalanine induced serotonin synthesis inhibition, Molec. Chem. [30] P. Lepetit, M. Touret, E. Grange, N. Gay, P. Bobillier, Decreased Neuropathol. 32 (1997) 195–211.
protein synthesis in hypothalamic nuclei following L-5-hydroxy- [48] P. Tagliaferro, A.J. Ramos, J.J. Lopez-Costa, E.M. Lopez, J. Pecci tryptophan in intact and p-chlorophenylalanine-pretreated rats, Saavedra, A. Brusco, Increased activity of NOS in a model of Neurosci. Lett. 122 (1991) 218–220. chronic serotonin synthesis inhibition, Arch. Pharmacol. 358 (Suppl. [31] Y. Li, J. Wang, J.G. Sheng, L. Liu, S.W. Barger, R.A. Jones, L.J. Van 1) (1998) 28.
Eldik, R.E. Mrak, W.S.T. Griffin, S100b increases levels of b- [49] I. Tohyama, M. Kameyama, H. Kimura, Quantitative morphometric amyloid precursor protein and its encoding mRNA in rat neuronal analysis of two types of serotonin-immunoreactive nerve fibres cultures, J. Neurochem. 71 (1998) 1421–1428. differentially responding to p-chlorophenylalanine treatment in the [32] L.H. Lin, L.J. Van Eldik, N. Osheroff, J.J. Norden, Inhibition of rat brain, Neuroscience 26 (1988) 971–991.
[51] P.M. Whitaker-Azmitia, R. Murphy, E.C. Azmitia, Stimulation of factor S-100b show neuronal cytoskeletal and behavioral signs of astroglial 5-HT1Areceptors releases the serotonergic growth factor, altered aging processes: implications for Alzheimer’s disease and protein S-100, and alters astroglial morphology, Brain Res. 528 Down’s syndrome, Brain Res. 776 (1997) 51–60.
(1990) 155–158. [54] C.C. Wilson, K.M. Faber, J.H. Haring, Serotonin regulates synaptic [52] P.M. Whitaker-Azmitia, C. Clarke, E.C. Azmitia, Localization of connections in the dentate molecular layer of adult rats via 5-HT1A 5-HT1A receptors to astroglial cells in adult rats: implications for receptors: evidence for a glial mechanism, Brain Res. 782 (1998) neuronal-glial interactions and psychoactive drug mechanism of 235–239.
action, Synapse 14 (1993) 201–205. [55] T. Yamamoto, A. Hirano, Nucleus raphe dorsalis in Alzheimer’s [53] P.M. Whitaker-Azmitia, M. Wingate, A. Borella, R. Gerlai, J. Roder, disease: neurofibrillary tangles and loss of large neurons, Ann.