Brain Research 888 (2001) 172–175
www.elsevier.com / locate / bres
Short communication
How central is nitric oxide (NO) to the activation of c-fos in spinal
neurones following noxious peripheral stimulation in the rat?
a b c c ,
*
M. Nazli , E.S. Hismiogullari , T. Thippeswamy , R. Morris
a
Department of Veterinary Anatomy, University of Kafkas, Kars, Turkey b
Department of Veterinary Pharmacology and Toxicology, University of Ankara, Ankara, Turkey c
Department of Veterinary Preclinical Sciences, University of Liverpool, Brownlow Hill and Crown Street, Liverpool, L69 3BX, UK
Accepted 10 October 2000
Abstract
Intrathecal application of high doses of NO-donor compounds in the anaesthetised rat was not found to cause any induction of c-fos in spinal neurones. Furthermore, intrathecal injection of a NO-synthase (NOS) blocking drug did not alter the numbers of c-fos positive neurones induced by noxious stimulation. Additionally very little colocalization between NOS and c-fos following noxious stimulation was found. Collectively these data give no support for a role for NO in the noxiously evoked induction of c-fos. 2001 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Pain modulation: pharmacology
Keywords: Nitric oxide; c-fos; Spinal cord; Pain; Nociception
Noxious peripheral stimulation causes the induction of urethane (1 mg / kg, i.p.) and prior to vascular perfusion the intermediate-early gene c-fos in the nuclei of cells in they were given a lethal dose of pentobarbitone (60 mg / the appropriate somatotopically related region of the spinal kg, i.p.). Animals were bred in the Biomedical Services cord, in particular in LII [6]. The neuronal isoform of nitric Unit of the University of Liverpool and all procedures oxide synthase (nNOS) is also located in many neurones in were performed under UK Home Office statutory regula-the inner part of LII [3]. This led to regula-the suggestion that NO tion. For studies involving intrathecal drug application, is involved in nociception [9] and may have an important large adult rats (over 300 g) were used and the intrathecal role in the induction of c-fos [7]. This is a very attractive cannula was inserted in the sub-dural space via a cut in the proposition as it would be an elegant mechanism linking atlanto-occipital membrane. The cannula was gently fed excitation of spinal neurones by nociceptors through the down this space to a level just rostral to lumbar enlarge-diffusion of NO to the induction opioids in intrinsic ment taking great care to avoid any spinal cord irritation or interneurones, resulting in a reduction in nociceptive damage which strongly activate c-fos.
signalling [2]. Several experiments have provided evidence The following treatments were used: 100 nmol of 3-for such a role of NO in c-fos induction following noxious morpholinylsydnoneimine chloride (SIN-1) (Tocris) (n5
peripheral stimulation [4,7,10], however, this has not been 6); 1 mmol SIN-1 to which was added 1 nmol of the consistently demonstrated [1]. The present experiments cGMP/ cAMP phosphodiesterase inhibitor, 3-isobutyl-1-have re-examined the role of NO in c-fos induction in methylxanthine (IBMX) (Sigma) (n51); 10 nmol of S-spinal neurones following noxious peripheral stimulation. nitroso-N-acetylpenicillamine (SNAP) (Tocris) (n52); ve-Adult rats (Wistar) of either sex were anaesthetised with hicle controls (n53). All injections were given in 10 ml, washed in with 10 ml of saline and repeated three times at half hour intervals. In a further 15 animals either injections
*Corresponding author. Tel.:144-151-794-4227; fax: 144-151-794- G
of N -nitro-L-arginine methyl ester (L-NAME) (10 nmol)
4243.
E-mail address: [email protected] (R. Morris). or its inactive isomer D-NAME (10 nmol) or vehicle
M. Nazli et al. / Brain Research 888 (2001) 172 –175 173
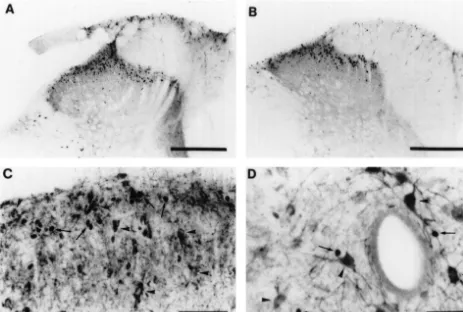
(saline) (n55 each treatment) were given. Injections were mustard oil treatment in animals treated with L-NAME 10 ml volume, washed in with 10 ml of vehicle and compared with those treated with D-NAME (Fig. 1A and repeated four times at 30 min intervals. Five minutes after B, Table 1). Neurones showing co-localisation of nNOS the first injection one paw was painted with 40% mustard and Fos irrespective of the type of stimulus used were rare oil (Allylisothiocyanate, Merck) in liquid paraffin. To (Fig. 1C and D). For example out of 5788 Fos positive examine the influence of cannulation and drug injection nuclei observed in LI–III in mustard-oil-treated animals controls were conducted in which both procedures were only one was in a nNOS positive cell. Sections were also omitted. In a further 23 rats one of the following stimuli made from more rostral lumbar levels in these studies and were applied to one hindlimb; topical application of 40% co-localisation of nNOS and Fos was slightly more mustard oil (12 animals), subcutaneous injection of for- frequent in the lateral horn (39 out of 631).
malin (5% or 10%, 50 ml into the plantar surface, three Collectively these data were unable to confirm any role animals each) or thermal stimulation (immersion of the for NO in c-fos induction in the spinal cord. The most paw in water at 48 or 528C for 20 s, three animals at each direct experiment of applying NO-donors to the spinal cord temperature). Two hours after the first noxious stimulus the caused at best a very weak induction of c-fos. The NO-animals were fixed by vascular perfusion with 4% parafor- donors were made up fresh and were able to induce maldehyde in 0.1 M phosphate buffered saline (PBS) (pH synthesis of cGMP. In a recent study microdialysis of 7.2). In all cannulated animals injection of 10 ml of SIN-1 in the middle of the spinal cord gave a significant pontamine sky blue dye was used to confirm the correct and clear induction of c-fos for up to 400 mm from the position of the cannula tip. In any animal in which damage dialysis fibre [13] and it is difficult to explain this to the spinal cord was visible at either a gross or a disparity.
microscopic level the data obtained were excluded (not We were also unable to confirm the inhibitory action of included in the above n-values). The lumbar tissue block NOS-blocking drugs on c-fos induction. The first study to was selected with reference to the L4 root entry zone and propose this mechanism used direct spinal injection of the was 2.5 mm rostral and caudal to this root (i.e. 5 mm antagonists in mice [7]. This and a subsequent study in the length). Sections (40 mm thick) were cut serially in the rat [10] used much higher NOS-blocker concentrations and transverse plane on a freeze knife microtome and pro- baseline levels of c-fos in vehicle controls were much cessed free floating. The sections were collected into 10 higher due to the route of drug administration. The doses tubes of PBS such that each tube contained sections at 400 of blocker used in the present study were based on those
mm intervals through the complete tissue block. Counts found to block thermal hyperalgesia in rats. However, in were carried out on all the sections from one tube thus another more recent study a clear dose-related suppression giving a systematic random sample from the whole block. of c-fos activation, induced by injection of formalin into Staining for nNOS and c-fos used standard immuno- one hindpaw, was seen using doses ranging from 0.01
v
cytochemical methods the sections being incubated in a nmol to 10 nmol of N -nitro-L-arginine as the NOS mixture of rabbit raised anti-Fos protein (CRB, 1:1000) inhibitor [4]. In this study c-fos induction, 1 h after the and sheep raised anti-nNOS (gift from P. Emson, 1:5000) noxious stimulus was investigated in animals anaesthetised overnight at 48C. They were then incubated in biotinylated throughout with sodium pentobarbital. The drugs were anti-rabbit (1:200, 1 h RT, Jackson), streptavidin HRP delivered by an intrathecal cannula inserted via a thoracic (1:1000, Amersham) and chromagen stained [11]. Fol- laminectomy. Unfortunately, no figures are given in this lowed by incubation in biotinylated anti-sheep (1:200, 1 h paper for numbers of Fos neurones in cannulated animals RT, Jackson), streptavidin HRP (1:1000) and incubation that had a vehicle injection that were not stimulated with with a second chromagen (Vector VIP kit). Counts were formalin, hence, it is difficult to assess c-fos induction due expressed as numbers of Fos nuclei / section / area. to surgical procedures. A role for NO in c-fos induction In the lumbar spinal cords of animals treated with following plantar injection of formalin is also supported by intrathecal SIN-1 or SNAP small numbers of c-fos positive the reduction in c-fos produced by intrathecal application nuclei were observed on either side of the cord. The counts of a cGMP-kinase Ia blocker [12]. This study was very obtained were: SIN-1, LI–LIII, 11.664.5, rest of cord, carefully conducted with chronically implanted cannula 6.261.6; SNAP, LI–LIII, 2.861.7, rest of cord, 7.063 and the figures for c-fos induction are comparable to those (mean6S.E.M., of numbers of positive nuclei per section / found in similar studies using plantar injection of formalin. 2). Increasing the concentration of SIN-1 and adding An increase in spinal cyclic GMP-dependent protein kinase IBMX made no difference and only very low numbers of was also shown in this study 96 h after formalin injection Fos positive nuclei were seen in this animal. Overall the into one hind paw, this elevation being reduced by numbers of Fos nuclei were slightly more frequent than in blockers of cGMP, NOS and NMDA receptor antagonist saline treated animals (LI–LIII, 5.262.3, rest of cord, MK801 [12]. Recently, using Western blot analysis a more
2.661.2). quantitative evaluation has been conducted. This
174 M. Nazli et al. / Brain Research 888 (2001) 172 –175
Fig. 1. Effects ofL-NAME andD-NAME on Fos induction by mustard oil stimulation (A and B) and colocalisation of Fos and nNOS following noxious
stimulation (C and D). (A, B) Typical sections from animals in which the paw ipsilateral to the section area shown was painted with 40% mustard oil. (A)
L-NAME and (B)D-NAME treated animals. (C, D) Sections showing the ipsilateral LI–LIII and central canal region of mustard-oil-treated animals. The
arrow heads indicate nNOS positive neurones and the arrows Fos positive nuclei. Scale bars, A and B, 250 mm; C, 100mm; D, 50mm.
The comparison of the distributions of NOS and Fos influence of neuronal injury due to the route of drug gave the very clear result that NO is not causing the administration has to be considered in some studies. induction of c-fos in the cells in which it is produced. This
confirms similar findings made following formalin
in-jections [5] but in another recent study 14% colocalisation Acknowledgements
between Fos and NOS was found following formalin
stimulation [8]. This work was supported by the Wellcome Trust and
In conclusion, whilst NO may be involved in c-fos Action Research. M.N. and E.H. were supported by synthesis following noxious peripheral stimulation the Turkish Government Scholarships.
Table 1
a Effects of NOS-blocker on c-fos induced by mustard oil stimulation
Noxious Intrathecal N-tested N-Fos nuclei / section / region of spinal cord counted stimulus drug
Ipsilateral to stimulus Contralateral to stimulus
LI–LIII Rest of cord LI–LIII Rest of cord
Mustard oil Saline 5 62.2614.7 9.363.5 19.869.4 4.161.6
L-NAME 5 65.4618.1 4.163.0 36.7619.1 2.762.0
D-NAME 5 54.3610.1 1.661.0 22.666.4 1.260.7
No catheter or drug 5 65.5612.2 12.964.1 34.1614.6 8.263.9
a
M. Nazli et al. / Brain Research 888 (2001) 172 –175 175
[7] J.H. Lee, G.L. Wilcox, A.J. Beitz, Nitric oxide mediates Fos References
expression in the spinal cord induced by mechanical noxious stimulation, Neuroreport 3 (1992) 841–844.
[1] D.A. Bereiter, D.F. Bereiter, N-methyl-D-aspartate and non-N- [8] S. Leong, H. Liu, J. Yeo, Nitric oxide synthase and glutamate methyl-D-aspartate receptor antagonism reduces Fos-like immuno- receptor immunoreactivity in the rat spinal trigeminal neurons reactivity in central trigeminal neurons after corneal stimulation in expressing Fos protein after formalin injection, Brain Res. 855 the rat, Neuroscience 73 (1996) 249–258. (2000) 107–115.
[2] G. Draisci, K.C. Kajander, R. Dubner, G.J. Bennett, M.J. Iadarola, [9] S.T. Meller, G.F. Gebhart, Nitric oxide (NO) and nociceptive Up-regulation of opioid gene expression in spinal cord evoked by processing in the spinal cord, Pain 52 (1993) 127–136.
experimental nerve injuries and inflammation, Brain Res. 560 [10] A.K. Roche, M. Cook, G.L. Wilcox, K.C. Kajander, A nitric oxide (1991) 186–192. synthesis inhibitor (L-NAME) reduces licking behavior and Fos-[3] N.J. Dun, S.L. Dun, U. Forstermann, L.F. Tseng, Nitric oxide labeling in the spinal cord of rats during formalin-induced
inflamma-synthase immunoreactivity in rat spinal cord, Neurosci. Lett. 147 tion, Pain 66 (1996) 331–341.
(1992) 217–220. [11] S.Y. Shu, G. Ju, L.Z. Fan, The glucose oxidase-DAB-nickel method [4] W.C. Gao, J.T. Qiao, Nitric oxide contributes to both spinal in peroxidase histochemistry of the nervous system, Neurosci. Lett.
nociceptive transmission and its descending inhibition in rats: an 85 (1988) 169–171.
immunocytochemical study, Neurosci. Lett. 240 (1998) 143–146. [12] Y.X. Tao, A. Hassan, E. Haddad, R.A. Johns, Expression and action [5] T. Herdegen, S. Rudiger, B. Mayer, R. Bravo, M. Zimmermann, of cyclic GMP-dependent protein kinase Ialpha in inflammatory Expression of nitric oxide synthase and colocalisation with Jun, Fos hyperalgesia in rat spinal cord, Neuroscience 95 (2000) 525–533. and Krox transcription factors in spinal cord neurons following [13] J. Wu, L. Fang, Q. Lin, W.D. Willis, Fos expression is induced by noxious stimulation of the rat hindpaw, Brain Res. Mol. Brain Res. increased nitric oxide release in rat spinal cord dorsal horn,
22 (1994) 245–258. Neuroscience 96 (2000) 351–357.