Pinitol accumulation in mature leaves of white clover in
response to a water deficit
Michael T. McManus
a,*
,1, Rod L. Bieleski
b, John R. Caradus
a,
David J. Barker
aaNew Zealand Pastoral Agriculture Research Institute Ltd(AgResearch Grasslands),Pri6ate Bag11008,
Palmerston North,New Zealand
bNew Zealand Horticultural and Food Research Institute,Mount Albert Research Centre,Pri6ate Bag92169,
Auckland,New Zealand
Received 24 March 1999; received in revised form 28 June 1999; accepted 30 June 1999
Abstract
In this study, two genotypes from the white clover breeding line ‘Syrian selection’, which demonstrates improved survival in summer drought, and two from the white clover cultivar ‘Grasslands Kopu’, a more drought-sensitive cultivar, are compared in terms of their responses to a water deficit. Plants of each genotype were grown in a temperature-controlled glasshouse and a water deficit imposed through water deprivation. Proline content and the relative abundance of soluble carbohydrates were determined in mature leaf tissue excised from water-sufficient plants, from plants after a short-term period of water deprivation (prior to the onset of a significant change in leaf water potential (ct)) and from plants after a longer period of water deprivation (after a significant change inct).
Proline accumulated in concert with the onset of a significant change inct; the highest content of 2.7 mg/g FW being measured in the Syrian selection genotypes compared with 2.4 mg/g FW measured in Kopu. In water-sufficient leaf tissue, pinitol was the major soluble carbohydrate present, with a significantly higher content (p=0.027) in terms of relative abundance in the Syrian selection genotypes when compared with the Kopu genotypes. After short-term water deprivation, pinitol was again an abundant soluble carbohydrate but the proportion of sucrose (in the Syrian selection genotypes) and fructose (in the Kopu genotypes) had increased to comprise significant levels in the leaf tissue. After a longer period of water deprivation, pinitol was again the major sugar present and represented a significantly higher proportion of leaf soluble carbohydrate (p=0.003) in the Kopu genotypes when compared with the Syrian selection genotypes. The results show that pinitol is the major soluble sugar present in mature leaves of white clover when subjected to a significant water deficit. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Trifolium repensL.; Pinitol; Proline; Water deficit; White clover
www.elsevier.com/locate/envexpbot
* Corresponding author. Tel.: +64-6-3506154; fax: +64-6-3505694. E-mail address:[email protected] (M.T. McManus)
1Present address: Institute of Molecular BioSciences, Massey University, Private Bag 11222, Palmerston North, New Zealand.
1. Introduction
Two major functions have been proposed for the water-soluble sugar alcohols and cyclitols that occur in higher plants (Bieleski, 1982; Drew 1983; Loescher, 1987). The first is as a transport and storage form of photosynthetically-derived sugars, for example sorbitol in apple fruits
(Loescher, 1987) and inositol in kiwifruit
(Bieleski et al., 1997). The second, and of partic-ular relevance to this study, proposes that these compounds act as compatible solutes during os-motic adjustment in plant tissues in response to a water deficit or high salinity. In this latter role, the cyclitol D-pinitol (1D-3O
-methyl-chiro-inositol) has received particular attention
(Bieleski, 1994a).
The postulated importance of pinitol in leaf tissues during osmotic adjustment is derived from three lines of experimental evidence. The first is evidence that plants which grow in saline environments have high levels of pinitol in their leaves (Drew, 1983; Bieleski, 1994b). The second is that some plants, when subjected to a water deficit, accumulate pinitol to relatively high con-centrations in leaf tissue (Ford and Wilson, 1981; Ford, 1984; Nguyen and Lamant, 1988; Keller and Ludlow, 1993). A similar increase in pinitol content has also been shown to occur in response to growing plants in conditions of high salinity (Gorham et al., 1981, 1988; Fougere et al., 1991; Adams et al., 1992). The third line of evidence is the demonstration that increased salinity induces the expression of genes, one of which has been shown to be involved in pinitol synthesis (Vernon and Bohnert, 1992).
As part of a larger study on the responses of the pasture legume white clover to a water deficit (Barker et al., 1993), we have examined the accumulation of pinitol and other soluble carbohydrates in leaf tissue. Pinitol has been measured previously in white clover (Smith and Phillips, 1980; Davis and Nordin, 1983; Phillips et al., 1984; Bieleski, 1994a; Turner and Pollock, 1998), and is present as the major soluble sugar carbohydrate in leaf tissue (Bieleski, 1994a). However, the accumulation of this cyclitol and other compatible solutes, including proline, in
response to a water deficit has not been re-ported. In this study, two genotypes from the cultivar ‘Grasslands Kopu’ and two from the breeding line ‘Syrian Selection’ are compared. Kopu is not considered to be particularly drought-tolerant (McFarlane et al., 1990), while the Syrian selection exhibits improved survival in summer drought particularly when compared with Kopu (Caradus et al., 1990). The responses of these genotypes in terms of proline accumula-tion and changes in the relative abundance of soluble carbohydrate in response to a water deficit are reported. We hypothesise that pinitol will be the major soluble sugar present in ma-ture leaves of white clover when subjected to a significant water deficit.
2. Materials and methods
2.1. Plant material
Two genotypes from the white clover (Tri
-folium repens (L.)) cultivar ‘Grasslands Kopu’ and two from the white clover breeding line ‘Syrian selection’ were established as apical cut-tings consisting of 2 to 3 nodes in sand – peat potting mix. When a significant root system had developed, each plant was transplanted into
18-litre capacity pots filled with a field soil
(Kairanga Silt Loam). Each pot, containing a single genotype, was randomly assigned to a po-sition in a temperature-controlled glasshouse (temperature range: 15 – 23°C) and grown under natural light. Each genotype was represented by two plants (pots) to provide a total of eight plants. When plants had reached a sufficient size, comprising at least 10 – 12 mature stolons, a water deficit was imposed by withholding water. At specific time intervals, the first fully ex-panded leaf (usually subtending from node 3 or 4) was excised at the petiole:stolon junction and used immediately for leaf water potential. In other leaves sampled, the petiole was removed at the pulvinule and the trifoliates used for os-motic potential measurements, or frozen in
liq-uid nitrogen and stored at −80°C for proline
2.2. Measurements of soil and plant water status
Soil water, to a 25 cm depth, was monitored electronically in all pots using a Time-Domain-Reflectometer (TDR; Soil Moisture Equipment, Santa Barbara, CA, USA), and converted to gravimetric soil water content (GSWC) by a pre-viously determined calibration. Leaf water
poten-tial (ct) was measured using a pressure bomb
apparatus as described by Scholander et al. (1965). Osmotic potential (cp) was measured
us-ing a thermocouple psychromoter (SC10A,
Decagon Devices, USA), by sealing the trifoliates in sample cups, freezing the tissue for 2 h at
−80°C, and then thawing. Turgor potential (cp) was calculated as ct-cp. Osmotic potential was
not adjusted for possible dilution of apoplastic water.
2.3. Measurement of leaf proline
Frozen (−80°C) leaf tissue was powdered in
liquid nitrogen and the proline content deter-mined using the method of Bates et al., (1973) essentially as described by Abernethy and Mc-Manus (1998).
2.4. Extraction and quantification of soluble sugars
Frozen (−80°C) leaf tissue was powdered in
liquid nitrogen and extracted in cold (−20°C)
methanol-chloroform-water (12:5:3; v/v) at −
20°C overnight. Subsequent extraction procedures and isolation of neutral sugars using ion-exchange chromatography was as described in Bieleski (1994b). Sugars were separated, identified and quantified by capillary GLC as described in Bieleski (1994b) and Bieleski et al., (1997).
2.5. Tissue sampling and statistical analysis
For the determination of ct, cp and proline
content, mean values were calculated from mea-surements from three stolons, each from duplicate plants from each of two genotypes from the Kopu
cultivar (to give n=12) and from the Syrian
selection (to give n=12). For the determination
of soluble carbohydrates at each sampling point, mean values were calculated from determinations made from mature leaf tissue excised from three stolons from each of duplicate plants from a single genotype (n=6).
Sample means, with an estimate of the standard error (S.E.) are calculated using the formulae described in Sokal and Rohlf (1987). Repeated measurements on the same experimental unit over time were analysed using the REPEATED option of the Statistical Analysis System (SAS Institute, North Carolina, USA).
3. Results
3.1. Changes in ct and cp in response to a water deficit
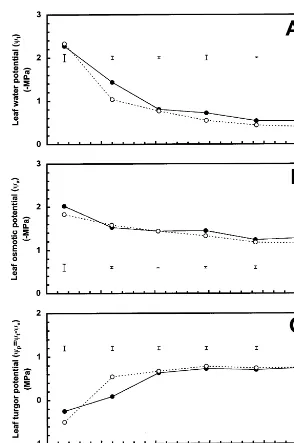
A significant decrease in ct after 20% GSWC
was observed in genotypes from both Kopu and the Syrian selection (Fig. 1). This was most marked in Syrian selection genotypes at 17% GSWC, but by 12% GSWC leaves from genotypes from both Kopu and the Syrian selection display
a similar ct of −2.3 MPa. This temporal
differ-ence in ct was also mirrored in leaf turgor
mea-surements with a significant decrease in cp(when compared with fully hydrated) observed for the Syrian selection genotypes at 17% GSWC (Fig. 1C). By 12% GSWC, genotypes from both Kopu and the Syrian selection displayed a significant
decrease in cp (when compared with fully
hy-drated) although no significant difference in cp
between genotypes was apparent. For these mea-surements, repeated measurements analysis found responses over time were statistically significant,
but no significant cultivar/selection by time
interaction.
3.2. Changes in proline content and soluble carbohydrates in response to a water deficit
At each sample point for the determination of
ct, proline content was also measured in leaf
Syrian selection and Kopu began to accumulate proline significantly at the same GSWC (20%). The highest content was measured in the Syrian
selection (2.7 mg/g FW), which was higher than
that measured for Kopu (2.4 mg/g FW) although
this difference is not statistically significant. For
these proline determinations, repeated measure-ments analysis again found responses over time were statistically significant, but no significant cultivar/selection by time interaction.
For soluble carbohydrate analysis, sampling
points before any significant change in ct had
Fig. 1. Changes in leaf water potential (ct; A), full turgor osmotic potential (cp; B) and leaf turgor (cp; C), plotted against
Fig. 2. Proline contents, plotted against gravimetric soil water content (GSWC), in leaf tissue from the cultivar Grasslands Kopu (--) and the breeding line Syrian selection (- -) in response to a water deficit. Bars denote9S.E.,n=12.
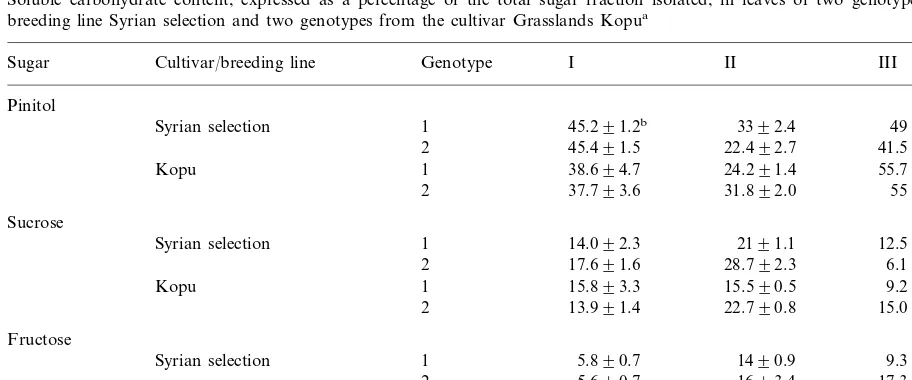
Table 1
Soluble carbohydrate content, expressed as a percentage of the total sugar fraction isolated, in leaves of two genotypes of the breeding line Syrian selection and two genotypes from the cultivar Grasslands Kopua
Genotype I II
Cultivar/breeding line III
Sugar
Pinitol
1 45.291.2b 3392.4 4992.2
Syrian selection
2 45.491.5 22.492.7 41.591.6
Kopu 1 38.694.7 24.291.4 55.793.6
2 37.793.6 31.892.0 5593.8
Sucrose
Syrian selection 1 14.092.3 2191.1 12.592.3
2 17.691.6 28.792.3 6.190.8
1 15.893.3
Kopu 15.590.5 9.290.7
2 13.991.4 22.790.8 15.091.8
Fructose
1 5.890.7
Syrian selection 1490.9 9.391.4
2 5.690.7 1693.4 17.391.8
Kopu 1 15.492.9 25.992.0 12.793.8
2 18.192.1 18.891.1 9.591.3
aLeaf tissue was excised from water-sufficient leaf tissue (I), after a short term period of water deprivation (II) and after a
prolonged period of water deprivation (III).
bValues are means9SE,n=6.
occurred (mean ct for all genotypes= −0.53
MPa; I), after a change in ct had occurred and
immediately at the point that wilting (meanctfor all genotypes= −2.4 MPa; II) and after
signifi-cant change inct(meanctfor all genotypes= −
4.2 MPa; III) were selected. In fully hydrated leaf
selection genotypes significantly higher (p=0.027) than the Kopu genotypes. After a period of mod-erate water stress (Table 1, II), the proportion of pinitol falls to about two-thirds its fully hydrated value while the proportion of sucrose and fructose rise 2- to 3-fold. In terms of content, sucrose and fructose have increased 3- to 4-fold, while there has been less than a 2-fold increase observed for
pinitol (data not shown). There is a cultivar/
breeding line effect in these trends, with sucrose the major sugar in the Syrian selection genotypes, while fructose is higher in the Kopu genotypes.
After a significant change in ct has occurred
(Table 1, III), pinitol again becomes the major soluble carbohydrate present, with falls in the proportions of sucrose and fructose. Again, no significant genotype differences were observed, but the Kopu genotypes had a significantly higher
pinitol content (p=0.003), as a proportion of
total soluble carbohydrate, when compared with the Syrian selection genotypes. Myo-inositol and sorbitol were also measured in this study but did not accumulate to any significant degree in
con-cert with significant changes in ct and cp (data
not shown).
4. Discussion
This study has examined changes in proline content and in the relative abundance of soluble carbohydrates in mature leaves of white clover when subjected to water deficit. Proline accumula-tion in response to a water deficit has been ob-served in many plant species (Rhodes, 1987), including many legumes (Ford, 1984), and is con-sidered part of the mechanism of osmotic adjust-ment (Delauney and Verma, 1993). This study, together with our previous findings (Barker et al., 1993), are the first reports of the accumulation of proline in white clover in response to a water deficit. The timing of this accumulation (in con-cert with significant changes in ct and cp) also suggests a role for proline in osmotic adjustment in leaf tissue of white clover.
The observation that pinitol is a major compo-nent of the soluble carbohydrate fraction in fully hydrated leaves is consistent with previous reports
(Davis and Nordin, 1983; Phillips et al., 1984; Bieleski, 1994a). However, after the imposition of a moderate water stress, its proportion falls and the proportion of fructose and sucrose rise markedly. Such accumulation of these sugars has been observed previously in another legume,
Phaseolus 6ulgaris. In bean leaves subjected to a
mild – short term water deficit, the starch – sucrose ratios changes to favour the accumulation of su-crose (Vassey and Sharkey, 1989; Castrillo, 1992). These authors suggest that mild water stress brings about a fall in sucrose-phosphate syn-thetase activity (SPS) and a shift of carbohydrate partitioning in favour of sucrose synthesis and starch remobilisation. In spinach leaves, short-term water stress again leads to stimulation of sucrose synthesis and starch depletion, but this time by activating SPS (Quick et al., 1989). The authors note that short-term water stress has little effect on photosynthesis, but that greater stress causes marked inhibition. Further, protein phos-phorylation of a specific serine residue (Ser434) has been proposed as part of the observed osmotic stress activation of SPS in spinach (Toroser and Huber, 1997). In white clover, sucrose/fructose was also observed to accumulate after a short-term water deprivation period (Table 1, II) in
which a change in ct had occurred (-2.4 MPa).
This change may reflect an alteration in carbon partitioning in favour of these sugars when com-pared with starch (Daie, 1988). However, we can-not yet say how this accumulation is modulated in white clover leaves in terms of changes in the key metabolic enzymes associated with starch hydrol-ysis and sucrose synthesis.
In response to a longer term water deficit, in
which a significant change in ct is observed (−
4.2 MPa), pinitol is again the major soluble car-bohydrate present in white clover leaves. In other legume species that accumulate pinitol in response to drought (Ford, 1984), the cyclitol is, as ob-served here, the major soluble carbohydrate when compared with regular metabolic sugars. How-ever, this is the first report in which pinitol has been shown to accumulate in terms of relative abundance in concert with significant changes in
ct and cp in leaf tissues of white clover. Total
fresh weight, accumulated in leaf tissue from all genotypes used in this study suggesting that pini-tol has also increased in terms of amount during the water-deprivation time course (data not shown).
In this study, we compared genotypes from a drought-sensitive cultivar Kopu with a more drought-tolerant Syrian selection breeding line to determine if the capacity to synthesise pinitol differed. In a comparable study with two popula-tions of maritime pine, Pinus pinaster, well-wa-tered seedlings of the more drought tolerant line (‘Tamjoute’) had 2- to 3-fold more pinitol in water sufficient leaf tissue when compared with the drought-sensitive line (‘Landes’) (Nguyen and Lamant, 1988). However, in response to a polyethylene glycol (PEG)-induced water deficit, both lines accumulated pinitol to the same relative degree, such that more pinitol was still present in the drought-tolerant line. In the present study with white clover, a cultivar/breeding line differ-ence was observed in terms of the proportion of pinitol in fully hydrated leaf tissue and in tissue demonstrating significant changes in ct. In com-mon with the genotype differences displayed by
Pinus pinaster, pinitol represented a significantly higher proportion of soluble carbohydrate in leaf tissue of the more drought-tolerant Syrian selec-tion genotypes. However, after a marked change
in ct, the Kopu genotypes accumulated
signifi-cantly more pinitol when expressed as a propor-tion of total soluble carbohydrate when compared with the Syrian selection genotypes. In terms of agronomic performance, the Syrian selection genotypes have been shown to be more adapted
to dryland sites when compared to Kopu
(Caradus et al., 1990). The results from the cur-rent study suggest that other plant characteristics observed in the Syrian selection genotypes (e.g. small leaf size) must act in concert with the in-creased proportion of pinitol to confer this adap-tive advantage in dryland sites, as Kopu (which accumulates pinitol as a higher percentage of total soluble carbohydrate) does not survive well in dryland sites (Caradus et al., 1990). Further study incorporating a greater range of white clover genotypes adapted to grow in different habitats is needed, therefore, to establish further the
contri-bution that a greater relative abundance of pinitol in leaves makes to dryland habitat survival.
Acknowledgements
We thank Vivienne Paterson for the GLC sepa-rations and analysis of soluble carbohydrates in leaf tissue extracts.
References
Abernethy, G.A., McManus, M.T., 1998. Biochemical re-sponses to an imposed water deficit in mature leaf tissue of Festuca arundinacea. Environ. Exp. Bot. 40, 17 – 28. Adams, P., Thomas, J.C., Vernon, D.M., Bohnert, H.J.,
Jensen, R.G., 1992. Distinct cellular and organismic re-sponses to salt stress. Plant Cell Physiol. 33, 1215 – 1223. Barker, D.J., Caradus, J.R., McManus, M.T., 1993.
Physio-logical responses of white cover genotypes to water deficit. In: Proceedings of the XVII International Grassland Con-gress, Palmerston North, New Zealand, pp. 67 – 68. Bates, L.S., Waldren, R.P., Teare, I.D., 1973. Rapid
determi-nation of free proline for water-stress studies. Plant Soil 39, 205 – 207.
Bieleski, R.L., 1982. Sugar alcohols. In: Loewus, W.T. (Ed.), Encyclopedia of Plant Physiology, New Series. Springer-Verlag, Berlin, pp. 158 – 192.
Bieleski, R.L., 1994a. It ain’t necessarily so: sugars, stress and shibboleths. N.Z. Biosci. 3, 3 – 6.
Bieleski, R.L., 1994b. Pinitol is a major carbohydrate in leaves of some coastal plants indigenous to New Zealand. N.Z. J. Bot. 32, 73 – 78.
Bieleski, R.L., Clark, C.J., Klages, K.U., 1997. Identification of myo-inositol as a major carbohydrate in kiwifruit, Ac-tinidia deliciosa. Phytochemistry 46, 51 – 55.
Caradus, J.R., Cooper, B., Widdup, K., Ryan, D., 1990. Breeding and selection for improved white clover produc-tion and persistence in New Zealand. Proc. Agron. Soc. N.Z. 20, 11 – 15.
Castrillo, M., 1992. Sucrose metabolism in bean plants under water deficit. J. Exp. Bot. 43, 1557 – 1561.
Daie, J., 1988. Mechanism of drought-induced alterations in assimilate partitioning and transport in crops. CRC Crit Rev. Plant Sci. 7, 117 – 137.
Davis, L.C., Nordin, P., 1983. Sugar and organic acid con-stituents in white clover. Plant Physiol. 72, 1051 – 1055. Delauney, A.J., Verma, D.P.S., 1993. Proline biosynthesis and
osmoregulation in plants. Plant J. 4, 215 – 223.
Drew, E.A., 1983. Sugars, cyclitols and seagrass phylogeny. Aquatic Bot. 15, 387 – 408.
Ford, C.W., Wilson, J.R., 1981. Changes in levels of solutes during osmotic adjustment to water stress in leaves of four tropical pasture species. Aust. J. Plant Physiol. 8, 77 – 91. Fougere, F., Le Rudulier, D., Streeter, J.G., 1991. Effects of
salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sati6aL.). Plant Physiol. 96, 1228 – 1236. Gorham, J., Hughes, L., Wyn Jones, R.G., 1981. Low
molecu-lar-weight carbohydrates in some salt-stressed plants. Phys-iol. Plant. 53, 27 – 33.
Gorham, J., Tomar, O.S., Wyn Jones, R.G., 1988. Salinity-in-duced changes in the chemical composition of Leucaena leucocephalaandSesbania bispinosa. J. Plant Physiol. 132, 678 – 682.
Keller, F., Ludlow, M.M., 1993. Carbohydrate metabolism in drought stressed leaves of pigeonpea (Cajanus cajan). J. Exp. Bot. 44, 1351 – 1359.
Loescher, W.H., 1987. Physiology and metabolism of sugar alcohols in higher plants. Physiol. Plant 70, 553 – 557. McFarlane, M.J., Sheath, G.W., McGowan, A.W., 1990.
Evaluation of clovers in dry hill country. 5. White clover at Whatawhata, New Zealand. N.Z. J. Agric. Res. 33, 549 – 556.
Nguyen, A., Lamant, A., 1988. Pinitol and myo-inositol accu-mulation in water-stressed seedlings of maritime pine. Phy-tochemistry 27, 3423 – 3427.
Phillips, D.V., Wilson, D.O., Dougherty, D.E., 1984. Soluble carbohydrates in legumes and nodulated nonlegumes. J. Agric. Food Chem. 32, 1289 – 1291.
Quick, P., Siegl, G., Neuhaus, E., Feil, R., Stitt, M., 1989. Short-term water stress leads to a stimulation of sucrose synthesis by activating sucrose-phosphate synthase. Planta 177, 535 – 546.
Rhodes, D., 1987. Metabolic responses to stress. In: Davies, D.D (Ed.), The Biochemistry of Plants, Vol XII. Academic Press, New York, pp. 210 – 241.
Scholander, P.F., Hammel, H.T., Bradstreet, E.D., Hem-mingsen, E.A., 1965. Sap pressure in vascular plants. Sci-ence 148, 339 – 346.
Smith, A.E., Phillips, D.V., 1980. Occurrence of pinitol in foliage of several forage legume species. Crop Sci. 20, 75 – 77.
Sokal, R.R., Rohlf, F.J., 1987. Biometry, second ed. W.H. Freeman, New York.
Toroser, D., Huber, S.C., 1997. Protein phosphorylation as a mechanism for osmotic-stress activation of sucrose-phos-phate synthase in spinach leaves. Plant Physiol. 114, 947 – 955.
Turner, L.B., Pollock, C.J., 1998. Changes in stolon carbohy-drates during the winter in four varieties of white clover (Trifolium repensL.) with contrasting hardiness. Ann. Bot. 81, 97 – 107.
Vassey, T.L., Sharkey, T.D., 1989. Mild water stress of Phase-olus6ulgarisplants leads to reduced starch synthesis and extractable sucrose phosphate synthase activity. Plant Physiol. 89, 1066 – 1070.
Vernon, D.M., Bohnert, H.J., 1992. A novel methyl trans-ferase induced by osmotic stress in the faculative halophyte Mesenbryanthemum crystallinum. EMBO J. 11, 2077 – 2085.