www.elsevier.com / locate / bres
Temporary inactivation of the retrorubral fields decreases the
rewarding effect of medial forebrain bundle stimulation
1
*
Meg Waraczynski , Mark Perkins
Department of Psychology, University of Wisconsin-Whitewater, 800 W. Main St. Whitewater, WI 53190, USA
Accepted 22 August 2000
Abstract
Prior studies indicate that lesioning the retrorubral fields (RRF) decreases the rewarding effect of medial forebrain bundle (MFB) stimulation, although these studies did not make the RRF their primary target. This study directly investigates the role of the RRF in MFB self-stimulation using transient lidocaine-induced inactivation of target tissue rather than permanent lesioning. In 18 rats with MFB stimulation electrodes, inactivation of the RRF via 0.5 and 1.0ml of 4% lidocaine produced immediate, substantial upward shifts in the frequency required to maintain half-maximal self-stimulation response rates whereas injecting comparable volumes of saline did not. Bilateral inactivation was particularly effective, especially at medium and high stimulation currents, although unilateral inactivation ipsilateral to the stimulation site was also effective. Contralateral inactivation alone did not substantially change the stimulation’s reward value, although contralateral inactivation appeared to contribute to the effectiveness of bilateral inactivation. The frequency required to maintain half-maximal responding returned to baseline levels by 15–20 min after lidocaine infusion. In seven rats whose infusion sites were not in the RRF, lidocaine inactivation did not consistently degrade the stimulation’s reward value. These results indicate that some neural elements located in the RRF contribute to the rewarding effect of MFB stimulation. Possible roles for these elements in the anatomical substrate for MFB self-stimulation are discussed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Motivation and emotion
Keywords: Brain stimulation; Rate–frequency curve shift; Lidocaine; Reward; Midbrain; Self-stimulation
1. Introduction median raphe lesion that strayed into the RRF was more effective than accurately targeted lesions in reducing MFB Previous investigations of the anatomical substrate for stimulation’s reward value [49]. Based on these findings, medial forebrain bundle (MFB) stimulation reward have this report directly investigates the role of the RRF in MFB indicated that the midbrain retrorubral fields (RRF) may be self-stimulation.
an important component. Lepore and Franklin, in targeting The RRF themselves support low rates of self-stimula-the nearby pedunculopontine tegmentum, noted that tion [25]. The RRF are also of potential interest because NMDA excitotoxin lesions were most effective in impair- the A8 dopamine cells are located there. MFB self-stimula-ing the acquisition of respondself-stimula-ing for MFB stimulation tion is clearly linked to midbrain dopamine systems, when those lesions strayed rostrally to affect the RRF [29]. although dopaminergic neurons are not typically directly Similarly, we have recently reported that a mistargeted activated by the stimulation [55]. Rewarding stimulation of both the MFB and midline mesencephalon increases activity in at least some midbrain dopamine cells
*Corresponding author. Tel.: 11-262-472-5415; fax: 11-262-472- [7,10,24,32], although it should be noted that recently
1863. several authors have questioned whether dopamine is
E-mail address: [email protected] (M. Waraczynski).
1 involved in the stimulation’s reward value per se or in
Current address: Department of Physiology, University of Wisconsin,
some other aspect of incentive motivation function (e.g.
Medical Science Building, 1300 University Avenue, Madison, WI 53706,
USA. [21]; see [8] for a thorough review). Most attention has
focused on the relationship between MFB self-stimulation cannulae were blocked with stylets made from 30 gauge and the A9 and especially A10 cells of the substantia nigra tubing. The rats were housed individually with food and and ventral tegmental area, respectively. Relatively little water continuously available in a day / night reversed attention has been paid to the A8 cells of the retrorubral colony. All testing was conducted during the dark phase of fields, possible because these cells only comprise about the cycle.
10% of mammalian midbrain dopamine cells [33]. These
cells have efferent control over both the A9 and A10 2.2. Procedures groups [6] and can be considered contiguous with the A9
and A10 groups [17]. A8 efferent projections often parallel 2.2.1. Rate–frequency testing
those of the A9 [19,31,46,51] and to some degree the A10 Rate–frequency testing was performed with the rat (e.g. [22] groups). A8 afference from the amygdala also placed in an operant chamber with a lever protruding from parallels amygdaloid afference to the lateral A9 group one wall. The rat was connected to a Stimtek ST1200 [23,47]. Thus, the links between midbrain dopamine and stimulation generator (San Diego Instruments) via a flex-self-stimulation also implicate the RRF as a target of ible cable and commutator (Plastics One). All experimental
interest. events were controlled by Stimtek ST1000 CPU and
We have previously suggested that temporary inactiva- ST1100 I / O boards in communication with a master PC. tion of targets thought to be relevant to MFB stimulation After 3–5 days postsurgical recovery, the rats were reward may be more effective, and more consistently trained to press a lever for a 0.5 s train of 0.1 ms cathodal effective, than permanent lesions [1,48,49]. In part, this pulses delivered by a constant current generator. The rats may be true because postlesion hyperexcitability in cells were trained using stimulation of either the LH or VTA surrounding a lesion, and / or synaptic plasticity in synapses site, whichever supported the more robust responding. surviving the lesion, compensate for the loss of lesioned Once the lever press response was reliably established (the cells. Neither would be a factor if, instead of lesioning, rats would press without coaching for stimulation delivered target tissue were rendered temporarily inactive via the on a VI 3-second schedule), the rat was tested at several injection of an anesthetizing agent. In this report, we use pulse frequencies at 200, 400 and 800 mA at both sites. lidocaine to temporarily inactivate cells in the RRF, both The site that yielded the lower and most consistent values ipsi- and contralateral to MFB self-stimulation sites. of the frequency required to maintain half maximal per-Changes in stimulation reward value are measured using formance at all three currents was chosen for all further the rate–frequency curve shift method. If the RRF are testing.
important to the stimulation’s reward effect, then their Each point in a single rate–frequency curve was de-inactivation should temporarily render MFB stimulation termined as follows: for 30 s, the rat was allowed to press
less rewarding. for a 0.5-s train of pulses of a given frequency, delivered
on a VI 3 s schedule. At the start of each 30-s trial, the rat received three non-contingent trains of the stimulation that
2. Methods would be available during that trial. Data from the first 10 s of the trial were discarded to allow response rate to
2.1. Subjects and surgery adjust to the presented frequency, and response rate over
the last 20 s was recorded.
For each rate–frequency curve, the frequency required positioning the tip at the desired target. Because the 11 mm to maintain half-maximal responding, called the ‘required cannulae were implanted such that the tips were 1 mm frequency’, was calculated by fitting a broken-line function dorsal to the injection target, the injectors had a collar to the curve. The broken line function fits three line placed 12 mm above their tips. Thus, the injector tip segments to the curve: two horizontal segments for the protruded exactly 1 mm below the end of the cannula and lower and upper asymptotes, respectively, and a third was prevented by the collar from sinking further ventral. segment connecting those two. The required frequency was Injectors were connected via flexible polyethylene tub-computed by interpolating the frequency corresponding to ing to the needle of a 10-ml Hamilton microsyringe which the midpoint of the connecting segment. Required fre- was mounted in a Harvard Apparatus dual programmable quency was averaged across curves at each current, syringe pump. Bilateral infusions were made simultaneous-yielding a daily average required frequency for each ly, using two syringes. Lidocaine (or saline) was infused at
current tested. a rate of 0.5ml / min. Once the infusion was complete the
Stable baseline was defined as 5 consecutive test days injector remained in place for 1 min to prevent diffusion with no apparent trend in required frequency at any back up the guide cannula. The injector was then removed current. Once this was reached, required frequency was no and replaced with the stylet, and behavioral testing re-longer averaged across currents each day. Instead, a daily sumed as soon as the rat was replaced in the operant session was divided into three separate passes through the chamber, within 1 min after the stylets were replaced. rat’s set of currents. The first pass was treated as a
warm-up condition and its data were discarded from 2.2.3. Histology
further analyses. On injection days, testing was paused At the end of postlesion testing the rats were euthanized after the first pass and the rat was removed for injection. using an overdose of sodium pentobarbital. They were Testing resumed immediately after the injection with a perfused transcardially with saline followed by 10% formal second and third pass through the current sets. The order of saline and the brains were harvested. The brains were fixed current presentation within a pass was randomized for each in 10% formal saline for at least 3 days followed by 1 day rat, but that order remained constant across passes. in 20% sucrose formalin. The brains were then quick Lidocaine and saline test days were always bracketed by frozen and cut in the coronal plane on a microtome non-injection test days. The effects of a given injection mounted in a cryostat. Alternate sections through the condition were assessed relative to the two injection days stimulation and lesion sites were saved for staining with that bracketed that condition. The order of test conditions formal thionin to visualize cell bodies and hematoxylin to
was randomized for each rat. visualize fiber tracts. The tissue was examined via a tissue
projector and the location of electrode tips and injection
2.2.2. Lidocaine injection sites were plotted on coronal plates from the Paxinos and
The lidocaine testing phase of the experiment began Watson [36] atlas of the rat brain. Electrode tip sites were with an initial 0.5ml bilateral saline injection to condition taken to be at the bottom of the electrode track left in the the target tissue. In preliminary work we found that the tissue after the implant was removed. The injector track initial bolus of any injectate often affected required typically appeared as a narrow, dark length of gliosis frequency, regardless of whether that injectate was saline extending approximately 1 mm below the end of the or lidocaine. Additional 0.5 and 1.0 ml bilateral saline cannula track. The infusion site was inferred to be at the injections were included in the randomly determined end of the injector track. Sites within 0.25 mm of the sequence of test conditions and served as control con- border of the retrorubral fields were considered on target ditions against which lidocaine’s effects were compared. In based on estimates of lidocaine’s spread of effectiveness as addition to saline, all rats received 0.5 and 1.0 ml of a function of infusion volume obtained by Tehovnik and lidocaine injected ipsi- and contralateral to the stimulation Summer [45]: 0.5 mm radius for 0.5ml and 0.65 mm for site and bilaterally, yielding a total of eight test injection 1.0 ml. These estimates were obtained using different conditions. Contralateral-only and ipsilateral-only saline injection parameters (2% lidocaine injected into cortex), injections were not tested in order to minimize the number but are consistent with other estimates of the spread of of times the injector tip was introduced into the tissue. effectiveness of 0.5 and 1.0 ml injections of 2 and 4% Thus, the effects of contra- and ipsilateral lidocaine lidocaine infused into subcortical structures infusions were compared with the effects of bilateral [2,14,30,37,39]. Thus, we use a conservative estimate in
injections of saline of comparable volume. assuming that infusions made within 0.25 mm of the
During the infusion the rat was loosely restrained in a retrorubral field border affected the retrorubral field. small towel and held in the experimenter’s hand. The
stylets were removed from the guide cannulae and replaced 2.2.4. Data analysis
bracketing the injection test day. Required frequency was calculated for each current in both the second and third passes through the current set on the day before and the day after the injection day. These data were averaged for each current and pass, yielding six values: mean non-injection required frequency at 200, 400 and 800mA in the second pass, and the same means for the third pass. These means were subtracted from the corresponding required frequency values obtained on the injection test day, yielding another six data points: shift in required frequency at each of the three currents, for both passes. Pilot testing and others’ work [2,45] indicate a roughly 20 min time course of effectiveness for the lidocaine injection parame-ters used here. Because each pass through a current set took 15–20 min, data from the second pass assessed the immediate effects of the lidocaine injection. Data from the third pass showed whether the rat returned to non-injection day levels of required frequency when lidocaine’s effects dissipated.
3. Results
Of the 25 rats tested, 11 had both injection sites in or within 0.25 mm of the border of the retrorubral fields. Six rats had only ipsilateral sites on target and one rat had only the contralateral site on target. For these rats we analyzed only the data from injection conditions in which the injection site was on target. For example, data from the rat whose contralateral site alone was on target were included only in the analysis of the effects of contralateral — not ipsi- or bilateral — lidocaine infusions. In the remaining seven rats neither injection site was in or within 0.25 mm of the border of the retrorubral fields; these rats comprised a control group against which the results of the other 18 rats were compared.
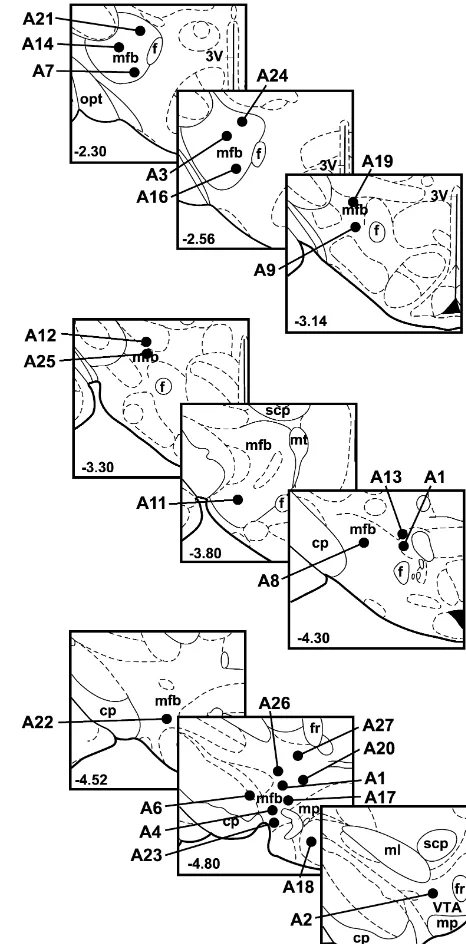
Figs. 1 and 2 show the stimulation sites and injection sites, respectively, for all 25 rats. Injection sites for the seven control rats are indicated by putting the rat’s identification number in italic print. The shaded areas in
the second plate in Fig. 2 show the relative location of the Fig. 1. Stimulation electrode tip locations for all 25 rats in the study. The number in the lower left hand corner of each plate gives the distance of
retrorubral fields, which begin approximately 0.25 mm
the plate from bregma, in mm. All plates in this figure and Fig. 2 are
behind that plate. Therefore, injection sites in that coronal
adapted from the Paxinos and Watson [36] atlas of the rat brain, used with
plane that fall within or adjacent to the shaded area were
permission. Abbreviations: 3V, third ventricle; cp, cerebral peduncle; f,
considered to be retrorubral injection sites. Although it fornix; fr, fasciculus retroflexus; mfb, medial forebrain bundle; ml, medial appears that rat A19 should be included in the retrorubral lemniscus; mp, mammillary peduncle; opt, optic tract; scp, superior
cerebellar peduncle; VTA, ventral tegmental area.
group as a ‘contralateral only’ rat, the asterisk indicating this rat’s injection site is a rough estimate of where injections may have gone. Upon postmortem examination
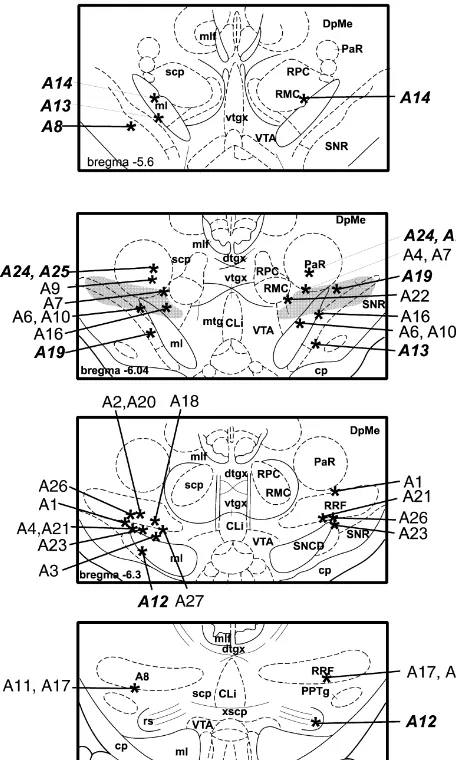
this rat was found to have a large region of heavy gliosis asterisks indicating these injection sites are also rough surrounding the injection track and the end of the guide estimates. It is possible that one or several of the injection cannula. The tissue was more suggestive of a large lesion conditions may have introduced infection into these rats’ than a well-localized injection site. Because of the am- brains, producing the extensive tissue damage, although biguity of this rat’s injection site, it was included in the their behavioral data were remarkably stable throughout control group. Similarly, large regions of heavy gliosis the experiment.
Fig. 2. Asterisks indicate estimated injection sites for all 25 rats. Injection sites for the 7 rats in the control group are indicated by placing the rat’s identification number in italic print. The number in the lower left hand corner of each plate indicates the distance from bregma, in mm. The shaded area in the second plate indicates the relative location of the retrorubral fields which begin approximately 0.25 mm behind this plane. Abbreviations: A8, A8 dopaminergic cell bodies; CLi, caudal linear
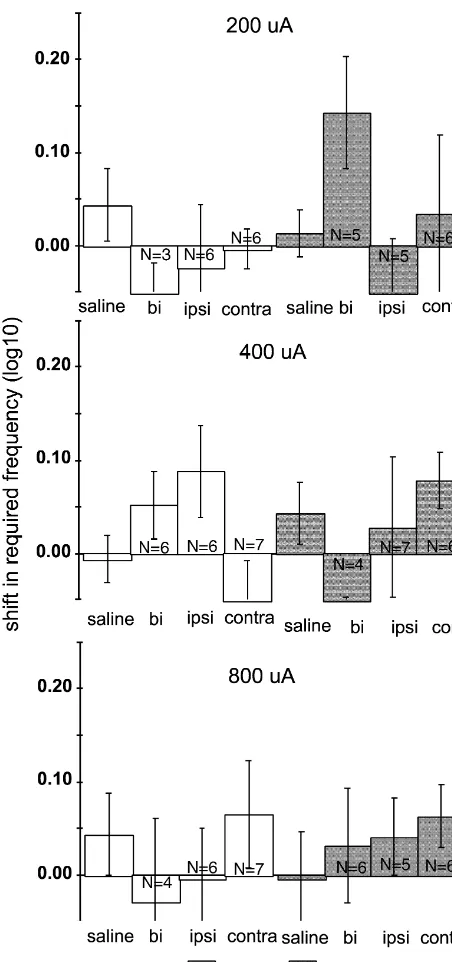
nucleus; cp, cerebral peduncle; DpMe, deep mesencephalic nucleus; dtgx, Fig. 3. Mean shifts in required frequency at each of three currents, dorsal tegmental decussation; ml, medial lemniscus; mlf, medial longi- relative to bracketing non-injection days, immediately following lidocaine tudinal fasciculus; mtg, mammillotegmental tract; PaR, pararubral nu- and saline injections in 18 rats with injection sites in the retrorubral fields. cleus; RPC, red nucleus, parvicellular; RPM, red nucleus, magnocellular; Error bars51 S.E.M. Asterisks indicate lidocaine-induced shifts that were RRF, retrorubral field; rs, rubrospinal tracts; scp, superior cerebellar significantly larger than the shifts induced by comparable volumes of peduncle; SNCD, substantia nigra, pars compacta; SNR, substantia nigra, saline (see text for discussion of the criterion for significant). The number pars reticulata; VTA, ventral tegmental area; vtgx, ventral tegmental of observations entering into each mean are indicated within or immedi-decussation; xscp, decussation of the superior cerebellar peduncle. ately above / below each bar; n518 for all saline conditions. All saline
injections were bilateral.
in the 18 rats in the retrorubral injection group are shown
conditions. The relatively small number of observations in frequently as large as 0.2–0.4 log units at a particular many test conditions accounts for the relatively large current.
S.E.M. values around those conditions’ means. In both averaged and individual data, 1.0-ml infusions As an alternative to traditional significance testing, we did not generally produce notably greater effects than evaluated the significance of the difference between (ob- 0.5-ml infusions, when valid curves were collected. The served) shifts produced by lidocaine and shifts produced rats were, however, more likely to fail to produce valid by saline by constructing a modified confidence interval curves following 1.0-ml infusions than they were following around the mean saline-induced shift. To do so, we added comparable 0.5-ml infusions. Note that the number of 2.53 saline S.E.M. to the relevant mean saline-induced observations entering into each mean lidocaine-induced shift. (Most standard tests of significance require differ- required frequency shift is frequently lower in 1.0-ml ences between means of 2.0–2.5 error units for those infusion conditions versus comparable 0.5-ml conditions. differences to be significant at the P50.05 level. Using our In this group of 18 rats, six were tested at the LH 2.53 S.E.M. criterion is equivalent to constructing a 98% stimulation site and 12 at the VTA site. There was some confidence interval, which is equivalent to using a con- indication that the effects of 0.5-ml infusions were some-servative P50.02 significance level.) Any mean lidocaine- what stronger in the rats stimulating at the LH site, but this induced shift that exceeded that value was considered pattern was not consistent and was not observed following significantly larger than the mean shift induced by the 1.0-ml infusions. The differences in average required comparable saline injection. These significant differences frequencies between the two groups were not statistically are indicated by asterisks over the appropriate bars in Fig. significant (using the confidence interval analysis explained
3. above) for any laterality / current condition except the
In general, lidocaine-induced shifts were more substan- contralateral 0.5-ml infusion at 800 mA.
tial at 400 and 800 mA than at 200 mA. At all three Fig. 5 presents third pass data from the 18 rats with currents, bilateral 0.5 and 1.0 ml lidocaine injections retrorubral injection sites. By the third pass, lidocaine produced the largest shifts in required frequency. These infusions did not produce any average shifts that were shifts were significantly larger than the shifts produced by significantly different from shifts following saline infusion. comparable volumes of saline in all but one case. Shifts With only a few exceptions, third pass required frequency following ipsilateral lidocaine infusions were generally on injection test days did not, on average, vary from smaller than shifts following bilateral infusion, and were required frequency on non-injection test days by more than significantly greater than bilateral saline-induced shifts in 0.05 log units. Also, in these conditions virtually all rats four out of six conditions. Contralateral infusions produced produced valid rate–frequency curves; at most, only 1 or 2 the smallest shifts, which were not significantly different rats failed to do so in any given condition. That is, the from shifts induced by comparable volume bilateral saline majority of rats that did not produce valid rate–frequency injections. However, in most test conditions the magnitude curves in the second pass were producing valid curves by of the average shift following bilateral lidocaine injection the third pass, which started 15–20 min after lidocaine is roughly equivalent to the sum of the average shifts infusion.
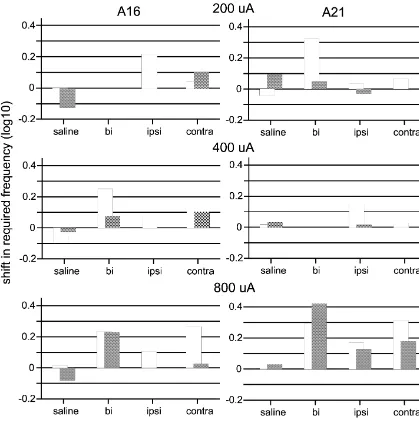
Fig. 4. Second pass shifts in required frequency, relative to bracketing non-injection days, from two individual rats. Conditions for which bars are missing are conditions in which the rat did not produce a valid rate–frequency curve.
800 mA, required frequency shifted downward by over shifted upward by 0.30 log units at 200mA and downward 0.20 log units), 1.0ml produced shifts of 0.20 log units at by approximately 0.15 log units at the other two currents. 400mA and 0.40 log units at 200mA. No valid curve was Of the three rats in the control group that had ambiguous collected in the second pass at 800 mA. This rat’s injection sites with extensive gliosis, one, rat A19, often contralateral injection site was approximately 5.2 mm but not consistently showed upward shifts in required caudal to bregma, at the ventral edge of the medial frequency. These were more commonly observed follow-lemniscus and midway between the VTA and substantia ing 1.0ml bilateral and ipsilateral infusions, particularly at nigra pars compacta (not shown in Fig. 2). Bilateral 200 and 400mA. On the other hand, rats A24 and 25 had infusions produced mixed results: following the 0.5-ml lesion-like injection tracks situated dorsal to the RRF infusion, valid curves could not be collected at 200 or 800 targets. These rats often but not consistently showed
Fig. 5. Mean third pass shifts in required frequency for all 18 rats Fig. 6. Mean second pass shifts in required frequency for seven rats receiving retrorubral lidocaine and saline injections; n518 for all saline whose injection sites were outside of the retrorubral fields; n57 for all
conditions. saline conditions.
inactivation, and that the temporary inactivation technique and / or terminating in the RRF but arising from other areas lends itself well to the rate–frequency curve shift are important to the reward effect. Each of these
possi-paradigm. bilities is viable.
Most attention to dopamine’s role in MFB stimulation
4.1. Reward versus performance effects reward has focused on the importance of that part of the
mesolimbic pathway from the ventral tegmental area to the In several test conditions, particularly following bilateral nucleus accumbens. As reviewed above, forebrain projec-lidocaine infusion, rats did not produce valid rate–fre- tions from the A8 cells tend to project to the striatum [19] quency curves immediately after the infusion although they rather than the accumbens, but also send inputs to both the did do so 15–20 min later. This was more likely to happen A10 cells of the ventral tegmental area and the A9 cells of following 1.0-ml infusions rather than 0.5-ml infusions, i.e. the substantia nigra [6]. Our data do not determine whether following a greater radius of inactivation spread. In these lidocaine’s effects depended on inactivating the A8 cells or cases we cannot definitively determine whether lidocaine on inactivating non-dopaminergic RRF cells.
inactivation rendered the stimulation so unrewarding that it The fact that lidocaine’s effects increased with stimula-failed to support responding or inactivation substantially tion current might imply a role for dopamine. On one impaired performance capacity. Although some, but not hand, this might have happened because at high currents, all, rats exhibited rapid rotation for the first several minutes especially 800 mA, the largest proportion of the reward following infusion, even these rats occasionally sampled substrate near the electrode tip was being stimulated, the lever throughout the second pass but never exhibited therefore it was most likely that any distal disruption of the sustained responding. (The rotation may have reflected an substrate would be detected. On the other hand, even imbalance in midbrain dopaminergic activity following though the stimulation parameters and electrode tips used inactivation; the possible role of A8 dopaminergic in- in this study do not typically stimulate dopaminergic fluence in these results is discussed below. Rats with axons, they may do so at 800mA [55]. Perhaps at this high midbrain dopaminergic lesions turn towards the lesioned current particularly, dopaminergic axons became an im-side, and such rotation can be used as an indirect measure portant part of the directly activated reward substrate and of interhemispheric imbalance in dopamine levels [4,16].) inactivating midbrain dopaminergic neurons had a greater Whether they rotated or not, the rats that failed to produce impact on that reward effect.
valid curves would occasionally respond for the highest It is tempting to suggest the use of dopamine-selective frequency or two at a particular current but their data did 6-OHDA lesions to tease this issue apart, but such lesions not meet criteria for a valid rate–frequency curve, there- would be permanent and would bring us back to the fore required frequency could not be calculated. problems with irreversible lesions discussed in the intro-The magnitude of shifts obtained from rats that did duction. Also, the present data indicate that strong effects produce valid second pass curves implies that the rats that would require bilateral lesioning. Irreversible bilateral did not respond reliably were at least in part experiencing lesioning of the A8 cells could so interfere with motoric reward degradation. Furthermore, most of the control rats ability (see [5] on the role of the A8 cells in motor produced valid rate–frequency curves during the second programming) that one might not be able to collect valid pass even though their injection sites were in or near psychophysical data. A better alternative might be to see motor-relevant structures such as the red nucleus and whether changes in forebrain dopamine release are corre-substantia nigra, and even though some of these rats also lated with the degree of reward degradation following showed rapid rotation following lidocaine infusion. inactivation of the retrorubral fields; we would welcome
Finally, rats with both RRF cannulae on target were the collection of such data.
more likely to fail to produce valid curves after bilateral or Recent investigations suggest that dopaminergic func-ipsilateral infusion than after contralateral infusion. Re- tion may be lateralized in both rat [3,44] and human [28] ward disruption is more likely to be hemisphere-specific brain, with the right hemisphere, especially right prefrontal than is performance impairment. Together, these facts cortex, being dominant for dopamine’s role in emotional suggest that failure to produce valid curves resulted at least responses and attention to emotionally relevant stimuli in part from effects on the stimulation’s reward effective- [3,44]. In the present study all stimulation sites were in the
ness. left hemisphere, therefore all of the ipsilateral infusions
were directed at the left RRF and all of the contralateral
4.2. What was inactivated? infusions, at the right RRF. Midbrain dopaminergic
right hemisphere infusions to have a greater effect than transience of lidocaine’s effects is supported by the third they did. While it would be interesting to see whether pass data shown in Fig. 5. By observing reward value ipsilateral infusions would still produce greater effects than degrade while lidocaine is physiologically active and contralateral infusions were stimulation sites moved to the return to baseline levels as its effects wear off, we observe right hemisphere, our results coupled with the recent a covariation between putative cause and effect that one findings about lateralization push us toward considering cannot observe using irreversible lesions. This strengthens
non-dopaminergic mechanisms. our conclusion that it was the inactivation of target tissue
Non-dopaminergic RRF cells are important to at least that led to reward reduction. Second, saline injections one motivational function: disconnecting the sexually reliably produced little if any effect on reward even though dimorphic nucleus of the gerbil hypothalamus from the these injections were randomly interspersed with lidocaine RRF disrupts male sexual behavior, and the A8 cells are test conditions. In some cases, saline injections were the not important to this effect [20,52]. (Numan and Numan very last condition to be tested. That saline produced [34,35] have also demonstrated that forebrain projections negligible shifts even when preceded by many lidocaine to the RRF participate in the neural control of maternal conditions — while lidocaine injections continued to behavior.) Axons passing through but not arising from the produce shifts — shows that the target tissue remains RRF could also be important to MFB self-stimulation. For stable over multiple injections. Thus, multiple conditions example, both the deep mesencephalic reticular field and may be validly tested within a single subject, gaining the the oral pontine reticular field send efference to the power inherent in within-subject experimental designs. forebrain that passes through both the RRF and MFB sites Finally, and most utilitarian, the required frequency shifts that support robust self-stimulation [26]. Therefore, it is observed in this study are among the most consistently relevant to distinguish whether cell bodies or axons or large shifts observed in this line of work in recent years, terminals in the RRF are responsible for its role in MFB suggesting that neural recovery and plasticity mechanisms
stimulation reward. may have obscured potentially informative results in past
It is tempting to address this issue using ‘cell body lesion studies. selective’ excitotoxins. However, this again would involve
permanent lesioning and, more to the point, such agents
have been shown to be less ‘cell body selective’ than the Acknowledgements nomenclature implies. Glutamatergic excitotoxins such as
NMDA, kainic acid, and ibotenic acid demyelinate axons This work was supported by NIMH grant MH52588 to passing through the injection site, a response that appears MW. The authors wish to thank Ashley Acheson, Jacqui to be at least partially mediated by an inflammatory Collins, Michael Messina, Toni Pann, and Wesley Kokott reaction to the toxin [11–13,18,43,50]. While the demyeli- for assistance in rat testing and histology and R.T. Busse nated axons may retain their integrity [40], demyelination for helpful suggestions on early drafts.
would impair their conduction ability. Especially in the context of studies of self-stimulation where reward signal conduction is an important factor, impaired conduction
References
would be functionally equivalent to lesioning those axons. A better approach would be to use the
electrophysiologi-[1] A. Acheson, M. Waraczynski, M. Perkins, Lesions and inactivation
cal approach pioneered by (among others) Rolls’, implicate dorsolateral hindbrain in MFB self-stimulation, Physiol. Yeomans’, and Shizgal’s research groups to determine (1) Behav. 71 (1-2) (2000) 159–171.
whether there is a direct, axonal connection between the [2] D.J. Albert, F.J. Madryga, An examination of the functionally effective spread of 4ml of slowly infused lidocaine, Behav. Neural
RRF and MFB self-stimulation sites and (2) whether single
Biol. 29 (1980) 378–384.
units in the RRF are activated by rewarding MFB
stimula-[3] S.L. Andersen, M.H. Teicher, Serotonin laterality in amygdala
tion and show conduction characteristics compatible with predicts performance in the elevated plus maze in rats, Neuroreport those known to characterize the directly-activated substrate 10 (1999) 3497–3500.
for MFB self-stimulation (see e.g. Refs. [4] G.W. Arbuthnott, T.J. Crow, K. Fuxe, U. Ungerstedt, Behavioral effects of stimulation in the region of the substantia nigra, J. Physiol.
[9,15,38,41,42,53,54]). We are beginning such work,
ad-210 (1970) 61–62.
dressing goal (1) by replacing traditional
behaviorally-[5] M.P. Arts, A.R. Cools, Bilateral 6-hydroxydopamine lesions in the
based collision techniques with direct recording of the dopaminergic A8 cell group produces long-lasting deficits in motor compound action potential evoked in distal sites by programming of cats, Behav. Neurosci. 112 (1998) 102–115.
rewarding MFB stimulation, an approach based on the [6] M.P. Arts, H.J. Groenewegen, J.G. Veening, A.R. Cools, Efferent projections of the retrorubral nucleus to the substantia nigra and
work of Kiss and Shizgal [27].
ventral tegmental area in cats as shown by anterograde tracing,
To close on a methodological note, the results presented
Brain Res. Bull. 40 (1996) 219–228.
here strongly support lidocaine inactivation as a technique [7] P. Bauco, R. Rivest, R.A. Wise, Extracellular nucleus accumbens to replace permanent lesioning in investigations of the dopamine and metabolite levels during earned and unearned lateral
[8] K.C. Berridge, T.E. Robinson, What is the role of dopamine in [28] R. Larisch, W. Meyer, A. Klimke, F. Kehren, H. Vosberg, H.W. reward: hedonic impact, reward learning, or incentive salience?, Muller-Gartner, Left-right asymmetry of striatal dopamine D2 Brain Res. Bull. 28 (1998) 309–369. receptors, Nucl. Med. Commun. 19 (1998) 781–787.
[9] C. Bielajew, M. Lapointe, I. Kiss, P. Shizgal, Absolute and relative [29] M. Lepore, K.B.J. Franklin, N-Methyl-D-aspartate lesions of the refractory periods of the substrates for lateral hypothalamic and pedunculopontine nucleus block acquisition and impair maintenance ventral midbrain self-stimulation, Physiol. Behav. 28 (1982) 125– of responding reinforced with brain stimulation, Neuroscience 71
132. (1996) 147–155.
[10] C.D. Blaha, A.G. Phillips, Application of in vivo electrochemistry to [30] Q. Li, J. Thornhill, A functional medial preoptic nucleus (MPO) is the measurement of changes in dopamine release during intracranial required for scrotal thermal stimuli to alter the neuronal activity of self-stimulation, J. Neurosci. Meth. 34 (1990) 125–133. thermoresponsive ventromedial hypothalamic (VMH) neurons,
Brain Res. 716 (1996) 134–140. [11] H. Brace, M. Latimer, P. Winn, Neurotoxicity, blood–brain barrier
breakdown, demyelination and remyelination associated with [31] O. Lindvall, A. Bjorkland, The organization of the ascending NMDA-induced lesions of the rat lateral hypothalamus, Brain Res. catecholamine systems in the rat brain as revealed by the glyoxylic Bull. 43 (1997) 447–455. acid fluorescence method, Acta Physiol. Scand. (Suppl. 412) (1974)
1–48. [12] P.J. Coffey, V.H. Perry, Y. Allen, J. Sinden, J.N.P. Rawlins, Ibotenic
acid induced demyelination in the central nervous system: a [32] J. Moisan, P.-P. Rompre, Electrophysiological evidence that a subset consequence of a local inflammatory response, Neurosci. Lett. 84 of midbrain dopamine neurons integrate the reward signal induced (1988) 178–184. by electrical stimulation of the posterior mesencephalon, Brain Res.
786 (1998) 143–152. [13] P.J. Coffey, V.H. Perry, J.N.P. Rawlins, An investigation into the
early stages of the inflammatory response following ibotenic acid- [33] E.L. Nelson, C.L. Liang, C.M. Sinton, D.C. German, Midbrain induced neuronal degeneration, Neuroscience 35 (1990) 121–132. dopaminergic neurons in the mouse: computer-assisted mapping, J.
Comp. Neurol. 369 (1996) 361–371. [14] K. Coleman-Mesches, J.L. McGaugh, Differential involvement of
the right and left amygdalae in expression of memory for aversively [34] M. Numan, M. Numan, A lesion and neuroanatomical tract-tracing motivated training, Brain Res. 670 (1995) 75–81. analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in [15] S.J. Cooper, E.T. Rolls, Relation of activation of neurones in the
rats, Dev. Psychobiol 29 (1996) 23–51. pons and medulla in brain-stimulation reward, Exp. Brain Res. 20
(1974) 207–222. [35] M. Numan, M.J. Numan, Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express [16] T.J. Crow, The relationship between lesion site, dopamine neurones,
Fos during maternal behavior in female rats, J. Neuroendocrinol. 9 and turning behavior in the rat, Exp. Neurol. 32 (1971) 247–255.
(1997) 369–384. [17] A. Dahlstrom, K. Fuxe, Evidence for the existence of
monoamine-containing neurons in the central nervous system, Acta Physiol. [36] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, Scand. 62 (Suppl. 232) (1964) 1–55. 4th Edition, Academic Press, San Diego, 1998.
[18] I. Dusart, S. Marty, M. Peschanski, Demyelination and remyelina- [37] A. Rashidy-Pour, F. Motamedi, Z. Motahed-Larijani, Effects of tion by Schwann cells and oligodendrocytes after kainate-induced reversible inactivations of the medial septal area on reference and neuronal depletion in the central nervous system, Neuroscience 51 working memory versions of the Morris water maze, Brain Res. 709
(1992) 137–148. (1996) 131–140.
[19] J.H. Fallon, R.Y. Moore, Catecholamine innervation of the basal [38] E.T. Rolls, Involvement of brainstem units in medial forebrain forebrain. IV. Topography of the dopamine projection to the basal bundle self-stimulation, Physiol. Behav. 7 (1971) 297–310. forebrain and neostriatum, J. Comp. Neurol. 180 (1978) 545–580. [39] T.M. Saleh, B.J. Connell, Modulation of the cardiac baroflex [20] P.D. Finn, P. Yahr, Projection of the sexually-dimorphic area of the following reversible blockade of the parabrachial nucleus in the rat,
gerbil hypothalamus to the retrorubral field is essential for male Brain Res. 767 (1997) 201–207.
sexual behavior: role of A8 and other cells, Behav. Neurosci. 108 [40] R. Schwarcz, T. Hokfelt, K. Fuxe, G. Jonsson, M. Goldstein, T. (1994) 362–378. Terenius, Ibotenic acid-induced neuronal degeneration: a mor-[21] P.A. Garris, M. Kilpatrick, M. Bunin, D. Michael, Q.D. Walker, phological and neurochemica study, Exp. Brain Res. 37 (1979)
R.M. Wightman, Dissociation of dopamine release in the nucleus 199–216.
accumbens from intracranial self-stimulation, Nature 398 (1999) [41] P. Shizgal, C. Bielajew, D. Corbett, R. Skelton, J.S. Yeomans,
67–69. Behavioral methods for inferring anatomical linkage between
re-[22] A. Gasbarri, M.G. Packard, A. Sulli, C. Pacitti, R. Innocenzi, V. warding brain stimulation sites, J. Comp. Physiol. Psych. 94 (1980) Perciavalle, The projections of the retrorubral field A8 to the 227–237.
hippocampal formation in the rat, Exp. Brain Res. 112 (1996) [42] P. Shizgal, D. Schindler, P.-P. Rompre, Forebrain neurons driven by
244–252. rewarding stimulation of the medial forebrain bundle in the rat:
[23] C. Gonzales, M.F. Chesselet, Amygdalonigral pathway: an antero- comparison of psychophysical and electrophysiological estimates of grade study in the rat with Phaseolus vulgaris leucoagglutinin refractory periods, Brain Res. 499 (1989) 234–248.
(PHA-L), J. Comp. Neurol. 297 (1990) 182–200. [43] J.R. Stellar, F.S. Hall, M. Waraczynski, The effects of excitotoxic [24] A. Gratton, B.J. Hoffer, G.A. Gerhardt, Effects of electrical stimula- lesions of the lateral hypothalamus on self-stimulation reward, Brain
tion of brain reward sites on release of dopamine in rat: An in vivo Res. 541 (1991) 29–40.
electrochemical study, Brain Res. Bull. 21 (1988) 319–324. [44] R.M. Sullivan, A. Gratton, Relationships between stress-induced [25] Y.H. Huang, A. Routtenberg, Lateral hypothalamic self-stimulation increases in medial prefrontal cortical dopamine and plasma cor-pathways in Rattus norvegicus, Physiol. Behav. 7 (1971) 419–432. ticosterone levels in rats: role of cerebral laterality, Neuroscience 83 [26] B.E. Jones, T.-Z. Yang, The efferent projections from the reticular (1998) 81–91.
formation and the locus coeruleus studied by anterograde and [45] E.J. Tehovnik, M.A. Sommer, Effective spread and timecourse of retrograde axonal transport in the rat, J. Comp. Neurol. 242 (1985) neural inactivation caused by lidocaine injection in monkey cerebral
56–92. cortex, J. Neurosci. Meth. 74 (1997) 17–26.
and adrenergic cell groups in the rat. Brain greater challenge, Res. [52] P. Yahr, P.D. Finn, N.W. Hoffman, N. Sayag, Sexually dimorphic cell Bull. 28 (1992) 447–454. groups in the medial preoptic area that are essential for male sex [48] M. Waraczynski, A. Acheson, M. Perkins, Temporary inactivation behavior and the neural pathways needed for their effects,
Psycho-may provide a greater challenge to MFB stimulation reward value neuroendocrinology 19 (1994) 463–470.
than does permanent lesioning, Soc. Neurosci. Abst. 25 (1999) [53] J.S. Yeomans, Quantitative measurement of neural post-stimulation
1373. excitability with behavioral methods, Physiol. Behav. 15 (1975)
[49] M. Waraczynski, M. Perkins, A. Acheson, Lesions of midline 593–602.
midbrain structures leave medial forebrain bundle self-stimulation [54] J.S. Yeomans, Absolute refractory periods of self-stimulation neu-intact, Behav. Brain Res. 103 (1999) 175–184. rons, Physiol. Behav. 22 (1979) 911–919.
[50] M. Waraczynski, J.R. Stellar, F.S. Hall, Ibotenic acid lesions and [55] J.S. Yeomans, N.T. Maidment, B.S. Bunney, Excitability properties lateral hypothalamic self-stimulation, Soc. Neurosci. Abst. 13 of medial forebrain bundle axons of A9 and A10 dopamine cells,
(1987) 1324. Brain Res. 450 (1988) 186–193.