www.elsevier.com / locate / bres
Research report
Persistent and delayed behavioral changes after nicotine treatment in
adolescent rats
*
J.A. Trauth, F.J. Seidler, T.A. Slotkin
Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, NC 27710, USA Accepted 8 August 2000
Abstract
Despite the increasing use of tobacco by adolescents, few animal studies have addressed the neurobehavioral consequences of nicotine exposure during this period. We administered nicotine to adolescent rats via continuous infusion on postnatal days (PN) 30 through 47.5, using a dosage regimen that maintains plasma levels similar to those found in smokers or in users of the transdermal nicotine patch. Behavior in a novel open field and learning a passive avoidance task were assessed during nicotine treatment and for 2 weeks post-treatment. On PN44, during nicotine exposure, female rats showed decreased grooming, an effect not seen in males; this effect is opposite to the effects of nicotine in adult rats. Two weeks after cessation of nicotine administration, females showed deficits in locomotor activity and rearing, whereas males again were unaffected; the behavioral deficits appeared at the same age at which gender-selective brain cell damage emerges. In contrast, nicotine exposure enhanced passive avoidance, with the effect intensifying and persisting throughout the post-treatment period. These results reinforce the concept that developmental vulnerability to nicotine extends into adolescence, with patterns of drug effects different from those in earlier or later periods. The correlation of neurochemical with behavioral effects strengthens the connection between adolescent nicotine exposure and persistent functional changes that may influence drug habituation, learning and memory. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Drugs of abuse: amphetamine and other stimulants
Keywords: Adolescence; Nicotine; Open field activity; Passive avoidance
1. Introduction receptor expression, accompanied by brain cell loss [35], hippocampal damage that appears after several weeks’ Recent estimates indicate that over one-third of U.S. delay [34,35], and persistent changes in cholinergic synap-high school students are using tobacco products and that tic activity, particularly in the hippocampus [33]. Initial nearly 3000 children under the age of 18 begin smoking behavioral studies with adolescent nicotine exposure also every day [20]. Early initiation of smoking leads to higher found long-term changes in the learning related to reward daily consumption of cigarettes and a reduced probability tasks [14] but there have been no systematic evaluations of of quitting [4,22,24]. Most life-long smokers begin their behavioral performance in the period during and immedi-habit as adolescents [20,22] and it is therefore somewhat ately after adolescent nicotine administration.
surprising that relatively little attention has been paid to Accordingly, the current study examines the effects of establishing animal models of adolescent nicotine expo- adolescent nicotine exposure on two sets of behaviors sure. Recently, we found that adolescent rats given nico- known to be affected by nicotine administration in adults: tine show gender-selective, persistent changes in nicotinic open field behaviors (locomotion, rearing, grooming) [3,5,13,36] and passive avoidance [23,29,37]. Nicotinic cholinergic pathways are essential in the acquisition of
Abbreviations: ANOVA, analysis of variance; PN, postnatal day
passive avoidance behaviors [19] and hippocampal *Corresponding author. Tel.: 11-919-681-8015; fax: 1
1-919-684-cholinergic pathways are specifically involved [1]. We used 8197.
E-mail address: [email protected] (T.A. Slotkin). an established minipump infusion model that produces and
maintains plasma nicotine levels within the range seen in for a 5-min period in a circular field (93-cm diameter3 45-typical smokers or in users of the nicotine transdermal cm height) divided into 10-cm squares. Scoring included patch and that, in the adolescent brain produces the locomotion (squares crossed), rears and grooms. The biochemical changes already described [33–35]. apparatus was cleaned in between animals with dilute Lysol and dried. The day after open field testing (PN45, PN51, or PN61), the animals were trained for passive
2. Methods avoidance using the Gemini Avoidance System (San Diego Instruments, San Diego, CA). The test apparatus contained 2.1. Animal treatments two chambers, each 21325.5316.5 (height) cm. Subjects were placed in the lighted chamber and allowed up to 10 All studies were carried out in accordance with the min to enter the darkened chamber, whereupon the door declaration of Helsinki and with the Guide for the Care closed and they received a mild foot shock (0.75 mA, 2 s).
and Use of Laboratory Animals as adopted and prom- Twenty-four hours later the animals were tested in the ulgated by the National Institutes of Health. Litters of apparatus and allowed up to 10 min to cross into the dark Sprague–Dawley rats (Zivic Laboratories, Pittsburgh, PA) chamber. For both types of behavioral tests, animals were were shipped with their dams by climate-controlled truck selected in a random order and were tested alternating (total transit time less than 12 h). At weaning, postnatal control and nicotine, male and female animals.
day (PN) 21, animals were housed individually and
allowed free access to food and water. Drug treatments 2.3. Data analysis were administered by osmotic minipump infusions
begin-ning on PN30. Each animal was anesthetized lightly with Data are presented as means and standard errors. ether, a 334 cm area on the back was shaved and an Treatment effects were analyzed initially using a repeated incision made to permit s.c. insertion of osmotic measures ANOVA across all three open field indices minipumps (type 1002, Alza Corp., Palo Alto, CA). Pumps (locomotion, rearing and grooming), or across the two were prepared with nicotine bitartrate (Sigma Chemical passive avoidance measures (training, retention) with three Co., St. Louis, MO) dissolved in bacteriostatic water factors (treatment, age, gender); data were square-root- or (Abbott Laboratories, N. Chicago, IL), to deliver an initial log-transformed because of heterogeneous variance. When dose rate of 6 mg / kg of nicotine per day [27,34,35]. The the initial analysis indicated an interaction of treatment incision was closed with wound clips and the animals were with the other variables, appropriate lower-order ANOVAs permitted to recover in their home cages. Control animals were conducted, followed by post-hoc evaluations of were implanted with minipumps containing bacteriostatic individual differences using Fisher’s Protected Least Sig-water and sodium bitartrate equivalent to the nicotine nificant Difference; post-hoc tests were not conducted bitartrate concentration. It should be noted that the pump, where there were only main treatment effects (i.e. without marketed as a two week infusion device, actually takes interactions with age or gender), in which case only the approximately 17.5 days to be exhausted completely main effect is reported. Significance was assumed at the (information supplied by the manufacturer) and thus the level of P,0.05 for main effects; for interactions, values nicotine infusion terminates on PN47.5. This paradigm of P,0.1 were examined for lower-order main effects after demonstrably activates central nicotinic receptors subdivision of the interactive variables [30].
[17,21,28,34] and produces plasma nicotine levels similar to those in typical smokers, approximately 25 ng / ml [35].
Cotinine levels are 6–8-fold higher [35], also as found in 3. Results
human smokers; cotinine is also a potential developmental
Table 1
Global statistical analyses
All open Locomotor Rearing Grooming Passive Training Testing
field avoidance
Treatment3gender NS NS NS NS NS NS NS
Treatment3measure NS – – – P,0.04 – –
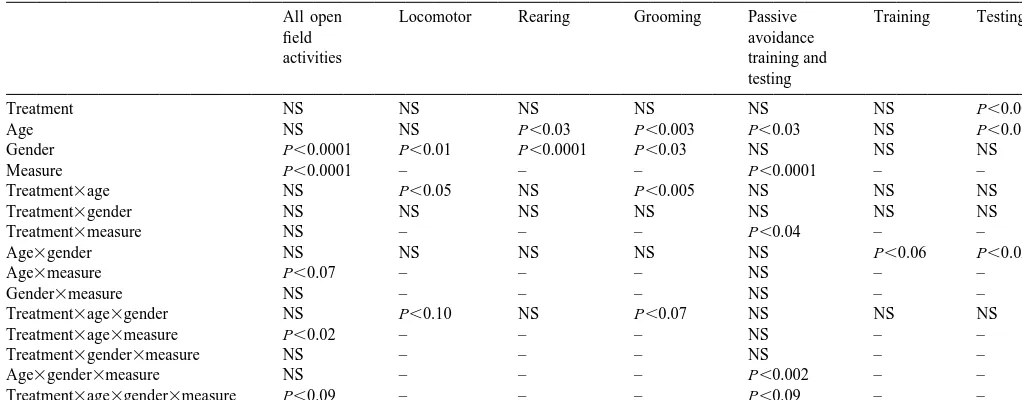
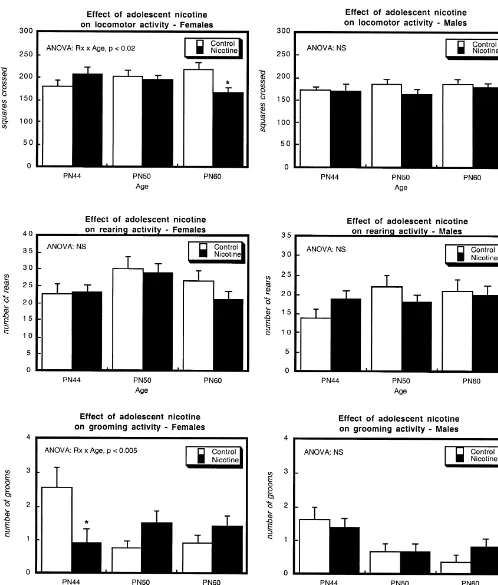
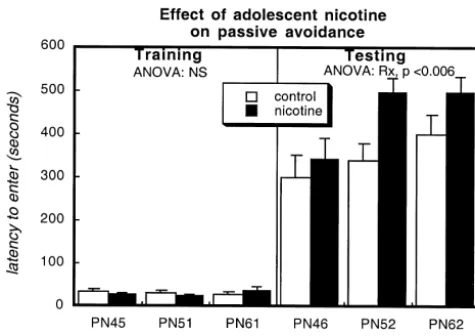
(locomotor activity and grooming but not rearing). There- adolescent nicotine exposure did not alter the latency to fore, results for open field activities are presented for each enter the dark compartment in which the animals received of the measures with subdivision by gender and age. the foot shock (Fig. 2), regardless of whether the training In female rats (Fig. 1, left panels), adolescent nicotine session was conducted during the period of drug treatment administration did not alter locomotor activity or rearing (PN45), shortly after the cessation of exposure (PN51) or during the period of nicotine administration (PN44), nor after 2 weeks (PN61). When these animals were retested was activity affected on PN50, immediately after the for retention, however, the nicotine-treated animals per-cessation of nicotine exposure. However, on PN60, nearly formed better overall (main effect of treatment); the effect 2 weeks after discontinuing nicotine, locomotor activity was not lost during the post-treatment period but rather was significantly reduced; rearing showed the same effect, intensified, superimposed on a general increase in latency and although the result did not achieve statistical signifi- with age (main effect of age).
cance due to greater variability for this measure, the overall effect of nicotine was not separable across the two
measures (no interaction of treatment3measure). Groom- 4. Discussion
ing showed a completely different pattern, with significant
Fig. 1. Effects of adolescent nicotine treatment (Rx) on open field activity. Data represent means and standard errors obtained from 10–12 rats in each group for each age and gender. ANOVA across all ages appears within the panels and asterisks denote individual values for which the nicotine group differs from the corresponding controls; individual tests were not conducted in the absence of a treatment3age interaction.
strate facilitated induction of long-term potentiation, an event associated with enhanced learning and memory [7,11]. However, nicotine enhances hippocampal learning through both stimulation and desensitization [8], and the adolescent brain shows a different temporal and regional pattern of nicotinic receptor upregulation from that seen in the adult [34], as well as displaying reductions in hip-pocampal cholinergic tone that persist into the post-treat-ment period [33]. It is thus likely that the differing patterns of neural activity and receptor expression of the adolescent brain produce distinct behavioral outcomes. Alternatively, the differences in passive avoidance behavior may again rest on the structural damage (cell loss, changes in cell size) evoked by adolescent nicotine exposure [34,35], or through unique effects on other neurotransmitter systems Fig. 2. Effects of adolescent nicotine treatment on latency to enter a or brain regions [18]. We are currently exploring the darkened chamber (left) and on retention of passive avoidance 24 h later impact of adolescent nicotine exposure on catechola-(right). Data represent means and standard errors obtained from 21–24
minergic systems and our preliminary results (manuscript rats in each group at each age. ANOVA across all ages appears within the
in preparation) indicate both immediate and delayed panel. Statistical tests were not carried out for individual ages because of
changes in synaptic activity as monitored by neurotrans-the absence of a treatment3age interaction.
mitter turnover; again, these include long-term effects on hippocampal noradrenergic systems that are selective for identifies adolescence as a unique, critical period for the female gender. Regardless of the mechanisms underly-effects of nicotine on brain function. ing the behavioral differences between adults and adoles-The apparent lack of effect of adolescent nicotine cents given nicotine, it should be emphasized that whereas exposure on open field activities in males was somewhat improved retention was observed with the passive avoid-surprising, given our previous finding that, in some brain ance paradigm, the same is not likely to be true for other regions, nicotinic receptor upregulation persists longer in learning or memory tasks, as nicotine is known to exert adolescent males than in females [34]. Indeed, males also dual actions, improving performance in some tasks while show suppression of hippocampal cholinergic tone that impairing it in others [10,16,25,31,32].
persists after the cessation of nicotine exposure [33]. Male The current findings indicate that the behavioral changes rats may simply be less susceptible to nicotine-induced elicited by adolescent nicotine exposure differ from those alterations in locomotor behavior, as adult males also do seen in the adult and may emerge well after the cessation not display differences under basal conditions, but do so of treatment. Along with earlier work demonstrating when subjected to an acute challenge [9]. Accordingly, effects on learning and memory [15] and on reward-situations that require an integrated response or learning directed behaviors lasting into adulthood [14], our results may be necessary to uncover the effects of adolescent indicate that the vulnerability of the developing brain to nicotine exposure in males [6,14]. In the current study, we nicotine extends into adolescence. Given the strong link of evaluated this possibility using passive avoidance, and in smoking initiation during adolescence with increased this case, the learning task evoked changes in both males addiction liability and poorer cessation rates, these be-and females. Unlike the results with open field activity, havioral studies indicate the need for greater concern about adolescent nicotine exposure actually enhanced passive the long-term consequences of adolescent tobacco use. avoidance 24 h after the training session. The temporal
pattern of the effect was particularly interesting, as the improvement was minimal during nicotine administration
Acknowledgements
but intensified in the post-treatment period; indeed, a preliminary report indicates that improved cognitive
per-Supported by a grant from the Smokeless Tobacco formance may persist for weeks or months after adolescent
Research Council and by a STAR fellowship from the US nicotine treatment [15]. In adults, cognitive tasks,
includ-Environmental Protection Agency. ing passive avoidance performance, show improvements
with nicotine administration, but unlike the situation seen here, the peak effects are seen during treatment
[12,16,23,25,29,32,37]. Again, these discrepancies may References reside in the unique neurochemical effects of nicotine on
avoidance learning and emotionality in rats, Life Sci. 64 (1999) chick telencephalon, following passive avoidance learning,
Neuro-1553–1561. sci. Lett. 270 (1999) 75–78.
[2] T. Audesirk, L. Cabell, Nanomolar concentrations of nicotine and [20] National Institute on Drug Abuse, Nicotine Addiction, NIH Publi-cotinine alter the development of cultured hippocampal neurons via cation Number 98-4342, Washington, DC, 1998.
non-acetylcholine receptor-mediated mechanisms, Neurotoxicology [21] H.A. Navarro, F.J. Seidler, R.D. Schwartz, F.E. Baker, S.S. Dob-20 (1999) 639–646. bins, T.A. Slotkin, Prenatal exposure to nicotine impairs nervous [3] R.M. Booze, M.A. Welch, M.L. Wood, K.A. Billings, S.R. Apple, system development at a dose which does not affect viability or
C.F. Mactutus, Behavioral sensitization following repeated intraven- growth, Brain Res. Bull. 23 (1989) 187–192.
ous nicotine administration: gender differences and gonadal hor- [22] D.E. Nelson, G.A. Giovino, D.R. Shopland, P.D. Mowery, S.L. mones, Pharmacol. Biochem. Behav. 64 (1999) 827–839. Mills, M.P. Eriksen, Trends in cigarette smoking among US [4] J. Chen, W.J. Millar, Age of smoking initiation: implications for adolescents, 1974 through 1991, Amer. J. Public Health 85 (1995)
quitting, Health Rep. 9 (1998) 39–46. 34–40.
[5] A.C. Collins, E. Romm, J.M. Wehner, Nicotine tolerance: an [23] A. Nitta, Y. Katono, A. Itoh, T. Hasegawa, T. Nabeshima, Nicotine analysis of the time course of its development and loss in the rat, reverses scopolamine-induced impairment of performance in passive Psychopharmacology 96 (1988) 7–14. avoidance task in rats through its action on the dopaminergic [6] W.A. Corrigall, S. Herling, K.M. Coen, Evidence for a behavioral neuronal system, Pharmacol. Biochem. Behav. 49 (1994) 807–812. deficit during withdrawal from chronic nicotine treatment, Phar- [24] J.P. Pierce, E. Gilpin, How long will today’s new adolescent smoker macol. Biochem. Behav. 33 (1989) 559–562. be addicted to cigarettes?, Am. J. Pub. Health 86 (1996) 253–256. [7] S. Fujii, Z. Ji, N. Morita, K. Sumikawas, Acute and chronic nicotine [25] E.J. Popke, A.J. Mayorga, C.M. Fogle, M.G. Paule, Effects of acute exposure differentially facilitate the induction of LTP, Brain Res. nicotine on several operant behaviors in rats, Pharmacol. Biochem.
846 (1999) 137–143. Behav. 65 (2000) 247–254.
[8] S. Fujii, Y.S. Jia, A.Z. Yan, K. Sumikawa, Nicotine reverses [26] T.A. Slotkin, Prenatal exposure to nicotine: What can we learn from GABAergic inhibition of long-term potentiation induction in the animal models, in: I.S. Zagon, T.A. Slotkin (Eds.), Maternal hippocampal CA1 region, Brain Res. 863 (2000) 259–265. Substance Abuse and the Developing Nervous System, Academic [9] Y.K. Fung, Y.S. Lau, Effect of nicotine pretreatment on striatal Press, San Diego, 1992, pp. 97–124.
dopaminergic system in rats, Pharmacol. Biochem. Behav. 32 [27] T.A. Slotkin, Fetal nicotine or cocaine exposure: which one is (1989) 221–226. worse?, J. Pharmacol. Exp. Ther. 285 (1998) 931–945.
[10] D.M. Gilliam, K. Schlesinger, Nicotine-produced relearning deficit [28] T.A. Slotkin, L. Orband-Miller, K.L. Queen, W.L. Whitmore, F.J. in C57BL / 6J and DBA / 2J mice, Psychopharmacology 86 (1985) Seidler, Effects of prenatal nicotine exposure on biochemical
291–295. development of rat brain regions: maternal drug infusions via
[11] S. Hamid, G.S. Dawe, J.A. Gray, J.D. Stephenson, Nicotine induces osmotic minipumps, J. Pharmacol. Exp. Ther. 240 (1987) 602–611. long-lasting potentiation in the dentate gyrus of nicotine-primed rats, [29] R.D. Smith, M.K. Kistler, M. Cohen-Williams, V.L. Coffin, Neurosci. Res. 29 (1997) 81–85. Cholinergic improvement of a naturally-occurring memory deficit in [12] V. Haroutunian, E. Barnes, K.L. Davis, Cholinergic modulation of the young rat, Brain Res. 707 (1996) 13–21.
memory in rats, Psychopharmacology 87 (1985) 266–271. [30] G.W. Snedecor, W.G. Cochran, Statistical Methods, Iowa State [13] E.T. Iwamoto, An assessment of the spontaneous activity of rats University Press, Ames, Iowa, 1967.
administered morphine, phencyclidine, or nicotine using automated [31] G.J. Spillich, L. June, J. Renner, Cigarette smoking and cognitive and observational methods, Psychopharmacology 84 (1984) 374– performance, Brit. J. Addict. 87 (1992) 1313–1326.
382. [32] I.P. Stolerman, N.R. Mirza, B. Hahn, M. Shoaib, Nicotine in an
[14] B.M. Kelley, L.D. Middaugh, Periadolescent nicotine exposure animal model of attention, Eur. J. Pharmacol. 393 (2000) 147–154. reduces cocaine reward in adult mice, J. Addict. Dis. 18 (1999) [33] J.A. Trauth, E.C. McCook, F.J. Seidler, T.A. Slotkin, Modeling 27–39. adolescent nicotine exposure: effects on cholinergic systems in rat [15] E.D. Levin, Persisting effects of chronic adolescent nicotine ad- brain regions, Brain Res. 873 (2000) 18–25.
ministration on radial-arm maze learning and response to nicotinic [34] J.A. Trauth, F.J. Seidler, E.C. McCook, T.A. Slotkin, Adolescent challenges, Neurotoxicol. Teratol. 21 (1999) 338. nicotine exposure causes persistent upregulation of nicotinic [16] E.D. Levin, A.H. Rezvani, Development of nicotinic drug therapy cholinergic receptors in rat brain regions, Brain Res. 851 (1999)
for cognitive disorders, Eur. J. Pharmacol. 393 (2000) 141–146. 9–19.
[17] W. Lichtensteiger, U. Ribary, M. Schlumpf, B. Odermatt, H.R. [35] J.A. Trauth, F.J. Seidler, T.A. Slotkin, An animal model of Widmer, Prenatal adverse effects of nicotine on the developing adolescent nicotine exposure: effects on gene expression and brain, Prog. Brain Res. 73 (1988) 137–157. macromolecular constituents in rat brain regions, Brain Res. 867 [18] C.G.A. Lorenzini, E. Baldi, C. Bucherelli, B. Sacchetti, G. Tassoni, (2000) 29–39.
Neural topography and chronology of memory consolidation: a [36] H. Welzl, B. Alessandri, R. Oettinger, K. Battig, The effects of review of functional inactivation findings, Neurobiol. Learn. Mem- long-term nicotine treatment on locomotion, exploration and mem-ory 71 (1999) 1–18. ory in young and old rats, Psychopharmacology 96 (1988) 317–323. [19] S. Mezey, A.D. Szekely, R.C. Bourne, P. Kabai, A. Csillag, Changes [37] M.R. Zarrindast, M. Sadegh, B. Shafaghi, Effects of nicotine on