Metabolite transporters in plastids
Ulf-Ingo Fl ¨ugge
Communication between plastids and the surrounding cytosol occurs via the plastidic envelope membrane. Recent findings show that the outer membrane is not as freely permeable to low molecular weight solutes as previously thought, but contains different channel-like proteins that act as selectivity filters. The inner envelope membrane contains a variety of metabolite transporters that mediate the exchange of metabolites between both compartments. Two new classes of phosphate antiporters were recently described that are different in structure and function from the known triose phosphate/phosphate translocator from chloroplasts. In addition, a cDNA coding for an ATP/ADP antiporter from plastids was isolated that shows similarities to a bacterial adenylate translocator.
Addresses
Botanisches Institut der Universit ¨at zu K ¨oln, Lehrstuhl II, Gyrhofstr. 15, D-50931 K ¨oln, Germany; e-mail: [email protected]
Current Opinion in Plant Biology1998,1:201–206 http://biomednet.com/elecref/1369526600100201 Current Biology Ltd ISSN 1369-5266
Abbreviations
3-PGA 3-phosphoglycerate
AAT ADP/ATP antiport system
DiT1 2-oxoglutarate/malate translocator
DiT2 glutamate/malate translocator
Glc6P glucose 6-phosphate
GPT Glc6P/phosphate translocator
PEP phosphoenolpyruvate
PPT PEP/phosphate translocator
TPT triose phosphate/phosphate translocator
Introduction
Like other eukaryotic cells, plants cells contain different organelles, amongst them plastids, a compartment that is specific for plants. Chloroplasts, chlorophyll containing plastids, are the site of photosynthesis during which process atmospheric carbon dioxide is assimilated into intermediates that are used within, as well as outside, the chloroplasts for a variety of metabolic pathways. Chloroplasts are also involved in nitrogen assimilation and the synthesis of amino acids, transitory starch and a series of secondary compounds. Plastids of non-photosynthetic tissues have to import carbon as a source of energy and biosynthetic pathways, for example, of fatty acids, amino acids as well as starch. All plastids are double membrane-bound organelles. The inner envelope membrane is the actual permeability barrier between the plastid and the surrounding cytosol and the site of different metabolite translocators that co-ordinate the metabolism in both compartments. In this review, I describe the molecular characterization of various transporters of plastidic
mem-branes and their role in plant metabolism, taking into account the recent progress achieved in this field.
Export of photoassimilates from chloroplasts
The triose phosphate/phosphate translocator (TPT) me-diates the export of the fixed carbon in the form of triose phosphate or 3-phosphoglycerate (3-PGA) from the chloroplast into the cytosol (Figure 1, no. 1). In the mature leaves of most plants, the photosynthates exported by the TPT are used in the formation of sucrose and amino acids which are the main products allocated to heterotrophic plant organs. Sucrose and amino acids are actively loaded into the sieve element/companion cell complex by specific H+/symporters (for reviews, see [1•,2•]).The TPT antiport system accepts as substrates inorganic phosphate, triose phosphates and 3-PGA which are transported via a ping-pong type of reaction mechanism [3,4]. Using the reconstituted system in which the concen-trations of phosphate in both the internal and the external compartments are accessible to experimental variations, it could be demonstrated that the TPT can function not only as an antiporter but also as a voltage-dependent ion channel, preferentially permeable to anions [5].
The spinach TPT was the first plant membrane trans-port system for which the primary sequence could be determined [6]. Meanwhile, TPT sequences from various plants are available that all have a high similarity to each other [7]. All TPTs are nuclear-encoded and possess amino-terminal transit peptides that direct the adjacent protein to the chloroplasts. In its functional state, the TPT forms a homodimer and belongs to the group of translocators with a 6+6 helix folding pattern, similar to mitochondrial carrier proteins [8].
The final proof for the identity of a transporter cDNA is the expression of the corresponding cDNA to produce the functional protein in heterologous systems, for example yeast cells, bacteria or oocytes. The recombinant TPT protein, produced in yeast cells, exhibited transport characteristics almost identical to those of the authentic chloroplast protein [9].
Figure 1
Mal Mal
Mal Mal OAA OAA
Pi
Pi
Pi Pi
Pi HCO3
PEP PEP
ADP
ADP
TrioseP
TrioseP TrioseP
Glc6P Glc6P
ADP-Glc STARCH AMP
Glucose
Gln Gln
Glu Glu
Glu
Glu Aminoacids
2-OG 2-OG
Sucrose
PEP PEP
e.g. Shikimic acid pathway
Plastid Cytosol MDH
GS GOGAT
GS
OPPP 9
4
10
5
11
2
8
7
6
1
3
Current Opinion in Plant Biology
–
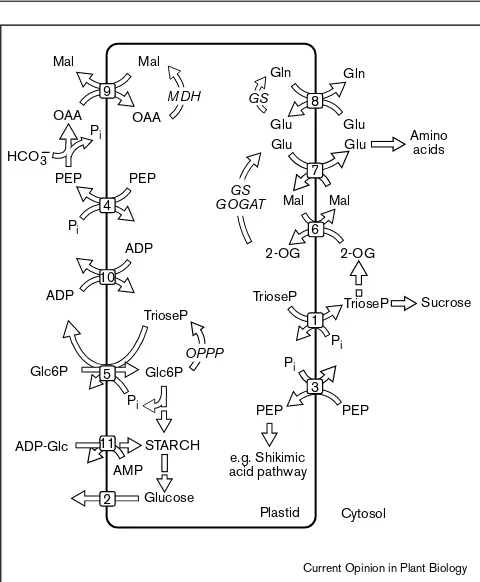
Translocators of the inner envelope membrane of plastids. 1, Triose phosphate/phosphate translocator, TPT; this translocator is primarily present in chloroplasts and mediates the export of the fixed carbon in form of triose phosphates and 3-PGA. 2, Glucose translocator; it exports the product of amylolytic starch breakdown, glucose, from plastids. 3, PEP/phosphate translocator, PPT; it provides plastids with PEP for the biosynthesis of PEP- and pyruvate-derived amino acids, fatty acids, or precursors of the shikimic acid pathway. 4, PEP/phosphate translocator present in mesophyll chloroplasts of C4-plants. It exports PEP as substrate for the PEP carboxylase. 5, Glucose 6-phosphate/phosphate translocator, GPT; it imports glucose 6-phosphate into plastids of heterotrophic tissues for the biosynthesis of starch and as a substrate for the oxidative pentose phosphate pathway. 6, 2-Oxoglutarate/malate translocator, DiT1. 7, Glutamate/malate translocator, DiT2. Both translocators, DiT1 and DiT2, are present in chloroplasts and are involved in ammonia assimilation and the refixation of photorespiratory ammonia. 8, Glutamine/glutamate translocator; it exports glutamine from chloroplasts as a source for assimilated nitrogen. 9, Oxaloacetate/malate translocator; this transporter is present in chloroplasts (corresponding activities are also found in other organelles) and mediates the indirect export of reducing equivalents in the form of malate. 10, ADP/ATP translocator providing plastids with ATP as driving force for biosynthetic
processes. 11, ADP-glucose/adenylate translocator; it is present in plastids of some starch-storing tissues and provides the plastids with ADP-glucose as substrate for starch biosynthesis. ADP-Glc, ADP-glucose; 2-OG, 2-oxoglutarate; Glc6P, glucose 6-phosphate; Gln, glutamine; Glu, glutamate; GS, glutamine synthetase; GOGAT, glutamate synthase; Mal, malate; MDH, malate dehydrogenase; OAA, oxaloacetate; OPPP, oxidative pentose phosphate pathway; Pi, inorganic phosphate; TrioseP, triose phosphates.
plastids and directed into the accumulation of starch. The deficiency in the TPT activity is obviously compensated for by mobilizing and exporting the major part of the daily accumulated carbon during the following night period [10].
This is in contrast to wild-type plants which generally export the major part of the fixed carbon during the day via the TPT. Most probably, the mobilization of the daily accumulated starch in the antisense TPT plants proceeds via the amylolytic starch breakdown resulting in hexoses which are subsequently exported via a hexose translocator (Figure 1, no.2). Interestingly, antisense TPT plants from tobacco accumulate starch as potato plants do, but mobilize the accumulated starch during ongoing photosynthesis. Hexoses are exported via a glucose transporter, the activity of which is two to three times higher in the transformants compared to the wild-type [11]. Little is known about this translocator that was first described twenty years ago [12]. Caspar et al. [13] described a starch-excess mutant from Arabidopsis, TC26, that is able to degrade starch but obviously unable to export the products of starch degradation from the chloroplasts via a glucose transporter [14]. The corresponding gene has not yet been identified.
The phosphoenolpyruvate/phosphate
translocator
Chloroplasts and nongreen plastids from most plants (depending on the developmental stage of the tissue) lack the complete set of glycolytic enzymes for the conversion of hexose phosphates and/or triose phosphate into phosphoenolpyruvate (PEP) and, therefore, rely on a supply of PEP from the cytosol [15,16]. In plastids, PEP can be used for the biosynthesis of PEP- and pyruvate-derived amino acids, fatty acids, or precursors for the shikimate pathway. The aromatic compounds synthesized from PEP via the shikimate pathway are not only constituents of proteins (aromatic amino acids) but are also utilized as precursors for the biosynthesis of a large number of secondary metabolites (e.g. alkaloids, flavonoids, and lignin) which are important in plant defense mechanisms and stress responses (for review, see [17]).
A transporter that enables the transport of PEP in plastids has recently been identified and corresponding cDNAs were isolated from nongreen and photosynthetic tissues of various plants ([18••], Figure 1, no.3). All of these clones were highly homologous to each other but the identities with members of the TPT family were only
In chloroplasts, in which both PPT and TPT are present, the combined action of both translocators would result in an exchange of triose phosphates with PEP without net phosphate transport. In heterotrophic tissues, erythrose 4-phosphate (another precursor for the shikimate pathway) and triose phosphates can be provided by the oxidative pentose phosphate pathway inside the plastids. Triose phosphate as the end products of this pathway could be exported from the plastids and converted into PEP, which subsequently could be imported via the PPT into the plastids.
A hexose phosphate/phosphate translocator
Nongreen plastids of heterotrophic tissues are carbohy-drate importing organelles and, in the case of amyloplasts from storage tissues, the site of starch synthesis. Due to the absence of plastidic fructose 1,6 bisphosphatase [19], these plastids rely on the import of hexose phosphates that are formed from sucrose delivered from source tissues. In nongreen tissues from most plants, glucose 6-phosphate (Glc6P) is the preferred hexose phosphate taken up. In amyloplasts from some tissues, however, both Glc6P and glucose 1-phosphate can be transported and subsequently used in biosynthetic processes [20,21].A Glc6P/phosphate translocator (GPT) was purified from maize endosperm and corresponding cDNA clones for GPTs from nongreen tissues of various plants were isolated ([22••], Figure 1, no.5). Analysis of the deduced GPT protein sequences revealed that the GPT proteins share only ∼38% identical amino acids with members of both the TPT and PPT families. Thus, the GPTs represent a third group of plastidic phosphate translocators. The expression of the corresponding cDNA in yeast cells and the subsequent reconstitution of the produced protein in artificial membranes revealed that inorganic phosphate, triose phosphates and Glc6P are about equally well accepted as counter substrates by the GPT, whereas PEP is only poorly transported. Other hexose phosphates, such as glucose 1-phosphate and fructose 6-phosphate, are virtually not transported by the GPT.
Inside the plastids, Glc6P is the precursor for starch biosynthesis during which process inorganic phosphate is released. In addition, Glc6P can be transformed to triose phosphates via the oxidative pentose phosphate pathway in which redox equivalents are delivered for the reduction of nitrite and for glutamate synthesis. Evidently, both substrates, triose phosphates and inorganic phosphate, can be used as counter substrates by the GPT in exchange with Glc6P.
It is worth mentioning that, until recently, it was believed that the transport of phosphate, triose phosphates, 3-PGA, PEP and hexosephosphates was mediated by a single transport system with a broad substrate specificity. Recent findings, however, clearly show that plastids contain instead a set of phosphate translocators with different
structures but overlapping specificities. Such a system enables the efficient uptake of individual phosphorylated substrates even in the presence of high concentrations of other phosphorylated metabolites, that would otherwise compete for the binding site of a single transport system.
Dicarboxylate translocators
C3-compounds exported from the chloroplasts can also serve as a source for the formation of α-keto acids (2-oxoglutarate). The resulting 2-oxoglutarate is imported into the chloroplasts for the assimilation of ammonia produced by nitrate reduction (which also requires the uptake of nitrite) or deriving from glycine decarboxylation during photorespiration. The stroma-located glutamine synthetase (GS2)/glutamate synthase reaction yields glu-tamate that is exported into the cytosol. Two different dicarboxylate antiport systems are involved in this process: the 2-oxoglutarate/malate translocator (DiT1) transport-ing 2-oxoglutarate (but no amino acids) and a gluta-mate/malate translocator (DiT2, a general dicarboxylate translocator) exporting glutamate (Figure 1, no. 6,7). As both translocators use malate as the substrate for counter-exchange, the resulting 2-oxoglutarate/glutamate transport proceeds without net malate transport [23]. Glutamate as the key compound of nitrogen metabolism in plants and other amino acids can then be further loaded into the sieve tubes via specific amino acid transporters.
Mutants ofArabidopsisdeficient in dicarboxylate transport activities are not viable under photorespiratory conditions, but they grow like wild-type plants in elevated carbon dioxide, that is to say primary ammonia assimilation is not impaired by the mutation. This phenotype is probably caused by a defect in DiT2 [24]. The corresponding gene has not yet been isolated.
DiT1 was identified as a 45 kDa component of the inner envelope membrane and a corresponding cDNA clone has been isolated [25,26]. The substrate specificities of heterologously expressed DiT1 were almost identical to the translocator purified from envelope membranes. DiT1 has a transmembrane topology with a 12-helix motif resembling that of other plasma membrane transporters from prokaryotes and eukaryotes that presumably all function as monomers. Database searches showed that DiT1 possesses no similarities to other proteins except for transporters from bacteria, for example, from Helicobacter pylori[27], that are as yet uncharacterized.
chloroplast-located GS2 of mesophyll cells, expression of GS1 is restricted to phloem companion cells that do not contain photosynthetically active chloroplasts [29,30]. The combined action of the glutamine/glutamate translocator and both DiT1 and DiT2 allows for the export of glutamine in exchange for 2-oxoglutarate with no net glutamate transport and to supply the cell with assimilated nitrogen.
Chloroplasts are also able to transport oxaloacetate (OAA) even in the presence of a large excess of other dicarboxy-lates [31] (Figure 1, no. 9). If the import is linked to an export of malate, this malate/OAA shuttle may play an im-portant role in the transfer of excess reducing equivalents from chloroplasts into the cytosol [32]. Imported OAA can also be used for transamination to aspartate which is, in turn, the precursor for the biosynthesis of various amino acids [33]. In mesophyll cells of most C4-plants, OAA, the product of the PEP carboxylase reaction, has to be imported into the chloroplasts for reduction to malate (Figure 1, no.9). The molecular structure of the OAA translocators is as yet unknown.
Chloroplasts of C3-plants also contain an antiport system for monocarboxylates, such as, glycerate and glycolate which are both intermediates of the photorespiratory cycle [34]. The molecular structure of this translocator also remains to be determined.
Transport of adenylates
Chloroplasts and nongreen plastid possess an ADP/ATP antiport system (AAT) that enables the supply of plastids with ATP as the driving force for biosynthetic processes, such as starch and fatty acid biosyntheses [35,36]. Kampfenkelet al.[37] isolated a partial cDNA clone from A. thaliana that exhibited similarities to the ADP/ATP translocase from the bacteriumRickettsia prowazekii. Isola-tion and characterizaIsola-tion of the full-length clone revealed no homology to the known mitochondrial adenylate trans-locators. The corresponding protein (AATP1) possesses an amino-terminal transit peptide that was able to target the adjacent protein to isolated chloroplasts. Interestingly, the AATP1 protein contains 12 potential transmembrane helices and thus belongs to the group of transporters with a 12 helix motif as is the case for DiT1. Expression of the AATP1 cDNA in heterologous systems and subsequent transport measurements demonstrates the function of AATP1 as an ADP/ATP translocator from plant plastids [38••].
It has been claimed that ADP-glucose can be transported via the AAT into both chloroplasts and amyloplasts [39]. The plastidic AAT, however, is obviously not able to transport the ADP-glucose [36,40]. Nonetheless, a mechanism for the transport of ADP-glucose is required in the seed endosperm of some cereals. It has been shown recently that, in these tissues, the ADP-glucose pyrophosphorylase is present mainly in the cytosol and
not in the plastids [41•,42]. The ADP-glucose formed in the cytosol is presumably transported into the plastids for starch biosynthesis via an ADP-glucose/adenylate antiporter that is distinct from the AAT. The direct import of ADP-glucose for starch biosynthesis circumvents the requirement for an AAT in these tissues. It is assumed, but not yet proven, that the Brittle 1 (Bt1) protein (which shows a significant similarity to the mitochondrial AAT) serves as an ADP-glucose/adenylate transporter [43,44]. The corresponding Bt1 mutation results in an accumulation of ADP-glucose and a reduction of starch biosynthesis in maize, probably reflecting the lack of the ADP-glucose/adenylate translocator.
Transport of amino acids
It is well established that chloroplasts are the major site of synthesis of many amino acids including those of the aspartate and the pyruvate family [33]. In addition, the plastid-located shikimate pathway leads to the formation of the aromatic amino acids phenylalanine, tyrosine and tryptophan. The resulting amino acids are either used for the plastid-located protein biosynthesis or are, in the main part, exported into the cytosol for intercellular nitrogen transport. Unfortunately, almost nothing is known about plastidic translocators involved in these processes.
Facilitators of metabolite transport in outer
envelope membranes
Until recently, it had been assumed that the outer envelope membrane of plastids is permeable to low molecular weight solutes because of the presence of porins. In chloroplast outer envelope membranes, a large conductance channel (Λ6720 pS, in 100 mM KCl) was found [45]. The primary sequence of a porin from nongreen plastids was recently obtained that resembles, structurally and functionally, the known mitochondrial porins (∼25% identical amino acids, [46,47]). More re-cently, the primary sequences of two outer envelope membrane proteins were obtained (OEP16, OEP24) that both possess channel-like activities [48••,49••]. OEP16 forms a slightly cation-selective, high conductance channel (Λ61.2 nS, in 1 M KCl). Surprisingly, this channel is selective for amino acids and molecules containing an amino acid backbone, but excludes triose phosphate and uncharged sugars. OEP 24 is a non-selective pore ((Λ62 pS, in 1 M KCl) that allows the flux of triose phosphate, dicarboxylates or adenylates but excludes sucrose. Neither OEP16 nor OEP24 show similarities to bacterial or mitochondrial porins. These results indicate the presence of selectivity filters in the outer envelope membrane and show that the intermembrane space might be not as freely accessible to small solutes as previously assumed.
Conclusions
the identity of the majority of these translocators is still rudimentary. There are multiple reasons for this. Bio-chemical approaches in the cloning of plastidic transport systems will become increasingly laborious as low-abun-dant translocators or translocators of tissues from which plastids are difficult to obtain, are studied. In addition, the identification of an unknown plastidic transporter by functional complementation of mutants defective for the transport of the corresponding metabolite does not appear feasible. This is mainly due to mistargeting of plastidic transporters in heterologous systems and to difficulty in setting up appropriate screening systems. Functional complementation of transport defective mutants, however, has been efficiently applied for the identification of sev-eral transporters of the plasma membrane. Furthermore, knowledge of transporter genes in other systems are of only limited value for the identification of plastidic transport systems by DNA-based strategies, because the primary sequences of plastidic translocators characterized to date have revealed only limited homologies to trans-porters known in other systems.
In the next few years, the various sequencing projects will provide the sequences of complete higher-plant genomes and will reveal numerous genes that presumably code for plastidic envelope transporters. However, even if a gene can be assigned as an envelope membrane transporter a challenging task will be to validate that conclusion and to elucidate the specific role of this particular gene in plant metabolism. This will require the combination of bioinformatic methods with genetical, biochemical and physiological approaches.
Acknowledgements
Work in the authors laboratory was funded by the Deutsche Forschungsge-meinschaft, the Fonds der Chemischen Industrie, the Bundesministerium f ¨ur Bildung und Forschung, and by the European Communities’ BIOTECH Programme, as part of the Project of Technological Priority 1993-1996.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest •• of outstanding interest
•
1. Ward JM, K ¨uhn C, Tegeder M, Frommer WB:Sucrose transport in higher plants.Intern Rev Cytol1997,178:41-71.
A review that summarizes studies on the molecular characterization of su-crose transporters as parts of the susu-crose transolcation pathway in higher plants.
•
2. Fischer W-N, Andr ´e B, Rentsch D, Krolkiewicz S, Tegeder M, Breitkreuz K, Frommer WB:Plant amino acid transport.Trends Plant Sci1998,3:188-195.
This review provides an excellent overview of the role of plasma membrane-located amino acid transporters of different organs that are involved in nitro-gen allocation in higher plants.
3. Fliege R, Fl ¨ugge UI, Werdan K, Heldt HW:Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts.Biochim Biophys Acta1978,502 :232-247.
4. Fl ¨ugge UI:Reaction mechanism and asymmetric orientation of the reconstituted chloroplast phosphate translocator.Biochim Biophys Acta1992,1110:112-118.
5. Schwarz M, Gross A, Steinkamp T, Fl ¨ugge UI, Wagner R:Ion channel properties of the reconstituted chloroplast triose phosphate/phosphate translocator.J Biol Chem1994,
269:29481-29489.
6. Fl ¨ugge UI, Fischer K, Gross A, Sebald W, Lottspeich F, Eckerskorn C:The triose phosphate-3-phosphoglycerate-phosphate translocator from spinach chloroplasts: nucleotide sequence of a full-length cDNA clone and import of thein vitrosynthesized precursor protein into chloroplasts.EMBO J1989,8:39-46. 7. Fischer K, Arbinger B, Kammerer K, Busch C, Brink S, Wallmeier
H, Sauer N, Eckerskorn C, Fl ¨ugge UI:Cloning andin vivo
expression of functional triose phosphate/phosphate translocators from C3- and C4-plants: evidence for the
putative participation of specific amino acids residues in the recognition of phosphoenolpyruvate.Plant J1994,5:215-226. 8. Walker JE, Runswick MJ:The mitochondrial transport protein
superfamily.J Bioenergetic Biomemembranes1993,25:435-446. 9. Loddenk ¨otter B, Kammerer B, Fischer K, Fl ¨ugge UI:Expression
of the functional mature chloroplast triose phosphate translocator in yeast internal membranes and purification of the histidine-tagged protein by a single metal-affinity chromatography step.Proc Natl Acad Sci USA1993,90 :2155-2159.
10. Heineke D, Kruse A, Fl ¨ugge UI, Frommer WB, Riesmeier JW, Willmitzer L, Heldt HW:Effect of antisense repression of the chloroplast triose-phosphate translocator on photosynthetic metabolism in transgenic potato plants.Planta1994,193 :174-180.
11. H ¨ausler RE, Schlieben NH, Schulz B, Fl ¨ugge UI:Compensation of decreased triosephosphate/phosphate transport activity by accelerated starch turnover and glucose transport in transgenic tobacco.Planta1998,204:366-376.
12. Sch ¨afer G, Heber U, Heldt HW:Glucose transport into spinach chloroplasts.Plant Physiol1977,60:286-289.
13. Caspar T, Lin T-S, Kakefuda G, Benbow L, Preiss J, Somerville C:Mutants ofArabidopsiswith altered regulation of starch metabolism.Plant Physiol1991,95:1181-1188.
14. Trethewey RN, ap Rees T:A mutant ofArabidopsis thaliana
lacking the ability to transport glucose across the chloroplast envelope.Biochem J1994,301:449-454.
15. Stitt M, ap Rees T:Capacities of pea chloroplasts to catalyse the oxidative pentose phosphate pathway and glycolysis.
Phytochemistry1979,18:1905-1911.
16. Miernyk JA, Dennis DT:A developmental analysis of the enolase isoenzymes fromRicinus communis.Plant Physiol 1992,
99:748-750.
17. Herrmann KM:The shikimate pathway: early steps in the biosynthesis of aromatic compounds.Plant Cell1995,7 :907-919.
••
18. Fischer K, Kammerer B, Gutensohn M, Arbinger B, Weber A, H ¨ausler RE, Fl ¨ugge UI:A new class of plastidic phosphate translocators: a putative link between primary and secondary metabolism by the phosphoenolpyruvate/phosphate antiporter.
Plant Cell1997,9:453-462.
The isolation and the molecular cloning of a new class of phosphate translo-cators are described. This transporter has only a low similarity to the triose phosphate/phosphate translocator and is present in all types of tissues. The possible function of this transporter in plant metabolism is discussed. 19. Entwistle G, ap Rees T:Lack of fructose-1,6-bisphosphatase
in a range of higher plants that store starch.Biochem J1990,
271:467-472.
20. Sch ¨unemann D, Borchert S:Specific transport of inorganic phosphate and C3- and C6-sugar-phosphates across the envelope membranes of tomato (Lycopersicon esculentum) leaf-chloroplasts, tomato chloroplasts and fruit-chromoplasts.Bot Acta1994,107:461-467.
21. Tetlow IJ, Bowsher CG, Emes MJ:Reconstitution of the hexose phosphate translocator from the envelope membranes of wheat endosperm amyloplasts.BiochemJ 1996,319:717-723. ••
The molecular cloning of a third class of plastidic phosphate translocators is described. This transporter is mainly present in nongreen tissues and imports glucose 6-phosphate into the plastids as a substrate for starch biosynthesis and the oxidative pentose phosphate pathway.
23. Woo KC, Fl ¨ugge UI, Heldt HW:A two-translocator model for the transport of 2-oxoglutarate and glutamate in chloroplasts during ammonia assimilation in the light.Plant Physiol1987,
84:624-632.
24. Somerville SC, Somerville CR:A mutant ofArabidopsisdeficient in chloroplast dicarboxylate transport is missing an envelope protein.Plant Sci Lett1985,37:217-220.
25. Menzlaff E, Fl ¨ugge UI:Purification and functional reconstitution of the 2-oxoglutarate/malate translocator from spinach chloroplasts.Biochim Biophys Acta 1993,1147:13-18. 26. Weber A, Menzlaff E, Arbinger B, Gutensohn M, Eckerskorn
C, Fl ¨ugge UI:The 2-oxoglutarate/malate translocator of chloroplast envelope membranes: molecular cloning of a transporter protein containing a 12-helix motif and expression of the functional protein in yeast cells.Biochemistry1995,
34:2621-2627.
27. Tomb J-F, White O, Kerlavage A, Clayton R, Sutton G, Fleischmann R, Ketchum K, Klenk H, Gill S, Dougherty Bet al.:The complete genome sequence of the gastric pathogenHelicobacter pylori.
Nature1997,388:539-547.
28. Yu J, Woo KC:Glutamine transport and the role of the glutamine translocator in chloroplasts.Plant Physiol1988,
88:1048-1054.
29. Edwards JW, Walker EL, Coruzzi GM:Cell-specific expression in transgenic plants reveals non-overlapping roles for chloroplast and cytosolic glutamine synthetase.Proc Natl Acad Sci USA
1990,87:3459-3463.
30. Carvalho H, Pereira S, Sunkel C, Salema R:Detection of a cytosolic glutamine synthetase in leaves ofNicotiana tabacum
L. by immunocytochemical methods.Plant Physiol1992,
100:1591-1594.
31. Hatch MD, Dr ¨oscher L, Fl ¨ugge U-I, Heldt HW:A specific translocator for oxaloacetate transport in chloroplasts.FEBS Lett1984,178:15-19.
32. Heineke D, Riens B, Hoferichter P, Peter U, Fl ¨ugge UI, Heldt HW:Redox transfer across the inner chloroplast envelope membrane.Plant Physiol1991,95:1131-1137.
33. Bryan JK:Advances in the biochemistry of amino acids biosynthesis.InThe Biochemistry of Plants, vol 16. Edited by Miflin BJ and Lea PJ. New York: Academic Press; 1990:161-195. 34. Howitz KT, McCarty RE:Substrate specificity of the pea
chloroplast glycolate transporter.Biochemistry1985,24 :3645-3650.
35. Heldt HW:Adenine nucleotide translocation in spinach chloroplasts.FEBS Lett1969,5:11-14.
36. Sch ¨unemann D, Borchert, S, Fl ¨ugge, UI, Heldt HW:ADP/ATP translocator from pea root plastids: comparison with translocators from spinach chloroplasts and pea leaf mitochondria.Plant Physiol1993,103:131-137.
37. Kampfenkel K, M ¨ohlmann T, Batz O, van Montagu M, Inz ´e D, Neuhaus HE:Molecular characterization of anArabidopsis thalianacDNA encoding a novel putative adenylate translocator of higher plants.FEBS Lett1995,374:351-355. ••
38. Neuhaus HE, Thom E, M ¨ohlmann T, Steup M, Kampfenkel K:
Characterization of a novel ATP/ADP translocator located in
the plastid envelope ofArabidopsis thalianaL.Plant J1997,
11:73-82.
This paper and [37] describe the isolation of a cDNA clone from Arabidop-sisthat exhibits a significant similarity to a bacterial adenylate translocator. Heterologous expression of the functional protein revealed the identity as a plant ADP/ATP translocator that is confined to plastidic envelopes and is distinct from mitochondrial adenylate translocators.
39. Pozueta-Romero J, Frehner M, Viale AM, Akazawa T:Direct transport of ADPglucose by an adenylate translocator is linked to starch biosynthesis in amyloplasts.Proc Natl Acad Sci USA
1991,88:5769-5773.
40. M ¨ohlmann T, Tjaden J, Henrichs G, Quick WP, H ¨ausler R, Neuhaus HE:ADP-glucose drives starch synthesis in isolated maize endosperm amyloplasts: characterization of starch synthesis and transport properties across the amyloplast envelope.
Biochem J1997,324:503-509. •
41. Thorbjørnsen T, Villand P, Denyer K, Olsen O-A, Smith AM:
Distinct isoforms of ADPglucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm.Plant J1996,10:243-250.
It has previously been thought that ADP-glucose pyrophosphorylase, as the key enzyme of starch biosynthesis, resides exclusively in the plastids. The authors re-examined the localization of this enzyme in non-photosynthetic tissues and found that most of this enzyme activity in developing endosperm of barley (and of maize [42]) is extraplastidial and is likely to be located in the cytosol. The cytosolic localization of this enzyme in some (but not all) cereals has a considerable impact on starch metabolism and plastid function 42. Denyer K, Dunlap F, Thorbjrnsen T, Keeling P, Smith AM:The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial.Plant Physiol1996,112:779-785. 43. Sullivan TD, Strelow LI, Illingworth CA, Phillips CA, Nelson
OE:Analysis of the maizebrittle-1alleles and a defective
Suppressor-mutator-induced mutable allele.Plant Cell1991,
3:1337-1348.
44. Sullivan T, Kaneko Y:The maize brittle1 gene encodes amyloplast membrane polypeptides.Planta1995,196:477-484. 45. Fl ¨ugge UI, Benz R:Pore forming activity in the outer membrane
of the chloroplast envelope.FEBS Lett1984,169:85-89. 46. Fischer K, Weber A, Brink S, Arbinger B, Sch ¨unemann D, Borchert
S, Heldt HW, Popp B, Benz R, Eckerskorn C, Fl ¨ugge UI:Porins from plants: molecular cloning and functional characterization of two new members of the porin family.J Biol Chem1994,
269:25754-25760.
47. Popp B, Gebauer S, Fischer K, Fl ¨ugge UI, Benz R:Study of structure and function of recombinant pea root plastid porin by biophysical methods.Biochemistry1997,36:2844-2852. ••
48. Pohlmeyer K, Soll J, Steinkamp T, Hinnah S, Wagner R:Isolation and characterization of an amino acid-selective channel protein present in the chloroplastic outer envelope membrane.
Proc Natl Acad Sci USA1997,94:9504-9509. See annotation for [49••].
••
49. Pohlmeyer K, Soll J, Grimm R, Hill K, Wagner R:A high conductance solute channel in the chloroplastic outer envelope from pea.Plant Cell1998, in press.