Changes in Activity Patterns and Intergroup
Relationships After a Significant Mortality Event
in Commensal Long-Tailed Macaques (
Macaca
Fascicularis
) in Bali, Indonesia
Fany Brotcorne1,2&Agustín Fuentes3&
I. Nengah Wandia4&Roseline C. Beudels-Jamar2&
Marie-Claude Huynen1
Received: 28 June 2014 / Accepted: 2 April 2015 / Published online: 24 May 2015 #Springer Science+Business Media New York 2015
Abstract Little is known regarding behavioral and social responses of free-ranging primates to demographic changes emerging from significant mortality events. Here, we report on the activity patterns and intergroup sociospatial relationships in a commensal population of long-tailed macaques (Macaca fascicularis) in Bali, Indonesia, that underwent a significant mortality event in summer 2012. During the period of interest, we noted heightened mortality in three of the five social groups present in this population, with adult females and juveniles experiencing higher mortality rates than adult and subadult males. Limited diagnostic data regarding pathogen identification and a lack of any conclusive etiology of the deaths prevent our ascertainment of the agent(s) responsible for the observed mortality, but given the characteristics of the event we assume it was caused by a transmissible disease outbreak. Comparing the pre- and post-mortality event periods, we found significant differences in activity patterns, including a decreased proportion of affiliation in adult females. This result is likely indicative of enhanced social instability induced by the high mortality of adult females that constitute the stable core of macaque social structure. A higher social tension between groups after the mortality event was indicated by more frequent and intense agonistic Int J Primatol (2015) 36:548–566
DOI 10.1007/s10764-015-9841-5
* Fany Brotcorne [email protected]
1
Primatology Research Group, Behavioural Biology Unit, University of Liège, 4020 Liège, Belgium
2 Conservation Biology Unit, Education and Nature, Royal Belgian Institute of Natural Sciences,
1000 Brussels, Belgium 3
intergroup encounters. Intergroup conflict success was inversely proportional to the rate of mortality a group suffered. Our results illustrate how changes in demographic structure caused by significant mortality events may have substantial consequences on behavior and social dynamics in primate groups and at the level of a population.
Keywords Anthropogenic habitat . Bali Monkey Forest . Behavioral impact . Intergroup relationship . Significant mortality . Social dynamics
Introduction
Pathogens are proposed as a significant ecological force in primate social systems and behavior, with important effects on survival and reproduction (Nunn 2012; Nunn and Altizer2006). In Old World monkeys, mass mortality episodes caused by infectious disease outbreaks have been reported (Bermejo et al. 2006; Hanamura et al. 2008; Kaur et al. 2008; Leendertz et al. 2006; Wallis and Lee
1999). Documenting the frequency of significant mortality in wild animals is of paramount importance for long-term population viability analyses (Young1994). Yet the behavioral and social responses of primates to significant disease-related demographic changes are still poorly documented. Sapolsky and Share (2004) described the long-term effects of a tuberculosis outbreak in a troop of olive baboons (Papio anubis) in Kenya, with the emergence of a particularly pacific culture following the death of the most aggressive males. A study comparing the pre-, ongoing, and post-Ebola outbreak periods in two gorilla populations (Gorilla
gorilla gorilla) showed that the immigrations and social dynamics were impacted
during the outbreak only to finally return to their initial state, suggesting a long-term resilience (Gentonet al. 2014).
Commensal primates are those that live in close association with humans and whose primary ecology is highly anthropogenic (Fuentes2012; Wheatley1999). There is a growing body of evidence that anthropogenic disturbance and increased habitat overlap of human and nonhuman primates influence primate behavioral ecology (Altmann and Muruthi1988; Gumert et al. 2011; Jaman and Huffman2013) and disease ecology through modifications of host–parasite relationship (Chapmanet al.2005; Lane2011). There is some support for increased prevalence of pathogen infection in primate populations living in anthropogenic environments (Chapman et al. 2005; Hussain
et al.2013; McLennan and Huffman 2012; Nunn and Altizer2006). By contrast, a
recent study conducted in Bali (Indonesia) argued that the quality of food consumed by long-tailed macaques (Macaca fascicularis) in anthropogenic context results in better nutritional conditions, and in turn, lessens the impact of gastrointestinal parasite infestation (Laneet al.2011). Yet, in 1994, a wide disease outbreak occurred in Bali, affecting farmed pigs and several populations of free-living long-tailed macaques, and
Streptococcus equi ssp. zooepidemicus was identified as the responsible infectious
agent (Soedarmantoet al.1996; Wheatley1999).
among individuals (Capitanio2012) and trigger period of social instability (Kaburu
et al.2013). It is well established that affiliative activities, especially allogrooming, serve an important social function of bonding and group cohesion (Dunbar 1991; Lehmannet al.2007). A decrease in affiliative activities may thus be an indicator of group instability and lower social cohesion (Beisneret al.2011). Similarly, agonistic interactions occurring within and between primate groups inform on the social tension at the group and population level, respectively (Schino et al. 1990). Demographic changes can also impact home range use by neighboring groups as well as their dominance relationships, by introducing temporary instability and leading to increased aggressions during intergroup encounters, as reported in capuchins (Cebus apella nigritus: Scarry and Tujague2012).
The main goals of the present study were to document the demographic and sociobehavioral impacts in long-tailed macaques (Macaca fascicularis) of a sig-nificant mortality event that occurred in a tourist Monkey Forest of Bali in 2012. We provide 26-yr demographic trends for the macaque population and analyze how changes in the demographic structure impacted activity patterns, intergroup relationships, and home range use of this population. Within this population, we contrasted affected groups in which many individuals died during the 2012 mortality event, and nonaffected groups in which virtually no monkeys died during this event.
Methods
Study Site and Subjects
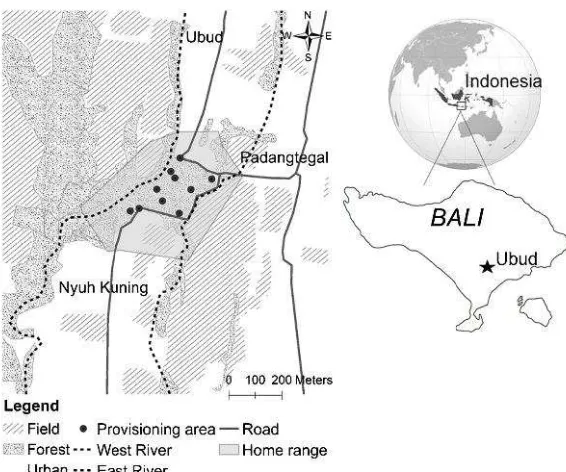
The Padangtegal Monkey Forest is located alongside the villages of Padangtegal, Nyuh Kuning and Ubud, in south-central Bali (8°31 S–155°15 E) (Fig. 1). It is a famous tourist site, visited by 205,000 tourists in 2012. The Monkey Forest consists of a Hindu temple complex within a 9-ha fragment of secondary mixed forest, surrounded by two rivers, roads, human habitations, and rice fields. Local people use the temples for ceremonies and cross the site to travel between villages (Fuenteset al.2011). The Padangtegal village committee manages the Monkey Forest and provisions the ma-caques several times a day with sweet potatoes and various fruits and vegetables. Tourists also provide them with bananas and other fruits (Fuenteset al.2005). The climate in Ubud District is tropical monsoonal, with a short dry season lasting from May to September and a wet season lasting from October to April. The average annual rainfall is 2244 mm (http://climate-data.org).
Long-tailed macaques (Macaca fascicularis fascicularis: Fooden1995) have been living in Padangtegal Monkey Forest for centuries (Fuentes et al. 2005; Wheatley
1999). Since 1986, the population has been the focus of a series of behavioral (Fuentes and Gamerl2005; Fuenteset al.2011; Wheatley1999), ethnoprimatological (Fuentes
et al. 2005; Lane-deGraaf et al. 2014; Lane et al. 2010; Loudon et al. 2006), and pathogen-related studies (Engelet al. 2006; Fuentes 2006; Jones-Engelet al.2008; Laneet al.2011). Since 2009, this population has been composed of five social groups (Cemetery, East, Michelin, Central, and Temple groups) with highly overlapping home ranges (Brotcorneet al.2011).
Data Collection
Demographic Patterns From 2009 to 2012, we conducted repeated demographic censuses of the overall population (October and June,N = 8 censuses). Because of the large groups at the site, we used procession counting (Kurita et al. 2008) to ascertain individual group size (N= 68 group counts between 2009 and 2012), counting the number of individuals during their collective travel across an open area. Population sizes before 2009 were collected by other authors using a similar counting method (Fuenteset al.2011; Loudonet al.2006; Wheatley1999). To assess the fluctuations in the composition of social groups, we assessed the representation of each of the five age–sex classes in each group: adult male (>6 yr), adult female (>3.5 yr), subadult male (4–6 yr), juvenile (sex unidentified, >1 yr), and infant (sex unidentified, < 1 yr). We determined age–sex classes by visual assessment of body size and development level of sexual organs. In addition to the overall population surveys, we kept track of the status, e.g. disappearance, age–sex class, pregnancy, of a subsample of individually identified macaques (N= 88).
Necropsy A significant mortality event occurred in the Padangetgal long-tailed ma-caque population in July–August 2012. Given the difficulty of encountering fresh carcasses and the logistical limitations imposed by onsite circumstances, we were not able to conduct a comprehensive diagnostic assessment on macaques that died during this 2012 event period. We collected only two fresh carcasses and sent them to the Veterinary Investigation Unit in Denpasar for necropsy in July 2012. To isolate the infectious agents, we collected nasal exudates and swabs from brain, thoracic, and Fig. 1 The study site Padangtegal Monkey Forest with the overall home range of the long-tailed macaque (Macaca fascicularis) population in 2012 and surrounding landscape and villages. The small dark circles represent the main food provisioning areas.
abdominal organs; streaked them onto Columbia sheep blood agar plates; and then incubated the plates at 37°C for one night. We then analyzed the morphology and chemical properties of suspect colonies with Gram staining (Hutabaratet al.1999).
Activity PatternsWe conducted behavioral observations for two periods of 3 mo each: 1) pre-event period corresponding to a 3-mo period of November 15, 2011– February 15, 2012 before the significant mortality event of July–August 2012; and 2) post-event period corresponding to a 3-mo period of September 15, 2012– December 15, 2012 subsequent to the mortality event. We followed each group for a mean of 3 d/mo, from 07:00 to 18:00 h when possible (Npre = 36 d,Npost = 26 d), or for half-days due to meteorological or other conditions (Npre= 10 d,Npost= 13 d). The minimum daily follow length included in the analyses of the focal data was≥4 h (Harrisonet al. 2009).
To compare the activity budget pre- and post-event, we used 20-min focal observa-tions (Altmann1974) collected on a total of 54 sexually mature individuals, i.e., focal individuals (27 males and 27 females), belonging to the five social groups and present for the two periods. We identified focal individuals by their physiognomy, body marks, and behavioral features. During focal samples, we selected individuals on a first-seen basis, avoiding resampling the same individual before all the other focal individuals of the group had been sampled. The analyses included 162 h of focal data for the pre-event period and 124 h for the post-pre-event period. To give equal weight to all focal individuals, the sampling effort was identical for each individual nested within each period (Periodpre= 3 h per individual; Periodpost= 2.3 h per individual).
We defined eight general activity categories (Fuentes et al. 2011): 1) Resting: individual inactive, sitting or lying alone including visual scanning of the environment;
2)Moving: solitary or collective (traveling) movement from place to place, excluding
foraging; 3)Feeding: looking for or handling food items including foraging, consum-ing, processing; 4)Affiliation: affiliative behaviors involving two or several individuals including allogrooming (or social grooming), huddling, lip-smacking, playing, and caring; 5)Agonism: agonistic behaviors involving two or several individuals including threat, submission, displacement, physical assault with or without contact, agonistic intergroup encounter; 6) Sexual behavior: copulation and sexual-related behaviors including presenting genitalia and visual inspection; 7) Self-directed behavior: self-grooming, object manipulation, solitary play; 8)Interaction with humans: human– macaque interactions involving physical contact or not, including begging, grabbing food, provisioning, agonistic interaction, and playing.
Agonistic Intergroup Encounters We defined intergroup encounters as agonistic when the proximity of a neighboring group induced one of the following reactions in members of the group under study: group displacement, chase, flight, collective fight, barking, and screaming vocalization (Scarry 2013; Wheatley 1999). We recorded intergroup encounters on anad libitumbasis (Altmann 1974) and noted the identity of the opponent groups as well as the level of intensity of the agonistic encounter (low-intensity = when the encounter involved the displacement of a group without other agonistic behavior vs. high-intensity = when any additional agonistic interaction between the opponent groups preceded the final group displacement) (Sugiuraet al.
2000). We determined the outcome of each intergroup encounter, i.e., the identity of
winners and losers, based on which group was ultimately displaced (Cooperet al.2004; Scarry2013).
Ranging PatternsDuring the daily group follows, we recorded the GPS location of the group at 20-min intervals (Npre= 1,056 GPS points,Npost= 623) to reconstruct the daily ranging patterns, using a handheld GPS Garmin 60CSx (≤10 m error).
Data Analysis
Demographic Patterns To analyze population trends over the last three decades, we combined data from previous studies (Fuentes et al. 2011; Loudon et al. 2006; Wheatley 1999) and our own 2009–2012 data. We calculated the intrinsic rate of increase (r) as a measure of the population growth rate over the surveyed years as follows (Cowlishaw and Dunbar2000):
r¼lnðNtÞ−lnðN0Þ
t
whereNtis the number of individuals in the final year,N0is the number of individuals in the beginning year,tis the number of years between surveys.
To calculate mortality rates (m) induced by the July–August 2012 event, we used two computational methods. For the first, we compared the number of individuals counted in each age–sex class for each group of the population before (census June 2012) and after (census October 2012) the event (excluding the infants born between both censuses). However, in an attempt to control for potential over- or underestimates stemming from using gross population census figures, we based the second mortality estimate only on the subsample of individually identified macaques (N= 88 in June 2012): we calculated the proportion of these that had disappeared after the event.
Activity PatternsFor each focal individual we calculated the duration (in seconds) spent in each of the eight activity categories, weighted by the total duration of time recorded for that individual during each period. The percentage of time each individual spent in an activity within each period was the unit of comparison (Harrisonet al.
2009). We used a multivariate analysis of variance (MANOVA, type III sum of square) to determine whether the overall activity budget varied between the pre- and post-event periods. We included as control predictors group identity and sex class, as well as the interaction of these predictors with the period, because they are likely to influence the activity budgets. To reduce heterogeneity of variances and increase the normality of residuals, we performed an angular transformation on the proportional activity data (Sokal and Rohlf1995). We checked model validity and stability (distribution of residuals, Shapiro–Wilk test, Levene’s test, leverage values, Cook’s distance), and the assumptions required for MANOVA techniques were respected (Quinn and Keough2002). We also performed univariate analyses of variance to analyze the effects on each activity category taken separately and employed contrast planned comparisons between sex classes and between groups (Sokal and Rohlf1995). In tables and graphs, we present the untransformed mean time percentages of activities with their standard errors.
Agonistic Intergroup EncountersWe used a Fisher exact test to compare the level of intensity of agonistic encounters between both periods. For each group and for each period, we calculated a win/loss ratio corresponding to the proportion of encounters won by a group on the total number of encounters observed for that group during that period, i.e., intergroup encounter success. We used a Spearman exact correlation test to examine the relationship between the win/loss ratio during the post-event period and the group’s mortality rate after the 2012 event.
Ranging PatternsWe used the accumulated GPS location points to generate the home range of each group for each period, by means of fixed kernel density estimates (Worton1989), implemented with Hawth’s Tools for ESRI ArcGIS 9.3.1. We calcu-lated the reference bandwidth (href) with adehabitatHR for R 3.0.1 (R Core Team2013). We defined the spatial centrality of the group home ranges as the access to the main provisioning areas within the study site (Fig.1). To calculate this, we first overlaid a grid of 5 × 5 m cell size on the study area and identified the cells that intersected the provisioning areas. Second, for each day of group follow (minimum daily follow fixed at≥7 h:Npre= 936 GPS points,Npost= 546), we counted the number of GPS points located within the cells of provisioning areas, divided by the total number of GPS points available for that day. We obtained a value of spatial centrality per group and per day. We compared this value for each group between periods using Mann–Whitney tests. Finally, we compared the daily centrality values between the affected vs. nonaffected groups for the post-event period, by means of a Welch’st-test (cf.unequal variances detected with anF-test) (Quinn and Keough2002).
We performed all statistical analyses (two-tailed statistics) with a significance level set at 0.05, using STATISTICA 10.0 and R 3.0.2 (R Core Team2013).
Ethical Note
This research followed all Indonesian laws for foreign research. Our study was conducted under research permission from the Indonesian Ministry of Research and Technology (#03B/TKPIPA/FRP/SM/III/2011, #355/SIP/FRP/SM/IX/2012), the Provincial Government of Bali, and the local authorities, and used protocols developed by Universitas Udayana Primate Research Center (UNUD-PKP) and the University of Notre Dame under University of Notre Dame IACUC (#07-001).
Results
Demographic Patterns
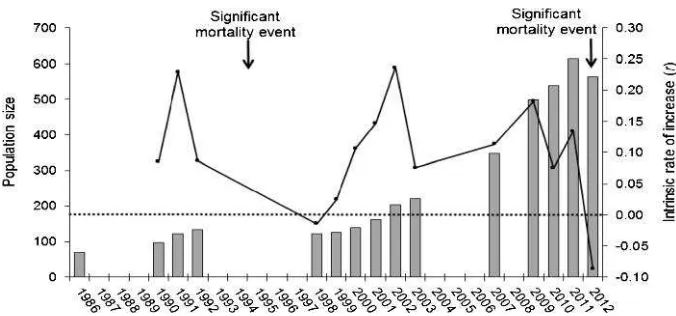
Population Demographic Trends over 26 yr. Between 1986 and 2012, the mean annual growth rate of the macaque population in Padangtegal Monkey Forest was 11%, increasing from 69 individuals in 1986 to 563 individuals in 2012 (Fig.2) (Fuentes
et al.2011; Loudonet al.2006; Wheatley1999). However, this dramatic growth has
not been constant over the 26 yr. The intrinsic rate of increase (r) was negative between
1992 and 1998 (r=–0.01) and in 2012 (r=–0.08), two periods corresponding to the occurrence of significant mortality events assumed to be associated with disease outbreaks (Fig.2).
Characterizing the Mortality Events In 1994, a disease outbreak caused by
Streptococcus equi ssp. zooepidemicus affected long-tailed macaques in the
Padangtegal Monkey Forest, but no detailed demographic data and mortality rates were available for that period (Soedarmantoet al.1996; Wheatley1999) (Fig.2). In summer 2012, several macaques at the same site showed clinical signs of illness including lethargy, severe movement difficulty suggesting polyarthritis, and substantial weight loss (indicated by observable body condition); and an unusual number of macaques were found dead during that period (N= 65; Wayan Gede Gunartha,pers. comm.). Bacteriological tests of swabs from two fresh carcasses of monkeys dead at the site revealed the presence of Gram-positive colonies of beta-hemolytic streptococci. Based on this result, the Denpasar Veterinary Investigation Unit diagnosed streptococ-cal infections (Streptococcussp.) as cause of both deaths, but no further bacteriological classification was conducted and no additional diagnostics were used to screen the monkeys at the site. This diagnosis of streptococci was communicated to the manage-ment staff and veterinarians at Padangtegal Monkey Forest who decided to treat the macaques with Amoxicillin (an antibiotic) delivered within a mixture of fresh eggs deposited at several locations in the site (during 5 days in July 2012 and 9 days in August 2012). Following this treatment, the overt signs of illness in macaques dimin-ished. Despite the assertion of Streptococcus sp. infection in this case, the lack of etiological, histopathological, and epidemiological data leave it unclear as to the presence and exact identity of pathological agent(s) that may have triggered the 2012 mortality event.
In 2012, based on the pre- and post-event censuses, the mortality rate (m) was 12.5% for the overall population (N = 77 missing individuals). This rate varied markedly between groups and age–sex classes (TableI). Only three of the five groups suffered a Fig. 2 Long-tailed macaque population size history in Padangtegal Monkey Forest: population size (bars) and intrinsic rate of increase (r; solid line) from 1986 to 2012. No data for 1987–1989, 1993–1997, 2004–2006, and 2008. 1986–1992: data from Wheatley (1999). 1999–2002: data from Fuenteset al. (2011). 2003: data from Loudonet al. (2006). 2007: unpublished data from Wayan Gede Gunartha. The statement of the significant mortality event in 1994 is based on Soedarmantoet al. (1996) and Wheatley (1999).
Table I Composition of the long-tailed macaque population in Padangtegal Monkey Forest, home range size (95 % kernel), and group density (total group size divided by the group’s home range area), before (Pre-mortality event period) and after (Post-mortality event period) a significant mortality event in summer 2012
Group Pre-mortality event period (census June 2012) Post-mortality event period (census October 2012)
AM SUB AF JUV Total group Home range (ha) Density (ind/ha) AM SUB AF JUV Total group Home range (ha) Density (ind/ha)
Cemetery 7 5 32 45 89 1.69 52.6 6 9 24 31 70 2.56 27.3
East 6 10 39 53 108 4.06 26.6 6 7 21 36 70 2.82 24.8
Michelin 7 8 38 60 113 2.85 39.6 8 11 28 46 93 2.03 45.8
Central 7 5 33 60 105 4.42 23.7 9 8 33 66 116 1.95 59.4
Temple 20 15 58 107 200 3.38 59.1 17 17 60 120 214 4.78 44.7
Total 47 43 200 325 615 8.7 70.6 46 52 166 299 563 10.16 55.4
Mortality rates per group Based on overall population (%) Based on known individuals (%)
Cemetery 24.7 31
East 37.9 29
Michelin 23.8 19
Central 0 0
Temple 0 0
Total 12.5 15.9
Mortality rates per age–sex class
AM 2.1 8.8
SUB 0 11
AF 22 21.6
JUV 13.5 25
Mortality rates per group (affected groups are in italic) and per age–sex class (AM = adult male; AF = adult female; SUB = subadult male; JUV = juvenile) computed via two methods: 1) based on the censuses of the overall population; 2) based on the censuses of identified individuals. The mortality estimation for juveniles excluded infants <4 mo born between both censuses.
55
6
F
.
Br
ot
cor
ne
et
al
substantial mortality (hereafter referred to as affected groups): Cemetery (m= 24.7%), East (m= 37.9%), and Michelin (m= 23.8%), while in the last two groups, Central and Temple, no monkeys died during that period (hereafter referred to as nonaffected groups: m = 0%). Among age–sex classes, the mortality rate was higher in adult females (m= 22%) and juveniles (m = 13.5%) than in adult males (m= 2.1%) and subadult males (m = 0%). The second mortality rate estimate including only the subsample of individually identified macaques showed that 8.8% of the known adult males and 11% of the subadult males had disappeared, as well as 25% of the juveniles and 21.6% of the adult females (TableI). Both assessment methods showed that adult females and juveniles suffered a higher mortality than adult and subadult males in this event.
Changes in Activity and Social Patterns Before and After the 2012 Mortality Event
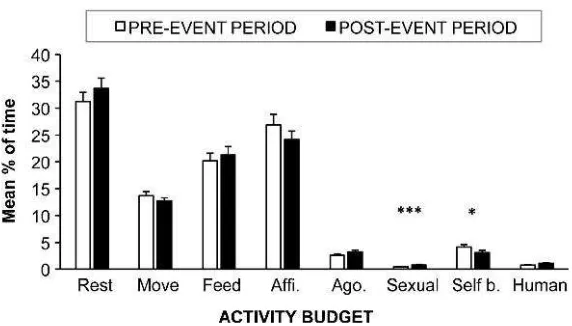
Activity Budget The activity budget of the overall population was significantly different in the pre- and post-mortality event periods (Table II: MANOVA). Univariate analyses on each activity category revealed that resting, moving, agonism, and human-macaque interactions did not significantly vary between periods while self-directed behaviors decreased after the event (Table II: ANOVAs, Fig.3). However, this effect of the period on the overall activity budget varied depending on the group (Table II: MANOVA). More precisely, univariate analyses on the proportion of feeding activity (TableII: ANOVAs) demonstrated that affected groups spent less time feeding after the mortality event than before, whereas the reverse was observed for nonaffected groups (contrast analysis:F = 10.25, d.f. = 1,P = 0.002). The effect of the period on affiliative behaviors was dependent on the age–sex class: adult females decreased the time engaged in affiliative behaviors after the mortality event (contrast analysis: F = 3.9, d.f. = 1,P= 0.04), but no change was seen in male affiliative behaviors (F= 1.39, d.f. = 1,P= 0.24). Finally, sexual behaviors increased after the mortality event, but only in the Cemetery, Michelin (cf.affected groups) and Temple (cf.nonaffected group) groups (contrast analysis:F= 26.57, d.f. = 1, P< 0.001) (TableII: ANOVAs).
Intergroup Agonistic EncountersWe observed a total of 77 agonistic intergroup encounters: 38 pre- and 39 post-mortality events. These encounters took place regularly in the vicinity of the main provisioning areas (Fig.1) and generally involved both males and females. The daily rate, i.e., for 12 h, of agonistic intergroup encounter increased from 1.19/d before the 2012 event to 2.09/d after. High-intensity agonistic encounters were significantly more frequent after the event (low-intensity/high-intensity ratio = 17:22) than before (27:11) (Fisher exact test: P = 0.02). The intergroup encounter success decreased after the event for the affected groups (win/loss ratio: Cemetery:xpre = 0.42,xpost= 0.38; East:xpre= 0.30,xpost= 0.26; Michelin:xpre= 0.76,xpost= 0.59) and conversely increased for the nonaffected groups (win/loss ratio: Central: xpre = 0.57,xpost= 0.64; Temple:xpre= 0.62,xpost= 0.75). Finally, the greater the mortality rate experienced by a group, the lower its intergroup encounter success after the event (Spearman exact correlation test:rs=–0.97,N= 5;P= 0.03).
Table II MANOVA multivariate analysis outputs (using Pillai–Bartlett statistics,λ) for the effects of all predictors and their interactions on the overall 2011–2012 activity budget of long-tailed macaques in Padangtegal Monkey Forest; and ANOVAs outputs for the effects of all predictors and their interactions on each activity category
Predictor Activity
MANOVA ANOVAs
Resting Moving Feeding Affiliation Agonism Sexual behavior Self-directed behavior Human interaction
Period λ= 0.45
The significantP-values are in bold type.
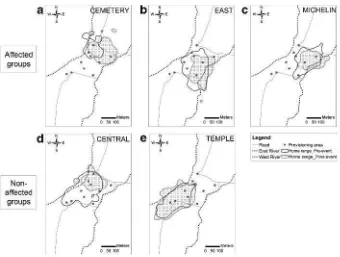
Home Ranges and Access to Provisioning AreasWe observed changes in the size (TableI) and the location (Fig.4) of the groups’home ranges before vs. after the 2012 event. Two of the three affected groups had significantly less frequent access to main food provisioning areas after the event than before: Cemetery (exact Mann–Whitney test:U= 2,P= 0.02,N= 12 days) and East (U= 1,P= 0.009,N= 11 d). The access to provisioning of the third affected group, Michelin, was not significantly modified (U= 6.5,P= 0.26,N= 10 d). For the nonaffected groups, the access to provisioning areas did not vary between periods: Central (U= 8,P= 0.13,N= 12 d) and Temple (U= 12,
P= 0.12,N= 14 d). These results and the analysis of the home range locations suggest that after the event, nonaffected groups kept ranging at the center of the Padangtegal Monkey Forest, while affected groups became slightly more peripheral and moved toward the eastern part of the site (Fig. 4), with a significantly lower access to provisioning areas than nonaffected groups (Welch’st-test conducted on ranked data:
t=–3.81, d.f. = 19.6,P <0.001).
Discussion
Long-Term Demographic Trends and Disease Outbreak Scenario
The long-term demographic pattern of the Padangtegal population reflects the influence of anthropogenic factors as well as the impact of at least two high-mortality events. The constant and abundant human food provisioning, combined with low predation pressure (Wheatley1999), resulted in a high population growth and large group sizes in this population. Similar positive trends have been frequently observed in food-enhanced cercopithecine primates (Cords 2012; Gumertet al.2011; Kuritaet al.2008).
Fig. 3 Comparison of the long-tailed macaque population’s activity budget before (Pre-event period) and after (Post-event period) a mortality event in 2012 in Padangtegal Monkey Forest. Values presented corre-spond to the mean time percentages (± SE) for each period, and the asterisks reveal the significant effects (*P< 0.05; ***P< 0.001) of the period on each activity (ANOVAs: TableII). Rest = resting; Move = moving; Feed = feeding; Affi. = affiliative behavior; Ago. = agonistic behavior; Sexual = sexual behavior; Self b. = self-directed behavior; Human = human–macaque interaction.
Regarding the significant mortality events, we can exclude starvation, poor nutri-tional conditions, and major habitat change as potential cause of mortality (Hanyaet al.
2004) because macaques were stably food provisioned in this tourist site over the periods. It is suggested that epidemic diseases can play a significant role in shaping the demographic trends of commensal primates, possibly by curbing population growth (Chapmanet al.2005; Fuenteset al.2011). In 1994, a wide outbreak ofStreptococcus
equi ssp. zooepidemicus affected free-living long-tailed macaques in Bali and was
assumed to be responsible for a high mortality event in the Padangtegal population that year (Soedarmanto et al. 1996; Wheatley 1999). Streptococcus equi ssp.
zooepidemicusis a bacterium belonging to the beta-hemolytic group C streptococci,
likely to cause infections in a wide variety of animals including horses, pigs, sheep, cows, primates, and humans and is transmitted by aerosols or wound contamination (Mätz-Rensinget al.2009; Soedarmantoet al.1996). Salasiaet al. (2004) confirmed the persistent occurrence of that commensal bacterium in 1998 in healthy Balinese macaques, suggesting the possibility that these populations acquired some immunity.
A similar infectious disease scenario might be the cause of the significant mortality event at Padangtegal Monkey Forest in summer 2012. High mortality over a short time period combined with what appeared to be symptoms of illness in macaques and the identification of bacterial infection in two monkeys that died during this period provides some support for the possibility that a pathogen-borne disease underlies the high mortality event at this site in 2012. Unfortunately, we lack etiological and epidemiological data to Fig. 4 Kernel home ranges of the long-tailed macaque groups in Padangtegal Monkey Forest (a:Cemetery;
b:East,c:Michelin,d:Central,e:Temple), before and after the 2012 mortality event. Empty buffer indicates the pre-mortality event home range and spotted buffer indicates the post-mortality event home range. The small dark circles represent the main food provisioning areas within the study site.
confirm such a hypothesis. Disease ecology in primates is very complex (Nunn and Altizer2006) and we cannot exclude the hypothesis of multiple infectious agents playing a mutual role in the immune status of the macaques (Jones-Engelet al.2006; Karlsson
et al. 2012). Previous studies reported the prevalence of different types of pathogens among Balinese temple macaques, such as gastrointestinal parasites (Laneet al.2011), commensal and pathogenic streptococcal bacterium (Salasiaet al.2004; Soedarmanto
et al.1996), and viral infectious agents (Engelet al.2002; Jones-Engelet al.2005). It is likely that some form of pathogen-borne disease played a core role in the 2012 mortality event, but we cannot support such an assertion with the available data. A comprehensive diagnostic assessment of this situation is critically lacking and would require further exhaustive investigations to understand the underlying epidemiological mechanisms, especially in human-dominated contexts such as the Padangtegal Monkey Forest, where anthropogenic factors can drive the occurrence and transmission of infectious disease (Chapmanet al.2005). Because we have limited knowledge of the specific causes of deaths during the 2012 event, our results must be taken as an overall assessment of the demographic and behavioral outcomes of a high-mortality event. However, it is crucial that the scientific and veterinary community respond more vigorously and thoroughly to these significant mortality scenarios in wild primate populations, by promoting an inter-disciplinary approach between medical and field research. The occurrence of such a mass mortality episode deserves development of a systematic screening and management response for successful diagnosis and pathogen detection (Kauret al.2008; Leendertz
et al.2006).
Variation in Mortality Rates
In 2012, three of the five social groups experienced marked mortality rates, and the adult females and juveniles were impacted more than adult and subadult males. If the mortality was related to some form of transmittable disease, the higher levels of physical contact and grooming interactions in females and juveniles (Cords 2012), particularly among close kin (Hanamuraet al.2008), may explain why they suffered a greater mortality. Social grooming is expected to increase the risk of exposure to and spreading of socially transmitted infections (MacIntoshet al.2012). Different immune responses to infections, according to social and nutritional stress levels of the individ-uals, might also explain the mortality differences observed between age–sex classes and groups (Chapmanet al.2005). Males generally have greater access than females and juveniles to contested clumped human foods of high nutritional content at this site (Fuentes and Gamerl2005), which is likely to improve body condition (Altmann and Muruthi1988; Kuritaet al.2008) and immunity against pathogens (Laneet al.2011; Weyheret al.2006). Males could also have preferentially benefited from better access to the antibiotics mixed with fresh eggs, a highly attractive food resource. Similarly, as two of the three affected groups (East and Cemetery) were already subordinate to the other groups before the event, individuals from these groups might have suffered higher levels of stress and lower nutritional status, making them more susceptible to the underlying causes of the mortality event, as well as less likely to access the antibiotics. The role of intertroop competition for resources has been proposed as a factor in troop decline in successive mass mortality episodes described in Japanese macaques (Macaca fuscata: Sugiuraet al.2002).
Although large group size and high density are expected to be linked to higher pathogen transmission and infection risk (Hussainet al.2013), this hypothesis is still poorly supported by empirical data (Chapmanet al.2009; Nunn2012). In June 2012, all the macaque groups in Padangtegal Monkey Forest were large (mean = 123 individuals/group) and characterized by high density (mean = 40.3 individuals/ha) compared to values generally reported for the species (group size range: 10–85 individuals/group; group density range: 0.1–1.49 individuals/ha) (Gumert et al.
2011). Because the affected groups were not necessarily the largest, nor had they the highest group density before the event in comparison with the nonaffected groups (TableI), our results do not support the prediction that pathogen infection risk varies as a function of group size and density.
Finally, ranging behavior is likely to impact infectious disease risk (Nunn2012), by influencing the probability of contacting infected areas (Chapmanet al.2009) and by sharing contaminated home ranges with other groups (Hanyaet al.2004). The home ranges of the three affected groups overlapped a river located in the eastern part of the site, while the nonaffected groups were never directly in contact with this river (Fig.4). Because some pathogens are known contributors to stream pollution (Weaveret al.
2005), this raises the question of whether some aspects associated to the river was related to the high mortality event in these groups (Lane-deGraafet al.2014). Finally, in a high-density anthropogenic setting such as the Padangtegal Monkey Forest, macaques are strongly terrestrial (data not shown), possibly increasing the risk of exposure to both feces and parasites in the soil (Nunn2012).
Impact on Sociobehavioral Patterns
Activity PatternsFood provisioning in Padangtegal Monkey Forest is stable year-round and we conducted the two periods of observation during the rainy season. As a result, we are confident that the behavioral differences we found were mainly attributable to the mortality-related consequences, and not to seasonality or variation in food availability.
In each group, the proportion of resting and moving remained unchanged, but we found indices of increased social tension after the mortality event, primarily as a decrease in time that adult females allocated to affiliative activities (Dunbar 1991). This change in social activities is to be read in light of general social patterns in macaques.Macaca fascicularishas a nepotistic dominance style (Thierry2007) with strict and stable matrilineal dominance hierarchies in a female-bonded system (van Noordwijk and van Schaik 1987). Consequently, the disappearance of a substantial number of adult females during the 2012 event might have weakened the matrilineal structure and induced in turn an overall group-level instability and lower social cohesion (Beisneret al. 2011). Social instability after a population decline in other taxa has also been reported in howlers (Alouatta seniculus: Pope1998) and gorillas (Gorilla gorilla gorilla: Gentonet al.2014).
The post-event increase in sexual activities suggests a peak in the number of estrous females at that period. However, we did not find an increase in the rate of agonism among adult males, which one would expect in conjunction with male mate competi-tion (Cooperet al.2004). Finally, stressed primates usually increase the proportion of self-directed behaviors such as self-grooming and self-scratching (Isbell and Young
1993; Sapolsky and Share2004). Surprisingly, the frequency of these anxiety-related
behaviors decreased after the 2012 mortality event, which does not support the assumption of higher post-event social tension. A study investigating the stress hor-mone levels among individuals would help to clarify this issue.
Intergroup Relationships and Access to Provisioning Areas InMacaca fascicularis, neighboring groups can have partially overlapping home ranges (van Schaik et al.
1983). However, intergroup competition over access to resources exists. Especially in anthropogenic habitats, the clumped nature of calorically rich and palatable human food (Altmann and Muruthi1988) generally increases the competitive behavior be-tween groups (Camperio Ciani1986; Jaman and Huffman2013; but see Cooperet al.
2004). We found that, after the mortality event, the affected groups were slightly more peripheral with lower access to the provisioning areas than the nonaffected groups. Moreover, the rate and intensity of agonistic intergroup encounters increased after the event, reflecting higher social tension between groups. Demographic changes may have created an instability in intergroup dominance relationships (Scarry and Tujague2012). After the mortality event, the ability of each group to win an intergroup encounter mirrored the mortality rate the group experienced. The post-event lower ranking status of the affected groups might be due to their numerical disadvantage, i.e., smaller group size than nonaffected groups (Scarry2013) and/or to the lower social cohesion within group. The fact that larger groups generally dominate smaller groups was also reported in Japanese macaques (Macaca fuscata: Hanyaet al.2004; Sugiuraet al.2000).
Conclusion
Our findings document the short-term consequences of a high-mortality event on the behavioral patterns and social structure of a primate population living in a highly anthropogenic habitat. Regardless of the underlying cause of the mortality event, there were clear socially and behaviorally important repercussions from differential mortality among groups in the population. Rapid and substantive mortality events can thus play significant roles shaping the social and ecological profiles of free-ranging primate groups and populations (Sapolsky and Share2004).
Acknowledgments We thank the Indonesian Ministry of Research and Technology, Bapak Sri Wahyono, the Padangtegal Wenara Wana Management Committee, and Bapak Wayan Selamet for permission to conduct this research in Indonesia. This study was carried out with the financial support of Belgian National Fund for Scientific Research, Foundation Marie Louise Leonard, Fund De Potter from the Royal Academy of Belgium, and the Ministry of Wallonia-Brussels Federation. We are grateful to Hery Kesumanegara and the Monkey Forest staff for their help during the field work. We are very grateful to the editor-in-chief and two anonymous reviewers for their very constructive comments on earlier versions of this manuscript, and to Michael A. Huffman for providing constructive comments and directing us to valuable references.
References
Altmann, J., & Muruthi, P. (1988). Differences in daily life between semiprovisioned and wild-feeding baboons.American Journal of Primatology, 15, 213–221.
Beisner, B. A., Jackson, M. E., Cameron, A. N., & McCowan, B. (2011). Detecting instability in animal social networks: Genetic fragmentation is associated with social instability in rhesus macaques.PloS One, 6(1), e16365.
Bermejo, M., Rodríguez-Teijeiro, J. D., Illera, G., Barroso, A., Vilà, C., & Walsh, P. D. (2006). Ebola outbreak killed 5000 gorillas.Science, 314(5805), 1564.
Brotcorne, F., Wandia, I. N., Rompis, A. L. T., Soma, I. G., Suatha, I. K., & Huynen, M. C. (2011). Recent demographic and behavioral data of Macaca fascicularisat Padangtegal, Bali, Indonesia. In M. D. Gumert, A. Fuentes, & L. Jones-Engel (Eds.), Monkeys on the edge: Ecology and management of long-tailed macaques and their interface with humans(pp. 180–182). Cambridge, U.K.: Cambridge University Press.
Camperio Ciani, A. (1986). Intertroop agonistic behaviour of a feral rhesus macaque troop ranging in town and forest areas in India.Aggressive Behavior, 12, 433–439.
Capitanio, J. P. (2012). Social processes and disease in nonhuman primates: Introduction to the special section. American Journal of Primatology, 74(6), 491–496.
Chapman, C., Rothman, J. M., & Hodder, S. A. M. (2009). Can parasite infections be a selective force influencing primate group size ? A test with red colobus. In M. A. Huffman & C. Chapman (Eds.), Primate parasite ecology: The dymanics of study of host-parasite relationships (pp. 422–440). Cambridge, U.K.: Cambridge University Press.
Chapman, C. A., Gillespie, T. R., & Goldberg, T. L. (2005). Primates and the ecology of their infectious diseases: How will anthropogenic change affect host-pathogen interactions?Evolutionary Anthropology, 14, 134–144.
Cooper, M., Aureli, F., & Singh, M. (2004). Between-group encounters among bonnet macaques (Macaca radiata).Behavioral Ecology and Sociobiology, 56(3), 217–227.
Cords, M. (2012). The behavior, ecology, and social evolution of cercopithecine monkeys. In J. C. Mitani, J. Call, M. Kappeler, R. A. Palombit, & J. B. Silk (Eds.),The evolution of primate societies(pp. 91–112). Chicago: University of Chicago Press.
Cowlishaw, G., & Dunbar, R. (2000).Primate conservation biology. Chicago: University of Chicago Press. Dunbar, R. I. M. (1991). Functional significance of social grooming in primates.Folia Primatologica, 57,
121–131.
Engel, G., Hungerford, L. L., Jones-Engel, L., Travis, D., Eberle, R., Fuentes, A., et al. (2006). Risk assessment: A model for predicting cross-species transmission of simian foamy virus from macaques (M. fascicularis) to humans at a monkey temple in Bali, Indonesia.American Journal of Primatology, 68(9), 934–948.
Engel, G. A., Jones-Engel, L., Schillaci, M. S., Suaryana, K. G., Putra, A., Fuentes, A., et al. (2002). Human exposure to herpesvirus B-seropositive macaques, Bali, Indonesia.Emerging Infectious Diseases, 8(8), 789–795.
Fooden, J. (1995). Systematic review of southeast Asian longtail macaques,Macaca fascicularis, (Raffles, [1821]).Fieldiana Zoology (New Series), 81, 1–206.
Fuentes, A. (2006). Human culture and monkey behavior: Assessing the contexts of potential pathogen transmission between macaques and humans.American Journal of Primatology, 68(9), 880–896. Fuentes, A. (2012). Ethnoprimatology and the anthropology of the human-primate interface.Annual Review of
Anthropology, 41, 101–117.
Fuentes, A., & Gamerl, S. (2005). Disproportionate participation by age/sex classes in aggressive interactions between long-tailed macaques (Macaca fascicularis) and human tourists at Padangtegal Monkey Forest, Bali, Indonesia.American Journal of Primatology, 66(2), 197–204.
Fuentes, A., Rompis, A. L. T., Arta Putra, I. G. A., Watiniasih, N. L., Suatha, I. K., Soma, I. G., et al. (2011). Macaque behavior at the human-monkey interface: The activity and demography of semi-free ranging Macaca fascicularisat Padangtegal, Bali, Indonesia. In M. D. Gumert, A. Fuentes, & L. Jones-Engel (Eds.),Monkeys on the edge: Ecology and management of long-tailed macaques and their interface with humans(pp. 159–179). Cambridge, U.K.: Cambridge University Press.
Fuentes, A., Southern, M., & Suaryana, K. G. (2005). Monkey forests and human landscapes: Is extensive sympatry sustainable forHomo sapiensandMacaca fascicularison Bali? In J. D. Patterson & J. Wallis (Eds.),Commensalism and conflict: The human-primate interface(Vol. 4, pp. 168–195). Norman, OK: American Society of Primatologists.
Genton, C., Pierre, A., Cristescu, R., Lévréro, F., Gatti, S., Pierre, J.-S., et al. (2014). How Ebola impacts social dynamics in gorillas: A multistate modelling approach.Journal of Animal Ecology. doi:10.1111/1365-2656.12268.
Gumert, M. D., Fuentes, A., & Jones-Engel, L. (2011).Monkeys on the edge: Ecology and management of long-tailed macaques and their interface with humans. Cambridge, U.K.: Cambridge University Press. Hanamura, S., Kiyono, M., Lukasik-Braum, M., Mlengeya, T., Fujimoto, M., Nakamura, M., et al. (2008).
Chimpanzee deaths at Mahale caused by a flu-like disease.Primates, 49, 77–80.
Hanya, G., Matsubara, M., Sugiura, H., Hayakawa, S., Goto, S., Tanaka, T., et al. (2004). Mass mortality of Japanese macaques in a western coastal forest of Yakushima.Ecological Research, 19, 179–188. Harrison, M. E., Vogel, E. R., Morrogh-Bernard, H. C., & van Noordwijk, M. A. (2009). Methods for
calculating activity budgets compared: A case study using orangutans.American Journal of Primatology, 71(4), 353–358.
Hussain, S., Ram, M. S., Kumar, A., Shivaji, S., & Umapathy, G. (2013). Human presence increases parasitic load in endangered lion-tailed macaques (Macaca silenus) in its fragmented rainforest habitats in Southern India.PloS One, 8(5), e63685.
Hutabarat, T. S. P. N., Putra, A. A. G., Widowati, N., & Heriyanto, A. (1999). Streptococcosis septikemik pada babi. InManual standar metode diagnosa Laboratorium kesehatan hewan(pp. 185–188): Direktorat bina Kesehatan Hewan, Direktorat Jenderal Peternakan, Departemen Pertanian.
Isbell, L. A., & Young, T. P. (1993). Social and ecological influences on activity budgets of vervet monkeys, and their implications for group living.Behavioral Ecology and Sociobiology, 32(6), 377–385. Jaman, M. F., & Huffman, M. A. (2013). The effect of urban and rural habitats and resource type on activity
budgets of commensal rhesus macaques (Macaca mulatta) in Bangladesh.Primates, 54(1), 49–59. Jones-Engel, L., Engel, G. A., Heidrich, J., Chalise, M., Poudel, N., Viscidi, R., et al. (2006). Temple monkeys
and health implications of commensalism, Kathmandu, Nepal.Emerging Infectious Diseases, 12(6), 900–
906.
Jones-Engel, L., Engel, G. A., Schillaci, M. A., Rompis, A., Putra, A., Suaryana, K. G., et al. (2005). Primate-to-human retroviral transmission in Asia.Emerging Infectious Diseases, 11(7), 1028–1035.
Jones-Engel, L., May, C. C., Engel, G. A., Steinkraus, K. A., Schillaci, M. A., Fuentes, A., et al. (2008). Diverse contexts of zoonotic transmission of simian foamy viruses in Asia.Emerging Infectious Diseases, 14(8), 1200–1208.
Kaburu, S. S. K., Inoue, S., & Newton-Fisher, N. E. (2013). Death of the alpha: Within-community lethal violence among chimpanzees of the Mahale Mountains National Park.American Journal of Primatology, 75(8), 789–797.
Karlsson, E. A., Engel, G. A., Feeroz, M. M., San, S., Rompis, A., Lee, B. P. Y. H., et al. (2012). Influenza virus infection in nonhuman primates.Emerging Infectious Diseases, 18(10), 1672–1675.
Kaur, T., Singh, J., Tong, S., Humhrey, C., Clevenger, D., Tan, W., et al. (2008). Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related Metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Moutains National Park, Western Tanzania.American Journal of Primatology, 70, 1–11.
Kurita, H., Sugiyama, Y., Ohsawa, H., Hamada, Y., & Watanabe, T. (2008). Changes in demographic parameters of Macaca fuscata at Takasakiyama in relation to decrease of provisioned foods. International Journal of Primatology, 29, 1189–1202.
Lane, K. (2011).Landscape dynamics: Genetic and parasitism in Balinese long-tailed macaques(Macaca fascicularis).Unpublished Ph.D. thesis, University of Notre Dame.
Lane, K., Holley, C., Hollocher, H., & Fuentes, A. (2011). The anthropogenic environment lessens the intensity and prevalence of gastrointestinal parasites in Balinese long-tailed macaques (Macaca fascicularis).Primates, 52(2), 117–128.
Lane, K., Lute, M., Rompis, A., Wandia, I. N., Arta Putra, I. G. A., Hollocher, H.,et al. (2010). Pests, pestilence and people: The long-tailed macaque and its role in the cultural complexities of Bali. In S. Gursky-Doyen & J. Supriatna (Eds.),Indonesian primates(pp. 235–248). Developments in primatology: Progress and prospects. New York: Springer Science+Business Media.
Lane-deGraaf, K. E., Putra, I. G. A., Wandia, A., Rompis, I. N., Hollocher, A. H., & Fuentes, A. (2014). Human behavior and opportunities for parasite transmission in communities surrounding long-tailed macaque populations in Bali, Indonesia.American Journal of Primatology, 76(2), 159–167.
Leendertz, F. H., Pauli, G., Maetz-Rensing, K., Boardman, W., Nunn, C., Ellerbrok, H., et al. (2006). Pathogens as drivers of population declines: The importance of systematic monitoring in great apes and other threatened mammals.Biological Conservation, 131(2), 325–337.
Lehmann, J., Korstjens, A. H., & Dunbar, R. I. M. (2007). Group size, grooming and social cohesion in primates.Animal Behaviour, 74(6), 1617–1629.
in assessing human-monkey co-existence in Bali, Indonesia. Ecological and Environmental Anthropology, 2(1), 1–13.
MacIntosh, A. J. J., Jacobs, A., Garcia, C., Shimizu, K., Mouri, K., Huffman, M. A., et al. (2012). Monkeys in the middle: parasite transmission through the social network of a wild primate.PloS One, 7(12), e51144. Mätz-Rensing, K., Winkelmann, J., Becker, T., Burckhardt, I., Van Der Linden, M., Köndgen, S., et al. (2009). Outbreak ofStreptococcus equisubsp.zooepidemicusinfection in a group of rhesus monkeys (Macaca mulatta).Journal of Medical Primatology, 38(5), 328–334.
McLennan, M., & Huffman, M. A. (2012). High frequency of leaf swallowing and its relationship to intestinal parasite expulsion in "village" chimpanzees at Bulindi, Uganda.American Journal of Primatology, 74, 642–650.
Nunn, C. L. (2012). Primate disease ecology in comparative and theoretical perspective.American Journal of Primatology, 74(6), 497–509.
Nunn, C. L., & Altizer, S. (2006).Infectious diseases in primates: Behavior, ecology and evolution. Oxford: Oxford University Press.
Pope, T. R. (1998). Effects of demographic change on group kin structure and gene dynamics of populations of red howling monkeys.Journal of Mammalogy, 79(3), 692–712.
Quinn, G., & Keough, M. (2002).Experimental designs and data analysis for biologists. Cambridge, U.K.: Cambridge University Press.
Core Team, R. (2013).R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Salasia, S. I. O., Teguh Wibawan, I. W., Pasaribu, F. H., Abdulmawjood, A., & Lämmler, C. (2004). Persistent occurrence of a singleStreptococcus equisubsp.zooepidemicusclone in the pig and monkey population in Indonesia.Journal of Veterinary Science, 5(3), 263–265.
Sapolsky, R. M., & Share, L. J. (2004). A pacific culture among wild baboons: Its emergence and transmis-sion.PLoS Biology, 2(4), 534–541.
Scarry, C. J. (2013). Between-group contest competition among tufted capuchin monkeys,Sapajus nigritus, and the role of male resource defence.Animal Behaviour, 85(5), 931–939.
Scarry, C. J., & Tujague, M. P. (2012). Consequences of lethal intragroup aggression and alpha male replacement on intergroup relations and home range use in tufted capuchin monkeys (Cebus apella nigritus).American Journal of Primatology, 74(9), 804–810.
Schino, G., Maestripieri, D., Scucchi, S., & Turillazzi, P. G. (1990). Social tension in familiar and unfamiliar pairs of long-tailed macaques.Behaviour, 113(3/4), 264–272.
Soedarmanto, I., Pasaribu, F. H., Wibawan, I. W. T., & Lammler, C. (1996). Identification and molecular characterization of serological group C Streptococci isolated from diseased pigs and monkeys in Indonesia.Journal of Clinical Microbiology, 34(9), 2201–2204.
Sokal, R., & Rohlf, F. (1995).Biometry. New York: W. H. Freeman.
Sugiura, H., Agetsuma, N., & Suzuki, S. (2002). Troop extinction and female fusion in wild Japanese macaques in Takushima.International Journal of Primatology, 23(1), 69–84.
Sugiura, H., Saito, C., Sato, S., Agetsuma, N., Takahashi, H., Tanaka, T., et al. (2000). Variation in intergroup encounters in two populations of Japanese macaques.International Journal of Primatology, 21(3), 519–
535.
Thierry, B. (2007). Unity in diversity: Lessons from macaque societies.Evolutionary Anthropology: Issues, News, and Reviews, 16(6), 224–238.
van Noordwijk, M. A., & van Schaik, C. P. (1987). Competition among female long-tailed macaques,Macaca fascicularis. Animal Behaviour, 35(2), 577–589.
van Schaik, C., van Noordwijk, M., Warsono, B., & Sutriono, E. (1983). Party size and early detection of predators in Sumatran forest primates.Primates, 24(2), 211–221.
Wallis, J., & Lee, D. R. (1999). Primate conservation: The prevention of disease transmission.International Journal of Primatology, 20, 803–826.
Weaver, R., Entry, J. A., & Graves, A. (2005). Numbers of fecal streptococci andEscherichia coliin fresh and dry cattle, horse, and sheep manure.Canadian Journal of Microbiology, 51, 847–851.
Weyher, A. H., Ross, C., & Semple, S. (2006). Gastrointestinal parasites in crop raiding and wild foraging Papio anubisin Nigeria.International Journal of Primatology, 27, 1519–1534.
Wheatley, B. P. (1999).The sacred monkeys of Bali. Prospect Heights, IL: Waveland Press.
Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home-range studies. Ecology, 70(1), 164–168.
Young, T. P. (1994). Natural die-offs of large mammals: Implications for conservation.Conservation Biology, 8(2), 410–418.