RESEARCH ARTICLE
The Role of Anthropic, Ecological, and Social Factors in Sleeping Site Choice
by Long
‐
Tailed Macaques (
Macaca fascicularis
)
FANY BROTCORNE1,2*, CINDY MASLAROV1, I. NENGAH WANDIA3, AGUSTIN FUENTES4, ROSELINE C. BEUDELS‐JAMAR2,
ANDMARIE‐CLAUDE HUYNEN1
1Primatology Research Group, Behavioural Biology Unit, University of Liège, Liège, Belgium
2Conservation Biology Unit, Education and Nature, Royal Belgian Institute of Natural Sciences, Brussels, Belgium 3Primate Research Center, Universitas Udayana, Bali, Indonesia
4Department of Anthropology, University of Notre Dame, Notre Dame, Indiana
When choosing their sleeping sites, primates make adaptive trade‐offs between various biotic and abiotic constraints. In human‐modified environments, anthropic factors may play a role. We assessed the influence of ecological (predation), social (intergroup competition), and anthropic (proximity to human settlements) factors in sleeping site choice by long‐tailed macaques (Macaca fascicularis) occupying a habitat at the interface of natural forests and human‐modified zones in Bali Barat National Park, Indonesia. Over the course of 56 nights, we collected data relating to physical features of sleeping trees, patterns of the use of sleeping sites within the home range, pre‐sleep behavior, diurnal ranging patterns and availability of natural and human food. Overall, the macaques used 17 sleeping sites with 37 sleeping trees. When the monkeys slept in forest zones, they selected sleeping trees that had larger trunks but were not significantly taller than surrounding trees. Though the macaques rarely re‐used sleeping sites on consecutive nights, they frequently re‐used four sites over the study period. The group favored sleeping within the core area of its home range, despite the occurrence of frequent agonistic intergroup encounters there. Macaques preferentially selected sleeping trees located within or near human‐modified zones, especially when human food was abundant and natural food was scarce. These results partially support the hypothesis that long‐tailed macaques choose their sleeping sites to avoid predation; proximity to human settlements appears to be the primary factor influencing sleeping site choice in this primate species. Our results reflect the strong influence that anthropic factors have on primates, which subsist in increasingly human‐dominated landscapes. Am. J. Primatol. 76:1140–1150, 2014. © 2014 Wiley Periodicals, Inc.
Key words: sleeping site; human proximity; human food; predation avoidance; Bali Barat National Park
INTRODUCTION
The choice of sleeping sites by diurnal primates reflects diverse factors and constraints that are mutually non‐exclusive and highly dependent on context and the species in question. Predation pressure, proximity of food, competition with con-specifics and physical comfort may influence the choice of sleeping sites and sleeping trees by primates [Anderson, 1998, 2000].
The predation avoidance hypothesis asserts that primates have evolved anti‐predator strategies at sleeping sites. To minimize detection by a predator, they should select tall emergent trees [Bernard et al., 2011; Fan & Jiang, 2008; Reichard, 1998] and behave in a cryptic manner (e.g. moving silently and rapidly) when entering sleeping trees [Heymann, 1995; Liu & Zhao, 2004; Smith et al., 2007]. Some primates may prefer open‐canopy trees located along
riverbanks [Fittinghoff & Lindburg, 1980; Matsuda et al., 2008] and connected to adjacent trees [Albert et al., 2011]. These conditions improve their chances of detecting approaching predators and facilitate
Contract grant sponsor: Belgian National Fund for Scientific Research; contract grant sponsor: Fondation Belge de la Vocation
Correspondence to: Fany Brotcorne, Primatology Research Group, Behavioral Biology Unit, University of Liège, 22 Quai Van Beneden, Liège 4020, Belgium. E‐mail: fbrotcorne@gmail. com
Received 22 November 2013; revised 9 April 2014; revision accepted 10 April 2014
DOI: 10.1002/ajp.22299
Published online 8 May 2014 in Wiley Online Library (wileyonlinelibrary.com).
American Journal of Primatology 76:1140–1150 (2014)
escape [Hankerson et al., 2007; Kurland, 1973]. Alternatively, sleeping trees with a crown not touching the neighboring trees may offer protection against arboreal mammalian predators [Barnett et al., 2012]. Some researchers argued that a dense canopy cover improves the concealment of primates [Anderson, 2000; Liu & Zhao, 2004]. Physical barriers such as large tree trunks, tall crowns, elevatedfirst branches, and the absence of lianas serve to impede the access of predators into trees [Barnett et al., 2012; Bernard et al., 2010; Di Bitetti et al., 2000; Ram-akrishnan & Coss, 2001; Teichroeb et al., 2012; Tenaza & Tilson, 1985]. As predators may memorize the sleeping refuges of their prey [Emsens et al., 2014], primates are expected to switch between several alternate sleeping sites, thus, making them less predictable for predators [Phoonjampa et al., 2010; Reichard, 1998; Zhang, 1995]. Alternatively, primates may re‐use the same sleeping sites repeat-edly because suitable sites are rare [Duarte & Young, 2011; Ramakrishnan & Coss, 2001; Tenaza & Tilson, 1985], or because familiarity with sleeping sites facilitates escape from predators [Di Bitetti et al., 2000; Hankerson et al., 2007; Li et al., 2011].
According to the food proximity hypothesis, primates select sleeping sites that maximize their access to food patches and at the same time minimize travel costs. Sleeping sites should be near the feeding sites used prior to or just after the period of rest [Chapman et al., 1989; Heymann, 1995; Li et al., 2011; Smith et al., 2007; Teichroeb et al., 2012].
Competition with conspecific groups may also influence the choice of sleeping site location within the home range. Seeping in areas of exclusive use may decrease the risk of intergroup encounters [Albert et al., 2011; Li et al., 2011; Phoonjampa et al., 2010; Smith et al., 2007; Von Hippel, 1998], while sleeping near range boundaries may allow these boundaries or nearby resources to be defended [Teichroeb et al., 2012], depending on the species’degree of territorial-ity [cf. risk hypothesis: Wrangham et al., 2007]. Additionally, factors such as parasite avoidance, thermoregulation and the search for comfort, stabili-ty, and social contact in trees may play a role in the choice of sleeping sites [Anderson, 1998; Di Bitetti et al., 2000; Fan & Jiang, 2008; Liu & Zhao, 2004; Zhang, 1995].
Primate habitat is becoming increasingly modified by humans and anthropogenic change is having large impacts on primate behavior and ecology [e.g. Fuentes & Hockings, 2010; McKinney, 2009; Strum, 2010]. Predation pressure may be lessened near human settlements as here predators have been persecuted by humans and their populations reduced [Bishop et al., 1981; Isbell & Young, 1993; Ramakrishnan & Coss, 2001; Stanford, 2002]. Studying the choice of sleeping sites in environments at the interface of forest and human‐influenced habitats contributes to a better understanding of how primates cope behaviorally with
human encroachment. In terms of predation risk, only a few studies have analyzed sleeping site choice in human‐dominated landscapes [Duarte & Young, 2011; Ramakrishnan & Coss, 2001]. In this study, we investigated the role of ecological, social and anthropic factors in the sleeping site choice of a group of long‐
tailed macaques (Macaca fascicularis) living in a partly human‐modified habitat within the Bali Barat National Park (BBNP), Indonesia.
The geographical range ofM. fascicularisextends across southeast Asia from Bangladesh to the Sunda Archipelago of Indonesia [Fooden, 1995]. Although in the past the species occurred mainly in riverine forests [Fittinghoff & Lindburg, 1980; Kurland, 1973], in more recent times its behavioral and ecologicalflexibility (including a generalist, opportu-nistic feeding strategy) has enabledM. fascicularisto colonize a wide variety of habitats [Fooden, 1995; Gumert et al., 2011]. In particular, the species has succeeded in exploiting fragmented forests and the edges of disturbed habitats [Gumert et al., 2011]. Although the species has long been associated with humans throughout its range [Fuentes & Hockings, 2010], recently it has increasingly entered into commensal relationships with humans, living in close association with people, taking advantage of human food to supplement its diet [Wheatley, 1999], and even competing with humans for spatial and dietary resources [Richard et al., 1989]. The island of Bali has a particularly intense and widespread human‐primate interface; long‐tailed macaques and humans have coexisted and interacted for centuries [Fuentes et al., 2005; Wheatley, 1999]. Fuentes [2010] suggested that the long‐term sympatry be-tween humans andM. fascicularisin Bali could have resulted in intertwined ecologies, with humans exerting selective pressures on the macaques.
The monkeys’ sleeping sites are one potential point of influence. Among the factors potentially influencing sleeping site choice, we focused on predation and intergroup competition avoidance, as well as proximity to human settlements and human food. We investigated the following predictions: (i) If avoidance of predators guides sleeping site choice, macaques should use multiple sleeping sites and alternate between them, and they should adopt cryptic pre‐sleep behavior to decrease the risk of detection by predators, (ii) Macaques could be safer when sleeping in human‐modified zones (cf. defi ni-tion in Methods) where natural predani-tion pressure was likely reduced. In this case, we expected a habitat‐specific choice of sleeping trees, with mac-aques selecting taller trees with larger trunks only when sleeping in forest zones, (iii) following the risk hypothesis [Wrangham et al., 2007], we predicted that the macaques should avoid sleeping in areas of their home range where they frequently encountered other groups, (iv) due to the long history of human‐
macaque interface on Bali, we predicted that
anthropic factors should influence the habitat use and sleeping site choice of the macaques. Particular-ly, we expected the macaques to use the human‐
modified zones and their surroundings because of the food left by people. The preference for certain sleeping site locations should also vary with food availability within the home range. During the months of high tourist activity, when the availability of human food was substantial, we expected the macaques to sleep more frequently near human‐modified zones.
METHODS
Study Site and Subjects
Bali Barat National Park (BBNP) is located in the north‐western part of the island of Bali, Indonesia (8°05’S–18°15’S, 114°25’E–114°56’E). The park cov-ers an area of 19,002 ha comprising dry deciduous monsoon forest, lowland rain forest, coastal forest, and mangrove. The climate is monsoonal, with a dry season lasting from May to September and a wet season lasting from October to April; the annual average rainfall is 1,198 mm. During the study period, sunset time ranged between 18:08 and 18:33, and sunrise was between 06:23 and 06:34 (http://www.timeanddate.com).
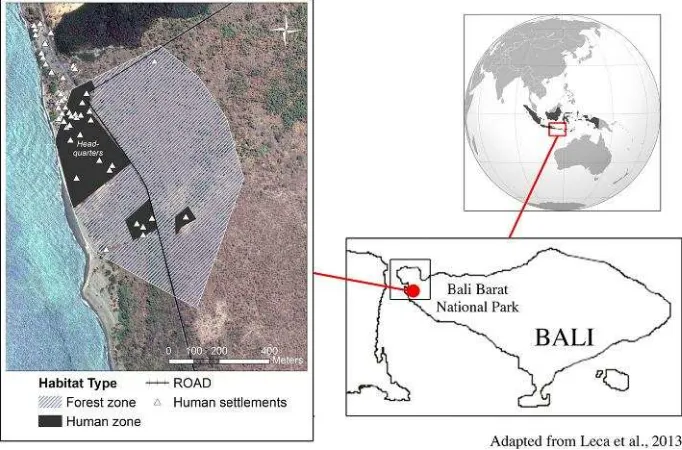
We conducted this study in the western sector of the park, an area of deciduous monsoon and coastal forests located around the park headquarters, which included a ranger station, tourist facilities, a camping area, and a Hindu temple. Two roadways crossed the study site (Fig. 1). Tourists visit the park throughout the year, with peak numbers from May to September (in 2013, monthly mean during
high tourist season¼7,715 visitors vs. low tourist season¼2,423 visitors). Human‐derived food (here-after referred to as human food), occasionally present in human‐modified zones and alongside roadways, mainly consisted of offerings (i.e. fruit or crackers) placed in temples, or refuse and leftovers (i.e. rice and fruits) in bins or scattered across the ground.
There were two non‐human primate species at BBNP, the long‐tailed macaque (M. fascicularis) and the ebony langur (Trachypithecus auratus) [Leca et al., 2013; Wheatley et al., 1993]. Although we did not quantify predator density in the study area, we observed several potential predators of M. fascicu-laris: the reticulated python (Python reticulatus), the water monitor lizard (Varanus salvator), diurnal and nocturnal raptors such as changeable hawk‐eagle (Nisaetus cirrhatus), Indian black eagle (Ictinaetus malayensis), barred eagle‐owl (Bubo sumatranus), as well as domestic dogs [Fam & Nijman, 2011; Fooden, 1995; van Schaik & Mitrasetia, 1990]. Tigers are known predators ofM. fascicularisat other sites [van Schaik et al., 1983], but the Bali tiger (Panthera tigris balica) has been extinct since around 1940 [Whitten et al., 1996]. The leopard cat (Prionailurus bengalensis), which has been reported as a potential predator of macaques [Palombit, 1992], is the sole wild felid that still occurred in the park (H. Kesumanegara, personal communication). Among the aforementioned predators, only the python, owl, and leopard cat represented a nocturnal threat to macaques.
We studied a group of long‐tailed macaques with 24–26 members including 3 adult males, 7 adult females, 3–4 subadult males, 6 juveniles, and
Fig. 1. Study site in Bali Barat National Park (Indonesia) with an outline of the study group home range, showing two habitat types: forest (natural zones with continuous tree canopy, 40 ha) and human zones (ranger station and grass patches with planted trees, 9 ha).
Am. J. Primatol.
4–6 infants. During the study, two births occurred and one subadult male disappeared.
Data Collection
We carried out the study over a period of four full months, from March to June 2011. We allocated the first month to habituating the macaques to observers, so the analyzed data set covers a 3‐month period. We followed the macaques 5 days per week (mean: 15 days per month; range: 14–17 days) from sleeping site (06:00) to sleeping site (18:30) when possible, resulting in 530 hr of observation. We ended our observations when visibility became too low for us to see the monkeys. We devoted some additional afternoons to locating sleeping site locations on days when we had not followed the study group (n¼13 days, with 11 successful attempts). In total, we recorded the location of sleeping sites for 56 nights.
We use the termsleeping treeto refer to trees in which macaques stayed overnight; sleeping site to refer to the circular plot delimited by a 20 m radius around the edges of the crowns of the sleeping trees used by the group (one sleeping site could be composed of one or more sleeping trees occupied simultaneously);control treeto refer to trees present within the sleeping site but never used for sleeping; re‐used sleeping siteto refer to sites used more than once; andfavored sleeping siteto refer to sites used more thanfive times by the group over the course of the study [Reichard, 1998].
For each sleeping site, we recorded the GPS location of the plot center (using a handheld GPS Garmin 60CSx;10 m error), as well as its distance to the nearest roadway and to any human settlement (using a 3 point‐scale: 0–20 m; 21–50 m;>50 m). We
also distinguished between two habitat types: the
“forest zones,” consisting of natural forested zones
with continuous tree canopy, and the “human‐
modified zones”(hereafter also referred as to“human zones”), consisting of roadways, the ranger station with its associated buildings, and grass patches with planted trees (Fig. 1) [Sha & Hanya, 2013]. For each sleeping tree (n¼37), we recorded the species (with the collaboration of a local botanist, H. Kesumanegara), diameter at breast height (DBH) and total tree height (visual estimate always made by the same observer). We took the same measures for control trees (n¼138), which were nearby trees (DBH
>10 cm) selected to represent the four quadrants of a
sleeping site equally. Additionally, we noted the time the macaques entered and left the sleeping site, the GPS location and nature of encounters with other primate groups during the day and at sleeping sites, and the occurrence of vocalizations such as loud collective contact calls and those associated with intra‐group aggression during retirement to sleeping sites on anad libitumbasis [Altmann, 1974].
During our daily follows of the macaques, we recorded the group’s GPS location at 20‐min intervals (n¼1,230) to reconstruct its daily ranging patterns. When possible, we took the GPS position from the group’s center (rough estimate of the typical group
spread¼30 m). When this was not possible, we
tolerated a maximum average distance of 10 m from the group’s periphery. In addition, we used
30 sec scans at 2.5 min intervals (n¼5,037)
[Altmann, 1974] to record the habitat type at the group’s location (i.e. forests vs. human zones), the distance between the group’s center and the nearest human settlement or roadway (using a 3 point‐scale: 0–20 m, 21–50 m,>50 m), and the presence of human
food in bins or elsewhere within ca. 30 m of the center of the group (this distance was a rough estimate always made by the same observer, corre-sponding to the maximum beyond which visibility was restricted).
To investigate whether preferences for sleeping site locations related to changes in natural fruit availability within the home range, we conducted a tree phenological survey. Across the center of the study area, we established a 1,200 m20 m transect running north‐south, along which we surveyed twice a month the phenological status of all trees with DBH
10 cm (0: absence of fruit, 1: presence of ripe fruit, 2: presence of sun‐dried fruit) [Newton, 2007]. This transect crossed all the habitat types present in the group’s home range.
Data Analysis
To assess whether sleeping trees had larger DBHs and were taller than nearby control trees within the sleeping site, we used nested ANOVAs (tree‐type nested within sleeping site). We tested for these differences in both habitat types, forest versus human zones. Since the residuals of the models did not conform to a Shapiro–Wilk normality test, the DBH was log‐transformed and height was square‐
root‐transformed [Sokal & Rohlf, 1995]. We used Levene’s test to confirm non‐deviance for homosce-dasticity [Dytham, 2003]. To test for randomness of sleeping site re‐use, we generated an expected frequency distribution based on Poisson lambda parameters [Sokal & Rohlf, 1995] that we compared with the observed frequency distribution of the sleeping site re‐use using a Kolmogorov–Smirnoff test for goodness‐of‐fit [Day & Elwood, 1999; Phoon-jampa et al., 2010]. The theoretical Poisson distribu-tion was appropriate for the counts of re‐use of sleeping sites, which are rare events [Agresti, 2007]. Using ArcGIS 9.3.1 (ESRI) and the extension Hawth’s Tool, we calculated the size of the home range, both on a monthly and daily basis, with thefixed Kernel method (95% kernels¼total home range; 50% kernels¼core area). We assessed the smoothing parameters href [Worton, 1989] with R
3.0.2 and the package adehabitatHR. We compared the daily home range sizes between months with a Kruskall–Wallis test. To compare the locations of sleeping sites and intergroup encounters within the home range, we used Binomial tests with expected values derived from the observed frequency of macaques inside the core and peripheral areas, which are, by definition, each used 50% of the time.
Using methods defined in previous studies
[Imaki et al., 2006; Sha et al., 2009], we established a human proximity gradient within the home range characterized by several buffer‐zones delimited by their distance to any roads and/or human settle-ments, with the 3‐level scale used on thefield: (i) 0–
20 m (including the human zones and their edges), (ii) 21–50 m (intermediary forest edge), and (iii) more than 50 m (forest interior). We tested for random buffer‐zone use by the macaques during their diurnal activities and for sleeping using G‐tests for goodness‐
of‐fit with Williams’s correction [Sokal & Rohlf, 1995] (with expected frequencies based on the surface area of the buffers within the home range) and the standardized residuals considered as significant when the absolute value exceeded 2 [Agresti, 2007].
To analyze the variation in natural food avail-ability, we used a fruit availability index (FAI) for each tree species k and for each month m [Albert et al., 2013]:
Month FAIkm¼DkBkPkm
where inDkis the density of a given speciesk(number of specimens on the phenology transect), Bk is the mean basal area of species k, and Pk is the fruit phenology score of species k in a given month m (calculated as the percentage of specimens holding ripe fruit on their crown). We used a Friedman test to compare the FAI between months.
To analyze the effect of the human food (HF) availability on the habitat type chosen for sleeping, we assigned each observation day to a category of HF availability (no/some HF vs. high HF), based on the overall median of the daily proportion of scans when HF was present around the macaque group (30 m radius from the center of the group). We examined variation in HF available among months using a Kruskall–Wallis test. We used a G‐test of indepen-dence to evaluate whether selection of habitat type for sleeping depended on month. Finally, we analyzed the relationship between daily home range size and daily HF availability with a Spearman correlation test.
We performed the nested ANOVAs using R 3.0.2. Other statistical tests were completed using STA-TISTICA 10.0 (two‐tailed statistics with a signifi -cance level set at 0.05). Data are presented as mean values with standard deviations.
Our study was approved by University of Liège, Belgium, the Indonesian Ministry of Research and Technology (#03B/TKPIPA/FRP/SM/III/2011), the Indonesian Directorate of Forest Protection and Nature Conservation (#SI.33/Set‐3/2011) and the Bali Barat National Park (#S.308/BTNBB‐1/2011), and using protocols developed by Universitas
Udayana Primate Research Center (UNUD‐PKP)
and The University of Notre Dame (USA) under University of Notre Dame IACUC (#07‐001). This research adhered to the American Society of Prima-tologists Principles for the Ethical Treatment of Non‐
Human Primates.
RESULTS
Physical Characteristics of Sleeping Trees
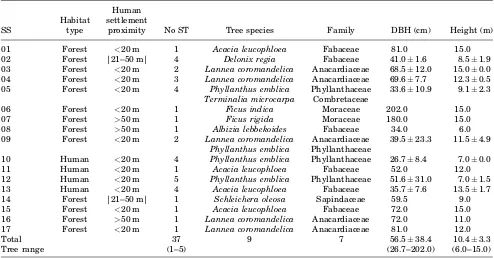
In total, macaques used 37 sleeping trees with a
mean DBH of 56.5SD 38.4 cm and a mean total
height of 10.4SD 3.3 m (Table I). In testing whether the monkeys selected the tallest trees with the largest trunks, we examined forest zones (82% of the home range) and human zones (18% of the home ranges) separately (Fig. 1). In forest habitat, the average DBH of sleeping trees was significantly larger than that of surrounding control trees (mST
¼66.5SD 43.3 cm,n¼23;mCT¼47.8SD 42.1 cm, n¼107; nested ANOVA: F13,104¼1.8, P<0.05). In contrast, in human zones, the average DBH of sleeping trees was not different from that of controls (mST¼40.0SD 21.1 cm, n¼14; mCT¼44.01SD 23.5 cm,n¼31; nested ANOVA:F4,37¼1.7,P¼0.16). The total height of sleeping and control trees did not differ significantly, either in forest (mST¼11.2SD
3.1 cm; mCT¼9.4SD 3.6 cm; nested ANOVA:
F13,104¼1.0, P¼0.37) or human zones (mST¼9.2
SD 3.3 cm; mCT¼10.3SD 4.5 cm; nested ANOVA: F4,37¼0.8,P¼0.52). We can thus conclude that when sleeping in forest zones, macaques selected trees with larger trunks that were not significantly taller than neighboring trees, while in human zones, sleeping trees did not differ from neighboring trees in either girth or height.
Macaques used nine species of tree for sleeping (Table I). The most frequently used werePhyllanthus emblica(n¼11, 30%),Lannea coromandelica(n¼8, 22%), andAcacia leucophloea(n¼7, 19%).
Patterns of Use and Re‐use of Sleeping Sites
Over 56 nights, the macaques used 17 sleeping sites and 37 sleeping trees (Table I). The group used 10 (59%) of the sleeping sites repeatedly (range: 2–9 times), but 4 of these sites accounted for 66% of the total nights, and the most favored site was re‐used nine times (Fig. 2). The other seven sleeping sites were each used only once. Sleeping sites were re‐used an average of 2.29 times (n¼17). Based on this mean
Am. J. Primatol.
(i.e. lambda), the expected re‐use frequency distribu-tion differed significantly from the observed re‐use frequencies (Kolmogorov–Smirnoff test: D¼0.31; P<0.05). The comparison between expected and
observed patterns showed two tendencies (Fig. 2). First, single use (¼0 re‐use) of sleeping sites was more common than expected, and some sleeping sites (n¼6) were re‐used less often than expected (range: 1–4 times). Second, four favored sleeping sites were re‐used equally or more often than expected (range: 5–9 times). Nonetheless, over the course of the study, macaques rarely used the same site on consecutive nights (n¼5 of 56 nights, 9%).
Sleep‐Related Behavior
The average time of day when macaques entered their sleeping trees was 18:00SD 25 min (n¼39; range: 16:45–18:35); the average time of departure was 06:41SD 25 min (n¼38; range: 06:10–08:00).
The monkeys spent an average of 12.40 hrSD
38 min per night in sleeping trees (n¼30; range: 11.40–13.45). Individuals in the group made loud contact calls while entering the sleeping site on 62% of the nights and intra‐group fights occurred upon ingress on 37% of nights. In summary, macaques usually entered their sleeping trees at dusk (sunset time for the study period: 18:08–18:33), seldom cautiously or silently.
Spatial Distribution of Sleeping Sites Within the Home Range and Intergroup Encounters
We recorded 38 inter‐group encounters involving at least four different long‐tailed macaque groups. These occurred both during diurnal activities and at sleeping sites. The average rate of encounter with conspecific groups was 0.07/hr and 50% of the 38 encounters were agonistic, i.e. including threats, charging or direct physical contact with members of the other group. We also observed two agonistic encounters between long‐tailed macaques and ebony langurs (Trachypithecus auratus), resulting in the displacement of the langur group. We noted that the TABLE I. Physical Characteristics (MeanSD) and Habitat Types of the Sleeping Trees Used by Long‐Tailed
Macaques at BBNP
SS
Habitat type
Human settlement
proximity No ST Tree species Family DBH (cm) Height (m)
01 Forest <20 m 1 Acacia leucophloea Fabaceae 81.0 15.0
02 Forest |21–50 m| 4 Delonix regia Fabaceae 41.01.6 8.51.9
03 Forest <20 m 2 Lannea coromandelica Anacardiaceae 68.512.0 15.00.0
04 Forest <20 m 3 Lannea coromandelica Anacardiaceae 69.67.7 12.30.5
05 Forest <20 m 4 Phyllanthus emblica Phyllanthaceae 33.610.9 9.12.3
Terminalia microcarpa Combretaceae
06 Forest <20 m 1 Ficus indica Moraceae 202.0 15.0
07 Forest >50 m 1 Ficus rigida Moraceae 180.0 15.0
08 Forest >50 m 1 Albizia lebbekoides Fabaceae 34.0 6.0
09 Forest <20 m 2 Lannea coromandelica Anacardiaceae 39.523.3 11.54.9
Phyllanthus emblica Phyllanthaceae
10 Human <20 m 4 Phyllanthus emblica Phyllanthaceae 26.78.4 7.00.0
11 Human <20 m 1 Acacia leucophloea Fabaceae 52.0 12.0
12 Human <20 m 5 Phyllanthus emblica Phyllanthaceae 51.631.0 7.01.5
13 Human <20 m 4 Acacia leucophloea Fabaceae 35.77.6 13.51.7
14 Forest |21–50 m| 1 Schleichera oleosa Sapindaceae 59.5 9.0
15 Forest <20 m 1 Acacia leucophloea Fabaceae 72.0 15.0
16 Forest >50 m 1 Lannea coromandelica Anacardiaceae 72.0 11.0
17 Forest <20 m 1 Lannea coromandelica Anacardiaceae 81.0 12.0
Total 37 9 7 56.538.4 10.43.3
Tree range (1–5) (26.7–202.0) (6.0–15.0)
SS, sleeping site ID; habitat type, forest versus human‐modified zones; No ST¼number of sleeping trees within the sleeping site.
Fig. 2. Observed and expected frequencies of sleeping site re‐use by the BBNP macaques. Expected frequencies were based on a Poisson distribution with lambda (i.e. arithmetic mean of re‐use) X¼2.29.
other primate groups also used two of the study group’s sleeping sites (both located alongside road-ways), although never simultaneously with our group. We once observed a bout of direct intergroup competition over access to one of these sleeping sites, resulting in the displacement of the study group. These two sleeping sites appeared to be, at least occasionally, contested resources.
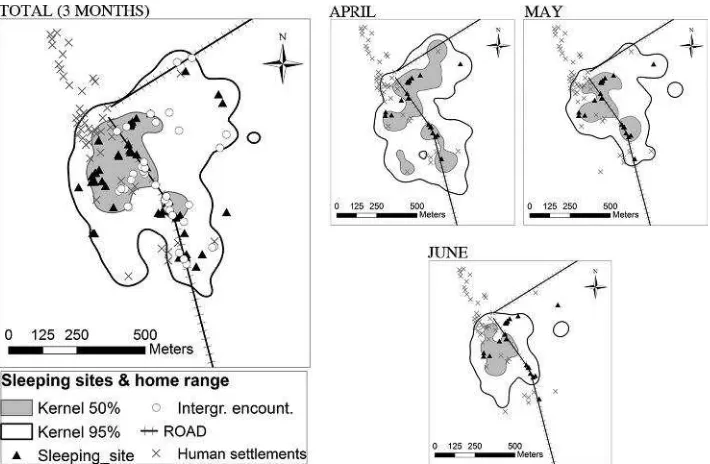
During the three study months, macaques used an overall home range of 32 ha. We compared the frequencies of the sleeping site locations between core and peripheral areas. The core area (7.72 ha) held a significantly higher number of sleeping sites than the peripheral area (Binomial test: Ncore¼36, Nperiph
¼20; P<0.05). However, the core area was not a
zone of exclusive use since intergroup encounters occurred at indistinguishable rates in the core and peripheral areas (Binomial test: Ncore¼22,
Nperiph¼16;P¼0.41) (Fig. 3).
Proximity to Human Settlements
During the day, the macaques used the human zones and their borders (buffer 0–20 m) substantially more frequently than expected by chance. They used zones >50 m distant from human settlements less
often than expected (GWilliams’s¼4,698; df¼2; P<0.001). Similarly, during the night, macaques
selected sleeping sites located near human settle-ments (buffer 0–20 m) significantly more often than expected, whereas, they chose sleeping sites deeper in the forest (buffer>50 m) less often than expected
(G‐test:GWilliams’s¼57.58; df¼2;P<0.001) (Fig. 4).
Furthermore, more than half of the 17 sleeping sites (56%) were located alongside roadways, and the favored sleeping sites were all located <50 m from
human settlement (Fig. 3). We found no clear difference between the frequency of use of the buffer‐zones for diurnal activities versus for sleeping (G‐test:GWilliams’s¼5.77; df¼2;P¼0.05), indicating consistency in habitat use between night and day.
Changes in Habitat Use and Food Availability
The mean daily range of the group decreased over the study months (Kruskall–Wallis test: H¼10.07, df¼2, n¼33, P<0.01). This result was consistent
with the parallel decrease of monthly home range size from April (44 ha) to May (27 ha) and June (16 ha) Fig. 3. Locations of sleeping sites and intergroup encounters of BBNP macaques within their overall home range, and variation in sleeping site locations between monthly home ranges (April–June 2011).
Fig. 4. Proximity to human settlements: observed and expected frequencies of nights spent by the macaques in different buffer‐ zones delimited by the distance to human roads and settlements. Adjusted residuals were significant when their absolute value exceeded 2 (i.e. for distances 0–20 m and>50 m).
Am. J. Primatol.
(Fig. 3). This decrease in mean daily range was also correlated with an increased availability of human food (rs¼ 0.49, P<0.05, n¼34). Human food was less available in April than in May and June (Kruskall–Wallis test: H¼11.81; df¼2, n¼46; P<0.01; post‐hoc: April–May: z¼2.74; P<0.05;
April–June: z¼3.16, P<0.01). While the increased
number of park visitors and campers in May (cf. Methods) led to an increase in food waste and leftovers available to macaques, the natural fruit availability index (FAI) also decreased significantly over the study months (Friedman test: x2¼9.33, df¼2,P<0.01,n¼8). In relation to these changes in the availability of natural and human food, we found a significant change between months in the monkeys’
selection of habitat types for sleeping (G‐test of independence: GWilliams’s¼6.37, df¼2, P<0.05): in April, macaques slept in forest habitat more often than expected while in June they slept more often in human zones.
DISCUSSION
Predation Avoidance Hypothesis
When sleeping in the human zones, macaques chose trees that did not differ from surrounding trees in these dimensions. Conversely, the sleeping trees chosen in forest zones had significantly larger trunks than neighboring trees, although they were not taller. Thus, the macaques did not seem to select sleeping trees based on height, no matter the habitat they occupied, but they selected trees with larger trunks when they slept in the potentially more risky forest environment. These results partially sup-ported the prediction of a difference in anti‐predator strategy between habitat types, based on tree defensibility characteristics [Ramakrishnan & Coss, 2001]. However, large trees may also facilitate the clustering of individuals, and therefore function to enhance group social cohesion at night [Albert et al., 2011; Anderson, 1998; Bernard et al., 2011; Di Bitetti et al., 2000]. In addition, Anderson [2000] emphasized that the availability of suitable sites is probably a major factor in choice of sleeping sites. Therefore, the low availability of large, tall trees, especially in the human zones, may provide another explanation for our results. Finally, human‐modified habitats may not be as safe as has been supposed, since certain potential primate predators are often associated with human settlements, such as reticu-lated pythons (Python reticulatus) and domestic dogs [Duarte & Young, 2011; Fooden, 1995; McKinney, 2009]. We were unable to assess the specific strategies that macaques might use against pythons [e.g. use of liana‐free trees: Tenaza & Tilson, 1985; Phoonjampa et al., 2010], or against nocturnal aerial predators such as owls [e.g. prefer-ence for open‐canopy cover for sleeping: Barnett
et al., 2012]. Large trunks and tall trees are advantageous for anti‐predator defense, increasing concealment and impeding access for terrestrial climbing predators [Albert et al., 2011; Barnett et al., 2012; Bernard et al., 2011; Day & Elwood, 1999; Di Bitetti et al., 2000; Fan & Jiang, 2008; Phoonjampa et al., 2010; Ramakrishnan & Coss, 2001; Reichard, 1998; Tenaza & Tilson, 1985]. Consequently, additional comparative data on the physical characteristics of trees (e.g. liana load, canopy cover density) and characteristics of actual sleeping places (e.g. grouping, distance to tree trunk) are needed to test the predator avoidance hypothesis by taking into account the attack mode of different predators. In addition, data on the current predation rates at this site are required to assess the degree of vulnerability of macaques in the two habitat types [Ramakrishnan & Coss, 2001].
Macaques clearly avoided reusing the same sleeping site on consecutive nights, possibly to be unpredictable to predators [Anderson, 2000; Emsens et al., 2014]. Although some sleeping sites were used only once, four sleeping sites located near human settlements were re‐used frequently. Some primato-logical studies reported a high level of site fidelity
with consecutive re‐uses [Chapman, 1989; Li
et al., 2011; Ramakrishnan & Coss, 2001; Tenaza & Tilson, 1985], while others documented several sleeping sites each with few subsequent re‐uses [Barnett et al., 2012; Fei et al., 2012; Phoonjampa et al., 2010; Reichard, 1998; Von Hippel, 1998; Zhang, 1995]. The pattern of sleeping site diversifi -cation and re‐use observed in our group might be an intermediate strategy. The avoidance of consecutive re‐uses of sleeping sites may serve to minimize detection by predators, but the frequent re‐use of a few favored sites over time may give the monkeys a familiarity with these sites, which may facilitate escape during night attacks [Albert et al., 2011; Day & Elwood, 1999; Di Bitetti et al., 2000]. It is notable that three of the four favored sleeping sites were located alongside roadways, probably enhancing the long‐distance visibility and detection of approaching terrestrial predators. This strategy is reminiscent of riverine refuging behavior of M. fascicularis and Nasalis larvatus, who tend to sleep in roosting trees with branches overhanging a river [Fittinghoff & Lindburg, 1980; Matsuda et al., 2008].
Over the course of our study, the macaques generally entered their sleeping sites at dusk and they were usually noisy. Such behavior contrasts with the cryptic pre‐retirement behavior reported in
many primate species [Saguinus mystax and S.
fuscicollis: Heymann, 1995; Smith et al., 2007; Hylo-bates lar: Reichard, 1998; Nomascus concolor jing-dongensis: Fan & Jiang, 2008; Trachypithecus leucocephalus: Li et al., 2011; Nomascus nasutus: Fei et al., 2012; Cacajao melanocephalus ouakary: Barnett et al., 2012], which minimizes risk of
detection by predators. Conversely, Reichard [1998] documented noisy pre‐sleep behavior in pig‐tailed macaques (M. nemestrina). While concealment from predators may be crucial for the survival of small‐
bodied species, or primates living in small groups [Heymann, 1995], the large average group size of macaques sleeping in the canopy may promote the early detection of predators [van Schaik et al., 1983]. Additionally, contact calls emitted by macaques as they enter sleep trees may enhance group cohesion [Chapman, 1989].
Overall, in relation to the predation avoidance hypothesis, our study provided conflicting results. A fuller understanding of human influence on night-time predation risk requires further research. Par-ticularly useful would be a comparative analysis with another macaque group at BBNP occupying a more risky zone remote from human settlements. Such studies would likely reveal different anti‐predator strategies within the repertoire of this species in response to diverse predation constraints.
Intergroup Competition Avoidance Hypothesis
Sympatry between long‐tailed macaques and ebony langurs has been reported in northern BBNP by Leca et al. [2013], and in Java (V. Nijman, personal communication). Our study confirms the frequent proximity of these two species in the western part of BBNP, near human settlements. The occurrence of agonistic intergroup encounters, as well as the use of the same sleeping sites by other groups of macaques and langurs, support the assumption of intergroup competition for access to sleeping sites as resources [Reichard, 1998]. Based on the risk hypothesis [Wrangham et al., 2007], we predicted that a non‐
territorial species such asM. fascicularis[van Schaik et al., 1983], which faces a significant risk of injuries and lethal aggression from neighboring conspecifics [Wheatley, 1999], should avoid sleeping in overlap areas of its home range to decrease the risk of competitive intergroup encounters. However, our results did not support this prediction. Macaques preferentially slept within the core area of their home range while frequent intergroup encounters occurred in this zone. This contrasts with the strategy of intergroup competition avoidance reported in other studies where monkeys preferentially slept in exclu-sively used areas of their home range [Albert et al., 2011; Li et al., 2011; Phoonjampa et al., 2010]. Conversely, Heymann [1995] suggested that the distribution of food resources may better account for the choice of sleeping site locations within home ranges. Since the study group’s core area was centered around human settlements, we similarly argue that proximity of sleeping sites and human food sources provides a better explanation for the concentration of sleeping sites within the core area, instead of avoidance of intergroup competition.
Proximity to Human Settlements and Human Food
The macaques preferentially exploited human zones and forest edges close to human settlements, both when engaged in diurnal activities and when sleeping. Thisfinding supports the hypothesis that M. fascicularis is a habitat generalist attracted to the edges of human‐modified habitats [Fuentes et al., 2005; Gumert et al., 2011; Sha et al., 2009]. Furthermore, this preference for edge habitats was increased by the distribution and availability of both natural and human food. The macaques shifted their habitat use patterns across months, favoring forest zones in April, when natural food was still abundant (as reflected by the FAI). Conversely, at the beginning of dry season, which coincided with the onset of the high tourist season in May,
macaques concentrated their now‐smaller home
range on the human zones. They also favored sleeping sites located near human settlements where more anthropogenic food, such as scraps and leftovers, was left by park visitors. This strategy mirrors the natural ability of M. fascicu-laris to exploit transitory resources opportunisti-cally [Kurland, 1973], even within increasingly human‐disturbed environments [Wheatley, 1999]. This flexibility in the ranging pattern of the group would be concurrent with an optimal foraging strategy minimizing the time and energetic costs
of traveling between foraging and sleeping
sites [Chapman et al., 1989; Smith et al., 2007; Teichroeb et al., 2012]. In addition, the consumption
of human‐provided food may in some cases
provide nutritional benefits to generalist and opportunistic primate species [Albert et al., 2013; Gumert et al., 2011; Sha & Hanya, 2013]. For example, Muruthi et al. [1991] reported that
baboons (Papio cynocephalus) who slept near
tourist lodges and fed partially from a garbage dump, spent less time feeding and yet still achieved a similar energy intake to that of their wild‐feeding counterparts. Baboons took advantage of human food, which was more spatially clumped, had a higher concentration of calories and was more
easily‐digestible in comparison to wild food
[Strum, 2010]. Since we observed the macaques consuming garbage food in human zones [Brotcorne et al., in preparation], we believe that they may obtain similar benefits, especially during the period of natural fruit scarcity. It is important, however, to note that negative impacts on health may also arise from eating human‐derived food sources. Sapolsky & Share [2004], for example, described a disease outbreak in a baboon population that erupted after the baboons fed on contaminated meat, which had been disposed in a dump.
In conclusion, the present study presents prelim-inary evidence of the important role played by
Am. J. Primatol.
anthropic factors in the choice of sleeping sites made by long‐tailed macaques inhabiting an environment
“on the edge.”We argue that the primary benefit to the macaques of sleeping near human settlements was the increased access this gave them to human food. The benefits of such behavior in relation to predation avoidance was, however, less clear. Never-theless, further interactions between ecological and social constraints certainly played a role and should be the subject of further investigation. Of particular interest would be a comparative study focusing on a macaque group living in a remote forest at BBNP with no access to human food. To critically examine the impact of food access, seasonal variations and overall foraging constraints, a further study should also cover a complete annual cycle and include data on the food resource distribution, the diet and the travel costs between the current foraging areas and sleeping sites [Chapman et al., 1989].
ACKNOWLEDGMENTS
We thank the Indonesian Ministry of Research and Technology, Bapak Sri Wahyono and BBNP authorities for permission to conduct research in Indonesia. This study was carried out with the
financial support of Belgian National Fund for
Scientific Research and the Fondation Belge de la Vocation. We are grateful to Thibaut Dosogne, Hery Kesumanegara, Tri Eliana Nurdian, Paryanto, and Febriansah for theirfield assistance. We thank Jean Van Campenhout for providing the satellite maps, as well as Yaëlle Bouyer and Pierre‐André Eyer for their advice regarding statistical analyses.
REFERENCES
Agresti A. 2007. Introduction to categorical data analysis. New Jersey: Wiley. 372 p.
Albert A, Huynen M‐C, Savini T, Hambuckers A. 2013.
Influence of food resources on the ranging pattern of
northern pig‐tailed macaques (Macaca leonina). Interna-tional Journal of Primatology 34:696–713.
Albert A, Savini T, Huynen M‐C. 2011. Sleeping site selection and presleep behavior in wild pigtailed macaques. American Journal of Primatology 73:1222–1230.
Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49:227–267.
Anderson JR. 1998. Sleep, sleeping sites, and sleep‐related activities: awakening to their significance. American Jour-nal of Primatology 46:63–75.
Anderson JR. 2000. Sleep‐related behavioural adaptations in free‐ranging anthropoid primates. Sleep Medicine Reviews 4:355–373.
Barnett A, Shaw P, Spironello W, MacLarnon A, Ross C. 2012. Sleeping site selection by golden‐backed uacaris,Cacajao melanocephalus ouakary (Pitheciidae), in Amazonian flooded forests. Primates 53:273–285.
Bernard H, Matsuda I, Hanya G, Ahmed A. 2011. Character-istics of night sleeping trees of proboscis monkeys (Nasalis larvatus) in Sabah, Malaysia. International Journal of Primatology 32:259–267.
Bishop N, Blaffer Hrdy S, Teas J, Moore J. 1981. Measures of human influence in habitats of south Asian monkeys. International Journal of Primatology 2:155–167.
Chapman C, Chapman L, McLaughlin R. 1989. Multiple central place foraging by spider monkeys: travel consequen-ces of using many sleeping sites. Oecologia 79:506–511. Chapman CA. 1989. Spider monkey sleeping sites: use and
availability. American Journal of Primatology 18:53–60. Day RT, Elwood RW. 1999. Sleeping site selection by the
golden‐handedTamarin Saguinus midas midas: the role of predation risk, proximity to feeding sites, and territorial defence. Ethology 105:1035–1051.
Di Bitetti MS, Vidal EML, Baldovino MC, Benesovsky V. 2000. Sleeping site preferences in tufted capuchin monkeys (Cebus apella nigritus). American Journal of Primatology 50:257– 274.
Duarte M, Young R. 2011. Sleeping site selection by urban marmosets (Callithrix penicillata) under conditions of exceptionally high predator density. International Journal of Primatology 32:329–334.
Dytham C. 2003. Choosing and using statistics. A biologist’s guide. Malden, MA: Blackwell. 248 p.
Emsens W‐J, Hirsch BT, Kays R, Jansen PA. 2014. Prey
refuges as predator hotspots: ocelot (Leopardus pardalis) attraction to agouti (Dasyprocta punctata) dens. Acta Theriologica 59:257–262.
Fam SD, Nijman V. 2011.Spizaetushawk‐eagles as predators of arboreal colobines. Primates 52:105–110.
Fan P‐F, Jiang X‐L. 2008. Sleeping sites, sleeping trees, and sleep‐related behaviors of black crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. American Journal of Primatology 70:153–160. Fei H‐L, Scott MB, Zhang W, et al. 2012. Sleeping tree selection
of Cao vit gibbon (Nomascus nasutus) living in degraded karst forest in Bangliang, Jingxi, China. American Journal of Primatology 74:998–1005.
Fittinghoff NA, Lindburg DG. 1980. Riverine refuging in east BorneanMacaca fascicularis. In: Lindburg DG, editor. The macaques: studies in ecology, behaviour and evolution. New York: Van Nostrand Reinhold Company. p 182–213. Fooden J. 1995. Systematic review of southeast Asian longtail
macaques, Macaca fascicularis, (Raffles, [1821]). Fieldiana Zoology (n.s.) 81:1–206.
Fuentes A. 2010. Naturalcultural encounters in Bali: monkeys, temples, tourists and ethnoprimatology. Cultural Anthro-pology 25:600–624.
Fuentes A, Hockings KJ. 2010. The ethnoprimatological approach in primatology. American Journal of Primatology 72:841–847.
Fuentes A, Southern M, Suaryana KG. 2005. Monkey forests and human landscapes: is extensive sympatry sustainable for Homo sapiens and Macaca fascicularis on Bali? In: Patterson JD, Wallis J, editors. Commensalism and conflict: the human‐primate interface. Norman: American Society of Primatologists. p 168–195.
Gumert MD, Fuentes A, Jones‐Engel L. 2011. Monkeys on the edge. Ecology and management of long‐tailed macaques and their interface with humans. Cambridge: Cambridge Uni-versity Press. 360 p.
Hankerson SJ, Franklin SP, Dietz JM. 2007. Tree and forest characteristics influence sleeping site choice by golden lion tamarins. American Journal of Primatology 69:976– 988.
Heymann EW. 1995. Sleeping habits of tamarins,Saguinus
mystax and Saguinus fuscicollis, in north‐eastern Peru. Journal of Zoology, London 237:211–226.
Imaki H, Koganezawa M, Maruyama N. 2006. Habitat selection and forest edge use by Japanese monkeys in the Nikko and Imaichi area, Central Honshu, Japan. Biosphere Conservation: For Nature, Wildlife, and Hu-mans 7: 87–96.
Isbell LA, Young TP. 1993. Human presence reduces predation
in a free‐ranging vervet monkey population in Kenya.
Animal Behaviour 45:1233–1235.
Kurland JA. 1973. A natural history of Kra macaques (Macaca fascicularis, Raffles, 1821) at the Kutai Reserve, Kalimantan Timur, Indonesia. Primates 14:245–262.
Leca JB, Gunst N, Rompis A, et al. 2013. Population density
and abundance of ebony leaf monkeys (Trachypithecus
auratus) in West Bali National Park, Indonesia. Primate Conservation 26:133–144.
Li D, Zhou Q, Tang X, Huang H, Huang C. 2011. Sleeping site use of the white‐headed langurTrachypithecus leucocepha-lus: the role of predation risk, territorial defense, and proximity to feeding sites. Current Zoology 57:260–268. Liu Z‐H, Zhao Q‐K. 2004. Sleeping sites ofRhinopithecus bieti,
at Mt. Fuhe, Yunnan. Primates 45:241–248.
Matsuda I, Tuuga A, Akiyama Y, Higashi S. 2008. Selection of river crossing location and sleeping site by proboscis monkeys (Nasalis larvatus) in Sabah, Malaysia. American Journal of Primatology 70:1097–1101.
McKinney T. 2009. Anthropogenic change and primate preda-tion risk: Crested caracaras (Caracara plancus) attempt predation on mantled howler monkeys (Alouatta palliata). Neotropical Primates 16:24–27.
Muruthi P, Altmann J, Altmann S. 1991. Resource base, parity, and reproductive condition affect females’feeding time and nutrient intake within and between groups of a baboon population. Oecologia 87:467–472.
Newton A. 2007. Forest ecology and conservation: a handbook of techniques. New York: Oxford University Press. 454 p. Palombit RA. 1992. A preliminary study of vocal
communica-tion in wild long‐tailed macaques (Macaca fascicularis). International Journal of Primatology 13:183–207.
Phoonjampa R, Koenig A, Borries C, Gale GA, Savini T. 2010. Selection of sleeping trees in pileated gibbons (Hylobates pileatus). American Journal of Primatology 72:617–625. Ramakrishnan U, Coss R. 2001. Strategies used by bonnet
macaques (Macaca radiata) to reduce predation risk while sleeping. Primates 42:193–206.
Reichard U. 1998. Sleeping sites, sleeping places, and presleep behavior of gibbons (Hylobates lar). American Journal of Primatology 46:35–62.
Richard AF, Goldstein SJ, Dewar RE. 1989. Weed macaques: the evolutionary implications of macaques feeding ecology. International Journal of Primatology 10:569–594.
Sapolsky RM, Share LJ. 2004. A pacific culture among wild baboons: its emergence and transmission. PLoS Biology 2:534–541.
Sha J, Gumert M, Lee B, et al. 2009. Status of the long‐tailed macaque (Macaca fascicularis) in Singapore and
implica-tions for management. Biodiversity and Conservation 18:2909–2926.
Sha J, Hanya G. 2013. Diet, activity, habitat use, and ranging of two neighboring groups of food‐enhanced long‐tailed mac-aques (Macaca fascicularis). American Journal of Primatol-ogy 75:581–592.
Smith AC, Knogge C, Huck M, et al. 2007. Long‐term patterns of sleeping site use in wild saddleback and mustached tamarins: effects of foraging, thermoregulation, predation, and resource defense constraints. American Journal of Physical Anthropology 134:340–353.
Sokal R, Rohlf F. 1995. Biometry. New York: WH Freeman. 887 p.
Stanford CB. 2002. Avoiding predators: expectations and evidence in primate antipredator behavior. International Journal of Primatology 23:741–757.
Strum S. 2010. The development of primate raiding: implica-tions for management and conservation. International Journal of Primatology 31:133–156.
Teichroeb J, Holmes T, Sicotte P. 2012. Use of sleeping trees by ursine colobus monkeys (Colobus vellerosus) demonstrates the importance of nearby food. Primates 53:1–10.
Tenaza R, Tilson RL. 1985. Human predation and Kloss’s
gibbon (Hylobates klossii) sleeping trees in Siberut Island, Indonesia. American Journal of Primatology 8:299–308. van Schaik C, Mitrasetia T. 1990. Changes in the behaviour of
wild long‐tailed macaques (Macaca fascicularis) after encounters with a model python. Folia Primatologica 55:104–108.
van Schaik C, van Noordwijk M, Warsono B, Sutriono E. 1983. Party size and early detection of predators in Sumatran forest primates. Primates 24:211–221.
Von Hippel FA. 1998. Use of sleeping trees by black and white
Colobus monkeys (Colobus guereza) in the Kakamega
Forest, Kenya. American Journal of Primatology 45: 281–290.
Wheatley B, Fuentes A, Harya Putra I. 1993. Primates of Bali. Asian Primates 3:1–2.
Wheatley BP. 1999. The sacred monkeys of Bali. Prospect Heights, IL: Wavelend Press, Inc.
Whitten T, Soeriaatmadja RE, Afiff SA. 1996. The ecology of Java and Bali. Singapore: Periplus. 969 p.
Worton BJ. 1989. Kernel methods for estimating the utilization distribution in home‐range studies. Ecology 70:164–168. Wrangham R, Crofoot M, Lundy R, Gilby I. 2007. Use of overlap
zones among group‐living primates: a test of the risk hypothesis. Behaviour 144:1599–1619.
Zhang SY. 1995. Sleeping habits of brown capuchin monkeys (Cebus apella) in French Guiana. American Journal of Primatology 36:327–335.
Am. J. Primatol.