Journal of Life Sciences
Volume 8, Number 10, October 2014 (Serial Number 78)
David Publishing Company www.davidpublishing.com
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 240 Nagle Avenue #15C, New York, NY 10034, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Prof. Dr. Fadel Djamel (Algeria), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Prof. Dr. Ismail Salih Kakey (Iraq), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available online at http://www.davidpublishing.com.
Editorial Office
240 Nagle Avenue #15C, New York, NY 10034, USA
Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374, 1-323-9080457 E-mail:[email protected], [email protected]
Copyright©2014 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA
Database of Cambridge Science Abstracts (CSA), USA Database of Hein Online, New York, USA
Ulrich’s Periodicals Directory, USA Universe Digital Library S/B, Proquest
Chinese Database of CEPS, American Federal Computer Library center (OCLC), USA China National Knowledge Infrastructure, CNKI, China
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China Index Copernicus, Index Copernicus International S.A., Poland
Google Scholar (scholar.google.com)
Subscription Information
Price (per year): Print $420, Online $300, Print and Online $560.
David Publishing Company
240 Nagle Avenue #15C, New York, NY 10034, USA
Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374, 1-323-9080457 E-mail: [email protected]
David Publishing Company www.davidpublishing.com
DAV ID P UBL ISH IN G
JLS
Journal of Life Sciences
Volume 8, Number 10, October 2014 (Serial Number 78)
Contents
Microbiology
799 Study of Biofilm Formed by Lactic Acid Bacteria on the Surface of Mild Steel Tsveteslava Veselinova Ignatova-Ivanova and Radoslav Iliev Ivanov
805 Biofilm Determination of Listeria monocytogenes That Isolated from Different Sources Srwa Ali Muhammed
811 Seroprevalence of Human Brucellosis in Kuku Dairy Scheme, Khartoum State, Sudan Tamador-Elkhansaa Elnour Angara, Adil Abdel Rahman Ali Ismail and Nageeb Suliman Saeed
Botany and Zoology
815 Detection of Mycoplasma synoviae Infection in Broiler Breeder Farms of Morocco Using Serological Assays and Real Time PCR
Nassik Saâdia, Aboukhalid Rachid, Azzam Falak, Rahmatallah Naoufal, Lahlou-Amine Idriss, Fassi-Fihri Ouafaa and El Houadfi Mohammed
822 Prebiotic Isomalto-oligosaccharide Production from Thai Rice
Premsuda Saman, Achara Chaiongkarn, Somporn Moonmangmee and Chantra Poonsiri
828 Influence of the Type of Fertilization on Nitrogen Balance of the Mountain Meadow Piotr Kacorzyk, Mirosław Kasperczyk and Wojciech Szewczyk
835 Effect of Phosphonate Fertilizers on the Growth of Soil Fungi
Samer Samir Mohd Habash, Mohammad Saleh Al-Bess, Ahmad Saleh Al-Bess and Luma Shareef AL
Banna
Interdisciplinary Researches
841 Ecological Research of Former Brown-coal Quarry—the Most Lake in the Czech Republic
848 Limited Efficacy of Aspirin in Patients with Peripheral Arterial Occlusive Disease Pavel Poredoš
Journal of Life Sciences 8 (2014) 799-804 doi: 10.17265/1934-7391/2014.10.001
Study of Biofilm Formed by Lactic Acid Bacteria on the
Surface of Mild Steel
Tsveteslava Veselinova Ignatova-Ivanova and Radoslav Iliev Ivanov
Department of Biology, University of Shumen, Shumen 9712, Bulgaria
Received: September 17, 2014 / Accepted: October 19, 2014 / Published: October 30, 2014.
Abstract: Biocorrosion processes at metal surfaces are associated with microorganisms, or the products of their metabolic activities including enzymes, exopolymers, organic and inorganic acids, as well as volatile compounds such as ammonia or hydrogen sulfide. It was proved that strain Lactobacillus delbrueckii B5 constituted biofilms in the presence of different amounts of carbohydrates (5% sucrose and a mixture of 5% lactose, 5% fructose and 5% maltose). The obtained information was used in a study treating the anticorrosive properties of microbial biofilms synthesized by the latter strain. The study of the corrosive stability of steel samples was conducted on the gravimetrique method. The rate of corrosion, the degree of protection, and coefficient of protection has been calculated. The structure of layer over steel plates was analyzed by Scanning Electron Microscopy (SEM) JSM 5510.
Key words: Biofilm, corrosion, inhibitor,lactic acid bacteria,SEM.
I. Introduction
Corrosion is the deterioration of a material, especially metal, as a result of chemical or electrochemical reactions on the metal/solution interface [1, 2]. The metallic corrosion is a spontaneous process which consists of the inverse of metallurgical processes, where the processed metals revert to their natural state of lower free energy, i.e., chemical compounds or minerals [1]. The metallic corrosion represents an economic burden for many industry sectors [3, 4]. According to the World Corrosion Organization [5], the annual cost of corrosion is greater than 3% of global GDP (Gross Domestic Product); however, governments and industries pay little attention to corrosion, except in high-risk areas, like aircrafts and pipelines. Kinetics of corrosion processes of metals, mineral and polymeric materials can be influenced by biofilms. Products of their metabolic activities including enzymes, exopolymers, organic and inorganic acids, as well as
Corresponding author: Tsveteslava Veselinova Ignatova-Ivanova, Ph.D., associate professor, research field: microbiology. E-mail: [email protected].
volatile compounds such as ammonia or hydrogen sulphide can affect cathodic and/or anodic reactions, thus altering electrochemistry at the biofilm/metal interface. This phenomenon is often referred to as “biocorrosion” or “microbially influenced corrosion”. The adoption of preventive measures that reduce or eliminate corrosion is financially costly and time consuming. In fact, corrosion prevention and treatment consume more than 20% of a typical industrial budget.
It was found that exopolysaccharides produced by bacteria were suitable candidates for such investigation [6]. Accelerated corrosion in which metal substrates were colonized by bacteria had been noted [7, 8]. However, in some cases, biofilms, composed of a secreted polymeric substance containing microbial populations, have shown to inhibit corrosion in metals [9, 10]. In most cases, the metal was submerged in an aqueous medium inoculated by a bacterial species. The bacteria colonised the metal substrate and produced a heterogenous extracellular polymeric substance that adhered to metal surfaces facilitated by the functional
D
Study of Biofilm Formed by Lactic Acid Bacteria on the Surface of Mild Steel
800
groups of the exopolymer substance. Corrosion inhibition was usually measured by loss-in-weight calculation and generally attributed to the diffusion barrier provided by the bacterial biofilm.
Work by Stadler et al. [11] concentrated on corrosion by sulphate-reducing bacteria but suggested that dextrans could prevent corrosion on metals. Van Leuven et al. [12] found an (1 → 3), (1 → 6)—linked D-glucan produced by Lactobacillus reuteri that inhibited corrosion while dispersed in a
electrolyte solution rather that as a coating [13]. In the authors’ previous studies [14-16], it showed how at the presence of high concentration of lactose (5 to 15%), high concentration of sucrose 4%, and mixture of sucrose 4% and 2% maltose, the strains
Lactobacillus delbrueckii B5, L. delbrueckii K27, L. delbrueckii B8, L. delbrueckii O43, L. delbrueckii K3,
L. delbrueckii K17 and L. delbrueckii K15 synthesized exopolysaccharides which can be used as corrosion inhibitor. It is well known that some lactobacillus strains as well genus Leuconostoc secreted transglucosidases after cultivation in the presence of sucrose. Glucose polymers and their corresponding oligosaccharides can be synthesized through enzymic transglucosylation by a wide variety of glycansucrases capable of using sucrose as substrate. Among this group, dextransucrases (EC 2.4.1.5) are glucansucrases (GSs) able to produce dextran, a glucose polymer linked mainly through a1-6 bonds, although a1-3, a1-6, a1-4 and a1-2 bonds are also found, in both the main chain and the branching linkages. Furthermore, glucose can also be transferred to molecules that can act as nucleophile acceptors; in the case of sugars such as maltose, a wide variety of low-molecular-mass glucose oligosaccharides are produced [17].
In this paper, data on the effect of EPS on corrosion of steel produced by Lactobacillus delbrueckii B5, cultivated on media with sucrose as donor and maltose, fructose, and lactose as acceptor are presented and discussed.
2. Materials and Methods
2.1 Strain
Strain Lactobacillus delbrueckii B5 was obtained from Collection of Department of General and Applied Microbiology, Sofia University.
2.2 Media Used
The strain cultivated in media of MRS (de Mann Rogosa Sharpe, Biolife 272-20128, Milano, Italia) in composition, per liter: Tween 80—1; pepton from casein—10.0; meat extract—8.0; yeast extract—4.0; K2HPO4—2.0; sodium acetat—5.0; amonium
citrate—2.0; MgSO4.7H2O—0.2 and MnSO4—0.05
g/L. The pH of media was adjusted to 6.5 with 1N NaOH. The basic media was sterilized by autoclaving at 121 ºC for 20 min, and carbohydrates supplemented were sterilized using 0.22 µm filters (Manisart®). The basic MRS broth was supplemented with 5% sucrose and 5% lactose; 5% sucrose and 5% fructose and 5% sucrose and 5% maltose to be tested.
2.3 Study of the Corrosive Stability
The study of the corrosive stability of steel samples was conducted on the gravimetrique method [18]. Before use, steel panels (10 × 4 × 0.2 mm) were treated with 70% C2H5OH, washed with water and dried in an
oven, cooled in a desiccator, weighed on a balance and kept in a desiccator unit used. The weight of the samples was measured using analytical balances. The dimensions of the samples measured with micrometer. Three types of experimental series were performed:
(1) Cultivation of the studied strain in mMRS media with 5% of sucrose and 5% fructose
(2) In mMRS media with 5% sucrose and 5% maltose
(3) In mMRS media with 5% sucrose and 5% lactose
Study of Biofilm Formed by Lactic Acid Bacteria on the Surface of Mild Steel 801
HCl as control probe and as inhibitor of the corrosion were added dilution (3:100) of the cultural media of the studied strain. The duration of the procedure was 120 h at 18 ºC. After the treatment the steel samples were washed with water and dried to constant weight. The structure of layer over steel plates was analyzed by Scanning Electron Microscopy (SEM) JSM 5510.
2.4 Parameters of Corrosion
Parameters of corrosion after retrieval, the corrosion products were removed using washed with water. They were dried in an oven. After the removal of corrosion, steel plates were cleaned and reweighed as above to estimate weight loss.
The rate of corrosion, the degree of protection, and coefficient of protection were calculated.
The corrosion rates К (g/cm2
.h) presented as follows:
К = ΔG/ S.τ, g/cm2
.h;
Where: К is the corrosion rate; ΔG—losses of mass consequence of corrosion, g; S—is the area of plates, m2; τ—is duration of the corrosion, h.
For track out of inhibitor properties of EPS, synthesized in media has been calculated the degree of protection (Z) and coefficient of protection (γ) using formulas:
Z = (K0–Ki)/K0 × 100%;
γ = K0/Ki;
Where: K0 is the corrosion rate in control media;
Ki—the corrosion rate in test media.
3. Results and Discussion
Strain L. delbrueckii B5 was cultivated in a media containing 5% sucrose and 5% fructose; 5% sucrose and 5% maltose and in a media containing 5% sucrose and 5% lactose for 12 h. After the cultivation the cells were removed and the obtained supernatants (deproteinized supernatant and cell free supernatants) were used for protection of the steel plates. The steel plates were placed in 10% HCl as control probe and as inhibitor of the corrosion were added dilution (3:100) of the cultural media of the studied strain. The received results are presented on Table1 and Table 2.
From the presented data in Table 1 the protective effect in all studied cases was proved. The coefficient of the protection of corrosion varied between 9.25 and 2.64. From the obtained results is clear that the protection of corrosion was higher for strain L. delbrueckii B5 in the presence of 5% sucrose and 5%
maltose were used during the fermentations process but were in the presence of the cells. The protection of corrosion was higher for strain L. delbrueckii B5 in the presence of 5% sucrose and 5% lactose were used during the fermentations process but were without the cells.
From the presented data in Table 2 the protective effect in all studied cases was proved. When used as inhibitor of the protection strain L. delbrueckii B5
cultivated in the presence of 5% sucrose and 5% lactose the protection of corrosion was highest.
Table 1 Characterization of the protective properties in cultural media.
№
sample Media Type of the tested inhibitor K.10
-5, g/cm2.h Z % γ
1. 5% sucrose + 5% lactose
Cell free supernatants 0.20 72.97 3.70
2. Deproteinised supernatant 0.13 82.43 5.69
3. 5% sucrose + 5% fructose
Cell free supernatants 0.33 55.40 2.24
4. Deproteinised supernatant 0.25 66.22 2.96
5. 5% sucrose + 5% maltose
Cell free supernatants 0.08 89.19 9.25
6. Deproteinised supernatant 0.28 62.16 2.64
7. control 0.74 - -
Study of Biofilm Formed by Lactic Acid Bacteria on the Surface of Mild Steel
802
Table 2 Characterization of the protective properties in HCl with added supernatant.
№
sample Media
The quantity of the supernatant in
10% HCl K.10
*The steel plates were photographed after washing. Results are mean ± SEM of three separate trails.
It could be underlined that 5% sucrose mixed with 5% maltose, or with 5% lactose in the media stimulated the process of protection of corrosion.
The structure of the layer over the steel plates was analyzed by Scanning Electron Microscopy.
The results from this procedure are shown in Fig. 1. The pictures in Fig. 1A show that there’s a biofilm formed on the steel surface which is an indicator of the good adhesive capacity of L. delbrueckiiB5 type.
The biofilm makes it not easily corrodible in 10% HCl, supplemented with cultivated ambient from the same strain grown in a composite of 5% sucrose and 5% lactose (Fig. 1B). Fig. 1C shows a picture of a steel surface sample treated directly with 10% HCL. The observed lamellae are most probably FeCl2
crystals, product of the corrosion.
The forms of corrosion which can be promoted by the interaction of microorganisms with metals are numerous, including general pitting, crevice attack, stress corrosion cracking, enhancement of corrosionfatigue, intergranular stress cracking and hydrogen embrittlement and cracking. Most cases of microbially-influenced corrosion (MIC) are associated with localized attack. The complexity of MIC reactions means that a broad range of techniques must be employed to relate the corrosion processes to the microbial activities at surfaces [19].
Microscope techniques provide information about
the morphology of microbial cells and colonies, their distribution on the surface, the presence of EPS (Fig. 1A and 1B) and the nature of corrosion products (crystalline or amorphous; Fig. 1C). They can also reveal the type of attack (e.g., pitting or uniform corrosion) by visualizing changes in microstructure and surface features after removal of the biofilm and corrosion products (Fig. 1C).
The role of EPS in MIC of stainless steel remains obscure. It has been postulated that they are not sufficient to induce biocorrosion of stainless steel unless aided by the presence of a biocatalyst of oxygen reduction, which could be oxido-reductase enzymes entrapped in the biofilm [20]. EPS has even been suggested to protect metal surfaces from corrosion.
A bacterial consortium consisting of a thermophilic
Bacillus sp. and Deleya marina produced metalbinding EPS that reduced the rate of corrosion of carbon steel by 94% [21]. Such a mechanism may be responsible for the protection microorganisms afford to mild steel under certain conditions [22].
4. Conclusions
Study of Biofilm Formed by Lactic Acid Bacteria on the Surface of Mild Steel 803
(A)
(B)
(C)
Study of Biofilm Formed by Lactic Acid Bacteria on the Surface of Mild Steel
804
Acknowledgments
The contributors express their gratitude for the funding by the project by Shumen University project RD 08-213/10.03.2014.
References
[1] Gentil, V. 2007. Corrosão. 5th ed. Rio de Janeiro, S. A.: Livros Técnicos e Científicos.
[2] Shi, X., Xie, N., and Gong, J. 2011. “Recent Progress in the Research on Microbially Influenced Corrosion: A Bird’s Eye View Through The engineering Lens.” Recent Patents on Corrosion Science. 11: 118-131.
[3] Demadis, K. D., Mantzaridis, C., and Lykoudis, P. 2006. “Effects of Structural Differences on Metallic Corrosion Inhibition by Metalpolyphosphonate Thin Films.”
Industrial & Engineering Chemistry 45: 7795-7800. [4] Hansson, C. M. 2010. “The Impact of Corrosion on
Society.” Metallurgical & Materials Transactions 42: 2952-2962.
[5] Geroge, F. H. 2012. Now is the Time. World Corrosion Organization.
[6] Finkenstadt, V., Cote, G., and Willett, J. 2008. “Agricultural Polymers for Corrosion Protection of Metals.” 236th National Meeting of the American Chemical Society.
[7] Beech, I. B., and Sunner, J. 2004. “Biocorrosion: Towards Understanding Interaction Between Biofilms and Metals.” Curr. Opin. Biotechnol. 15: 181-186. [8] Beech, I. B., and Gaylarde, C. C. 1991. “Microbial
Polysaccharides and Corrosion.” Int. Biodeterior 27: 95-107.
[9] Chongdar, S., Gunasekaran, G., and Kumar, P. 2005. “Corrosion Inhibition of Mild Steel by Aerobic Biofilm.”
Elecrtrochim Acta 50: 4655-4665.
[10] Fang, H. H. P., Xu, L. C., Chan, K. Y. 2002. “Effects of Toxic Metals and Chemicals on Biofilm and Biocorrosion.” Water Res. 36: 4709-4716.
[11] Stadler, R., Fuerbeth, W., Harneit, K., Grooters, M., Woellbrink, M., and Sand, W. “Electrochim.” Acta 54: 91-99.
[12] van Leeuwen, S., Kraly, S., Van Geel-Shutten, I., Gerwig, G. J., Dijkhuizen, L., and Kamering, J. P. 2008. “Structural Analysis of the a-D-glucan (EPS180)
Produced by the Lactobacillus reuteri Strain 180 Glucansucrase GTS180 Enzyme.” Carbohydr Res. 343: 1237-1250.
[13] Penninga, N., Kraly, S., Euverink, G. J., van der Maarel, M., van Geel-Shutten, I., and Dijkhuizen, L. 2002. “GTF180: A Glycosiltransferase Producing a Glucan with Anti-corrosive properties.” In Proceedings of the Nederlandse Vereniging voor Medische Microbiologie, 22.
[14] Ignatova-Ivanova, Ts., Ivanov, R., Iliev, I., and Ivanova, I. 2009. “Study Anticorrosion Effect of EPS from Now Strains Lactobacillus delbruecii.” Biotechnol. & Biotechnol.EQ Special edition/on line 23 (1): 705-708. [15] Ignatova-Ivanova, Ts., Ivanov, R., Iliev, I., and Ivanova, I.
2011. “Study of Anticorrosion Effect of Exopolysaccharides Produced Lactobacillus delbrueckii B5 Cultivated on Different Carbohydrates.” Biotechnol. & Biotechnol. EQ Special edition/on line 26 (1): 224-227. [16] Ignatova-Ivanova Ts., Ivanov, R. 2013. “Anticorrosion
Effect of Biofilm Forming by Lactobacillus Strains on Metal Surfaces.” Bulgarian Journal of Agricultural Science 19 (2): 83-85.
[17] Clarita Olvera, Jose´ Luis Ferna´ndez-Va´ zquez, Luis Ledezma-Candanoza and Agustı´n Lo´ pez-Munguı´a. 2007. “Role of the C-terminal Region of Dextransucrase from Leuconostoc Mesenteroides IBT-PQ in Cell Anchoring.” Microbiology 153 (12): 3994-4002.
[18] Raychev, R., Fachikov, L., and Zaprjanova, V. 2002.
Corrosion and Protection of the Materials—Handbook for Laboratorial Exercises. Sofia.
[19] Iwona, B., Beech, Christine, C., and Gaylarde 1999. “Recent Advances in the Study of Biocorrosion. An Overview.” Revista de Microbiologia 30: 177-190. [20] Lai, M. E., Scotto, V., and Bergel, A. 1999. “Analytical
Characterization of Natural Marine Biofilms.” Presented at 10th Internat. Congress on Marine Corrosion and Fouling, Melbourne, Australia.
[21] Edyvean, R. G. J., Benson, J., Thomas, C. J., Beech, I. B., and Videla, H. 1998. “Biological Influences on Hydrogen Effects in Steel in Seawater.” Mat. Perform 37: 40-44. [22] Soracco, R. J., Berger, L. R., Berger, J. A., Mayack, L. A.,
Journal of Life Sciences 8 (2014) 805-810 doi: 10.17265/1934-7391/2014.10.002
Biofilm Determination of
Listeria monocytogenes
That
Isolated from Different Sources
Srwa Ali Muhammed
Department of Microbiology, Faculty of Science and Health, Koya University, Daniel Mitterrand Boulevard, Koya KOY45 AB64 46017, Kurdistan Region, Iraq
Received: September 18, 2014 / Accepted: October 13, 2014 / Published: October 30, 2014.
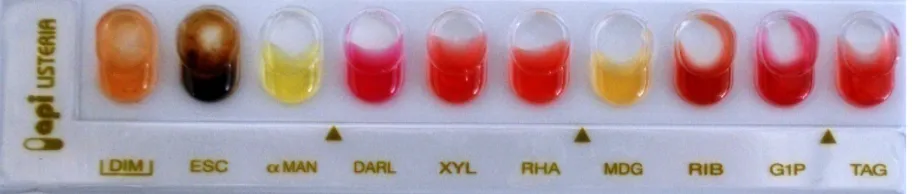
Abstract: The study was conducted for the detection of Listeria monocytogenes bacteria from different sources (CSF and blood) obtained from patients in Pediatric hospital and from food sources like (yogurt, raw vegetables and raw milk, sausage). Ten isolates were isolated from 150 specimens one of them from CSF and one isolate isolated from blood samples the others isolated from food specimens 6 isolates isolated from sausage and 2 from raw vegetables. Isolates were identified traditionally involved culture methods based on selective enrichment and plating followed by the characterization of Listeria spp. based on colony morphology, sugar fermentation and haemolytic properties, identification by Api listeria was done. Determine the isolates that produce biofilm by tissue culture plate method. The highest biofilm forming strains of Listeriamonocytogenes isolates appear in No. (D10, E1, E5 and E7) OD reading each of them is (0.13, 0.09, 0.11 and 0.19) respectively, the lowest or poor biofilm forming strains appear in No. (D11, D12, E2, E3, E4 and E6) that optical density (OD) reading are (0.04, 0.03, 0.05, 0.04, 0.05 and 0.03) respectively by comparing with control prepared in well (A12) that stained by crystal violate without putting any isolates and the OD reading is (0.003). Confirmation by PCR was done only for four isolates that produce biofilm (D10 and E1) that obtained from CSF and blood sample and for (E5 and E7) that obtained from food samples.
Key word: Listeria monocytogenes, biofilm, Api, PCR, OD.
1. Introduction
Listeria monocytogenes is the bacterium that causes the infection listeriosis. It is a facultative anaerobic bacterium, capable of surviving in the presence or absence of oxygen. It can grow and reproduce inside the host’s cells and is one of the most virulent food-borne pathogens, with 20 to 30 percent of clinical infections resulting in death [1].
Listeria monocytogenes is a gram-positive food-borne pathogen that causes life-threatening infections in fetuses, newborns, and immunocompromised people. It also causes a severe flu-like illness in pregnant women and self-limited gastrointestinal infections in immune-suppressed
Corresponding author: Srwa Ali Muhammed, M.D., research fields: bacteriology, microbial genetic and the effect of medicinal plants on bacterial virulence factors. E-mail: [email protected].
people [2, 3].
L. monocytogenes successfully contaminates processed foods because it persists on food-processing surfaces in the form of biofilms [4]. Unlike most other food-borne pathogens, L. monocytogenes grows during refrigeration.
Thus, a small inoculums at the time of packaging can lead to a significant burden of organisms by the time it reaches the consumer [4].
Biofilm-coated surfaces are particularly difficult to decontaminate, since bacteria in biofilms are more resistant to detergents, biocides, and antibiotics than are their planktonic counterparts [5, 6].
Very little is known about the molecular mechanisms of L. monocytogenes biofilm formation. Flagellum-mediated motility is important for biofilm formation by several gram-negative bacteria [7]. In contrast, flagella are implicated as surface adhesions
D
Biofilm Determination of Listeria monocytogenes That Isolated from Different Sources
806
early in L. monocytogenes surface attachment, but a role for motility in biofilm formation has not been examined [8].
2. Materials and Methods
2.1 Specimens’ Collection
A total of 150 samples were collected from the different sources of human infections (CSF and Blood) obtained from patients that bedded in Pediatric hospital in Sulaimania city, and also isolated from food sources like (yogurt, raw vegetables andraw milk, sausages).
2.2 Isolation and Identification
Microscopic examination with Gram stain was done. Biochemical test (Catalase test, Oxidase test, heamolysis, Sugar Fermentation test) in addition to biochemical testes Api for listeria also was done. For confirmation used colony PCR, PCR identification, Gel Electrphorsis detection.
2.3 Confirmation of L. monocytogenes by Colony PCR
Preparation of colony PCR production for L. monocytogenes PCR identification, 2 primers were selected based on the invasive association protein gene (hlyA) [9] as shown in Table 1.
All PCR reactions were performed in a final volume of 24 μL using 2 μL of distilled water was added to one PCR tube as negative control. Each reaction mixture contained 1 μL (10 µM) of forward primer; 1 μL (10 µM) of reverse primer and 15 μL of
Ultra-Pure DNase/RNase-Free distilled water: 1 μL of Mgcl; 1 μL of dNTps; 4 μL of reaction buffer. Then added one single colony of the sample and put under 95 ˚C for 5 min for destroying the cell wall. After that put 1 μL of DNA polymerase, the DNA amplification reactions were performed in thermal cycler. The cycling conditions for PCR illustrated in Table 2.
The presence of genomic DNA in all prepared samples was confirmed by 0.8% agarose gel electrophoresis followed by staining with ethidium bromide [10]. Listeria monocytogenes isolates were detected by PCR assay using primer sequence mentioned in Table 1.
2.4 Screening of Biofilm Production
Isolates were sub cultured in tryptic soy broth (TSB) for 18 h at 37 ºC. Before the experiments, all the strains were vortex for 5 min and the optical density adjusted to 1-1.5 at 600 nm by spectrophotometer according to McFarland scale to the overnight culture was standardised to a concentration of 3 × 108 Cfu/mL (PRO-LAB Diagnostics).
All isolates were studied for their capability to produce biofilm in a modified microplate quantitative assay. These strains were previously isolated from cerebrospinal fluid, blood and foods. Then each strains diluted by obtain 50 µL of sample to 950 µL broth aliquots of 200 µL transferred to pre-sterilized, 96-well polystyrene microtiter plates commercially available (Deltalab S. L., Spain), then incubated for 24 h at 37 ºC. After incubation, discard the bacterial
Table 1 Primer sequence for detection of L. monocytogenes.
Reference
(Ritu Arora et al.,2007) 456
Table 2 Thermal cycling protocols for detection of L. monocytogenes.
Species Initial
Denaturation Denaturation Annealing Extension
Extension Final Hold
L. monocytogenes 94 ˚C
Biofilm Determination of Listeria monocytogenes That Isolated from Different Sources 807
suspension totally, each well was washed with 200 µL sterile phosphate buffer solution (PBS) to remove the planktonic cells, then put on the hot plate for one hour at 60 ºC for fixation, 25 µL of 1% Crystal Violet was added to each well, shaking the plates three times to help the colorant to get the bottom of the well. After 15 min at room temperature, each well was washed with 200 µL sterile PBS to remove the planktonic cells and stain not adhered to the well. Only the adhered bacteria forming the biofilm were kept on the surface of the well. To determine the degree of biofilm formation, the absorbance was determined at wave length (450 to 630) nm in an ELISA microtiter (Botic England). Controls were performed with Crystal Violet binding to the wells exposed only to the culture medium without bacteria. The data obtained were used to classify the strains as high producers (OD between 0.19 and 0.09) or poor producers (OD lower than 0.09) [11].
3. Results
3.1 Isolation and Identification of Listeria Species
150 samples were obtained from hospitalized patients in pediatric hospital in sulaimanya city and also from different food sources. The samples obtained and transferred to the laboratory and L. monocytogenes
were identified by colony identification. Palcam listeria selective agar is recommended for the isolation of Listeria monocytogenes from foods, while inhibiting Gram-negative and most of the Gram-positive accompanying bacteria. The selectivity of this medium results from its content of polymyxin, acriflavin, ceftazidime, and lithium chloride. L.
monocytogenes breaks down the esculin in the medium to glucose and esculetin which reacts with ferric citrate producing a brownish black precipitate around the colonies of Listeria monocytogenes [12].
Biochemical tests: listeria species are positive for catalase test, and give a typical umbrella growth pattern in the motility test medium which is semisolid medium near the microaerophllic subsurface of the medium at (25 °C) [13]. Green colour colonies surrounded by a black zone on PALCAM agar plates, indicating esculinase positive, were detected for the hemolytic activity, by 330 streaked on 7% blood agar plates, and appearance of a clear zone around the growth area ,after incubation, was considered as a positive reaction [14]. All Listeria species hydrolyzed esculin to esculetin [15]. Listeria species are differentiated on the bases of sugar fermentation [16]. The result showed that L. monocytogenes fermented salcin, lactose, galactose and rhamnose, and did not ferment manitol, xylose, sucrose, and melibiose. L.
monocytogenes produced narrow zone of β-hemolysis [17], in addition to biochemical test Api of Listeria
spp. also was done as shown in Fig. 1. 6010 according Api listeria system for the identification of listeria.
Thus according of above study 10 isolates was obtained from 150 samples one isolate isolated from CSF and one from blood sample, six isolated from sausage and two isolates isolated from raw vegetable.
3.2 PCR Identification of L. monocytogenes
Four isolates of L. monocytogenes were identified by biochemical tests and produced biofilm subjected to PCR, and these four isolates were successfully
Biofilm Determination of Listeria monocytogenes That Isolated from Different Sources
808
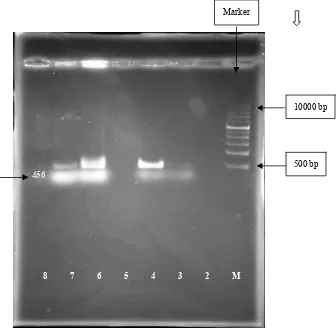
amplified the desired amplicon of 456 bp for listeria monocytogenes (E1 and E4) that isolated from CSF and blood sample and for 8 and E10 that isolated from food samples as shown in Fig. 2. The PCR was performed using hlyA primer pair of gene. Listeria monocytogenes has several important virulence markers. Among them, Listeriolysin O (LLO) is one of the important markers encoded by hlyAgene and is essential for disruption of phagocytic vacuole and release of bacteria into cytoplasm [9].
3.3 Screenning of (L. monocytogenes) Isolates That Produce Biofilm Using Trypticase Soy Broth (TSB
Difco) with 1% Glucose
Preparing the samples for detecting biofilm according [18] the absorbance was determined at wave length (450 to 630) nm in an ELISA
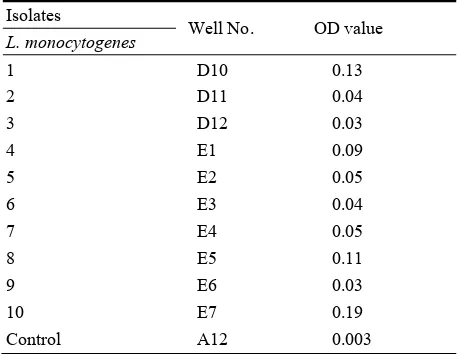
microtiter (Botic England) as show in Fig. 3. Fig. 3 show the capability of L. monocytogenes isolates from clinical sources and food appear in No. (D10, E1, E5 and E7) OD of each of them (0.13, 0.09, 0.11 and 0.19) respectively as shown in Table 3, the lowest or poor biofilm forming strains appear in No. (D11, D12, E2, E3, E4 and E6) that optical density (OD) reading are (0.04, 0.03, 0.05, 0.04, 0.05 and 0.03) respectively by comparing with control No.1 prepared in well (A12) that stained by crystal violate without putting any isolates and the OD reading is (0.003).
4. Discussion
Ten isolates L. monocytogenes were identified by biochemical tests and four of them that produced biofilm subjected to PCR, and all these isolates were
Fig. 2 Agarose gelelectrophoresis of the PCR amplication products of L. monocytogenes colonies amplified hlyA 456 bp sequence. Lane 1: 1Kb DNA ladder; Lane 2: negative control; Lane (3-4-6-7): samples positive for L. monocytogenes with hlyA 456 bp.
Marker
10000 bp
500 bp
8 7 6 5 4 3 2 M
Biofilm Determination of Listeria monocytogenes That Isolated from Different Sources 809
Fig. 3 Biofilm formation of L. monocytogenes were isolated from hospitalized patients and different food sources by using TSB.
Table 3 Determination of biofilm with a micro ELISA using Microtitre plate and reader using TSB medium supplemented with 1% glucose for(L. monocytogenes).
Isolates
Well No. OD value
L. monocytogenes
1 D10 0.13
2 D11 0.04
3 D12 0.03
4 E1 0.09
5 E2 0.05
6 E3 0.04
7 E4 0.05
8 E5 0.11
9 E6 0.03
10 E7 0.19
Control A12 0.003
successfully amplified the desired amplicon of 456 bp as shown in Fig. 2. The PCR was performed using primer pair of hlyAgene, there are many studies used hlyA gene as primer [19], used hlyA gene as PCR—target for the species specific detection of L. monocytogenes from fish samples.
The present study determined the ability of Listeria monocytogenes for producing biofilms. There are many studies that determined the ability of listeria to produce biofilm [20] was treated, the development of microbial biofilms of three pathogens (Listeria monocytogenes, Pseudomonas aeruginosa and
Candida albicans).
5. Conclusions
The results show that the Listeria monocytogenes
isolated from CSF and blood sample in pediatric hospital and also isolated from food samples specially isolated from sausage samples. The results show that
Listeria monocytogenes produce biofilm from 10 isolates four of the isolates produce high amount of biofilm.
References
[1] Ramaswamy, V., Cresence, V. M., Rejitha, J. S., Lekshmi, M. U., Dharsana, K. S., Prasad, S. P., and Vijila, H. M. 2007. “Listeria—Review of Epidemiology and Pathogenesis.” J. Microbiol. Immunol. Infect 40 (1): 4-13. PMID 17332901.
[2] Farber, J. M., and Peterkin, P. I. 1991. “Listeria monocytogenes, a Food-borne Pathogen.” Microbiol. Rev.
55: 476-511.
[3] Lorber, B. 2005. “Listeria monocytogenes.” In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, edited by Mandell, G. L., Bennett, J. E., and Dolin, R. Philadelphia, PA: Elsevier Churchill Livingstone.
[4] Moretro, T., and Langsrud, S. 2004. “Listeria monocytogenes: Biofilm Formation and Persistence in Food-processing Environments.” Biofilms 1: 107-121. [5] Hogan, D., and Kolter, R. 2002. “Why are Bacteria
Refractory to Antimicrobials?” Curr. Opin. Microbiol. 5: 472-477.
[6] Fux, C. A., Costerton, J. W., Stewart, P. S., and Stoodley, P. 2005. “Survival Strategies of Infectious Biofilms.”
No. 10 Control 1
Biofilm Determination of Listeria monocytogenes That Isolated from Different Sources
810
Trends Microbiol. 13: 34-40.
[7] O’Toole, G., Kaplan, H. B., and Kolter, R. 2000. “Biofilm Formation as Microbial Development.” Annu. Rev. Microbiol. 54: 49-79.
[8] Vatanyoopaisarn, S., Nazli, A., Dodd, C. E., Rees, C. E., and Waites, W. M. 2000. “Effect of Flagella on Initial Attachment of Listeria monocytogenes to Stainless Steel.”
Appl. Environ. Microbiol. 66: 860-863.
[9] Ritu, A., Alka, P., and Sant P. 2006. “A Comparative Study of Conventional Culture and PCR Method the Detection of Listeria monocytogenes from Artificially Inoculated Milk.” Indian Journal of Dairy Science 60 (5). [10] Dmitriy, V., Joseph, G., Christine, A., Robert, E. D., and Anthony, D. H. 2006: “Discovery of Natural Atypical Nonhemolytic Listeria seeligeri Isolates.” Applied and Environ. Microbiol. 72:2439-2448.
[11] Danhorn, R. M., Hentzer, M., Givskov, M. R., Parsek, and Fuqua, C. 2004. “Phosphorus Limitation enhances Biofilm Formation of the Plant Pathogen Agrobacterium Tumefaciens Through the PhoR-PhoB Regulatory System.” J. Bacteriol. 1186: 4492.
[12] Walter, F. 2000. “Food Borne Listeriosis.” Clinic Infect Disease 31: 770-775.
[13] Brugere-Picoux, O. 2008. “Ovine Listeriosis.” Small Ruminant Research 76: 12-20.
[14] Lazar, V. 2003. Microbial Adherence. Bucharest: Romanian Academy Publishing House.
[15] Feresu, S., and Jones, D. 1988. “Taxonomic Studies on Brochottrix, Erysipelothrix, Listeria and Atypical Lactobacilli.” J. Gen. Microbiol. 134: 1165-1183.
[16] Pagotto, F., Daley, E., Fraber, J., and Warburton, D. 2001. “Isolation of Listeria monocytogenes from All Food and Environmental Samples.” In Health Products and Food Branch Ottawa, Canada, MFHPB-30 method.
[17] Robinson, R. K., Batt, C. A., and Patel, P. D. 2000.
Encyclopedia of Food Microbiology. San Diego, CA: Academic Press.
[18] Mendez, C., Garza, E., Gulati, P., Morris, P. A., and Allen, C. A. 2005. “Isolationand Identification of Micro-organisms in JSC Mars-1 Simulant Soil.” Lunar and Planetary Science 36: 1-2.
[19] Swetha, C. S., Madhava Rao, T., Krishnaiah, N., and Kumar, A. V. 2012. “Detection of Listeria monocytogenes in Fish Samples by PCR Assay.” Annals of Biological Research 3 (4): 1880-1884. ISSN 0976-1233 CODEN (USA): ABRNBW. scholarsresearchlibrary.com.
Journal of Life Sciences 8 (2014) 811-814 doi: 10.17265/1934-7391/2014.10.003
Seroprevalence of Human Brucellosis in Kuku Dairy
Scheme, Khartoum State, Sudan
Tamador-Elkhansaa Elnour Angara1, Adil Abdel Rahman Ali Ismail2 and Nageeb Suliman Saeed3
1. Department of Development Studies & Extension, Sudan University of Science and Technology (SUST), Khartoum 11111, Sudan 2. Department of Preventive Veterinary Medicine, University of Bahri, Khartoum 11111, Sudan
3. Department of Microbiology, Faculty of Medicine, University of Khartoum, Khartoum 11111, Sudan
Received: May 20, 2014 / Accepted: October 28, 2014 / Published: October 30, 2014.
Abstract: A seroprevalence investigation of human brucellosis was carried out in Kuku Dairy Scheme, Sudan. A total of 176 serum samples were collected and screened by Rose Bengal Plate Test (RBPT). The positive sera were further examined using Tube Agglutination Test (TAT) and c-Elisa. The seropositivity was 15.9%, 14.8% and 11.4% using RBPT, TAT and c-Elisa respectively. Whereas, the active infection based on seropositivity and clinical signs were 4.6%, 4.6% and 2.3% in case of RBPT, TAT and c-Elisa respectively. Based on c-Elisa result the infected individuals were further subjected to clinical examination and treated with streptomycin and doxocycline for six weeks.
Key words: Malta fever, in contact persons, agricultural workers, Kuku Scheme.
1. Introduction
Brucellosis is one of the most common zoonosis in the world [1]. The disease is caused by organisms belonging to the genus Brucella, gram-negative, non-spore-forming, facultative, intracellular bacteria [2]. The centers for Disease Control and Prevention (CDC) classify B. abortus, B. melitensis and B. suis as “agents of mass destruction” and as category B organisms [3]. The occurrence of the disease in human is largely dependent on animal reservoir [4]. The disease is transmitted to humans by ingestion of infected food products, direct contact with an infected animal, or inhalation of aerosols [5]. Cattle are the most important livestock source of human brucellosis [6, 7]. Although the definite diagnosis of brucellosis is made by isolation of the organism from blood samples or other clinical specimens [8]. Human brucellosis is diagnosed on the basis of clinical findings and laboratory studies [9].
Corresponding author: Tamador-Elkhansaa Elnour Angara, Ph.D., associate professor, research field: public health economics. Email: [email protected].
Serodiagnosis is most commonly made on the basis of the Tube Agglutination Test (TAT). Other assays, including the (RBT) and the anti-brucella Coombs test. The ELISA is more sensitive than the TAT in diagnosis of brucellosis [10]. Studies on the infection in Saudi Arabia and Oman revealed that 20% of the population had serological evidence of exposure in southern Saudi Arabia [11] while 1% of healthy residents of Dhofar, Oman (mainly children) had serologic evidence of exposure [12]. The disease in agricultural workers was investigated in Bari, Southern Italy by standard tube agglutination test. None of the subjects examined had antibodies to Brucella [13]. In Castellon, Spain, the seroprevalence of brucellosis in agricultural workers was 3.1% based on Wright and Coombs tests; all sera were negative to Rose Bengal [14].
A 12.7% prevalence rate was reported in nomads community in Darfur states [15] using serological and bacteriological tests. In Chad brucellosis was investigated in three nomadic communities of Chad (Fulani cattle breeders, and Arab camel and cattle
D
Seroprevalence of Human Brucellosis in Kuku Dairy Scheme, Khartoum State, Sudan
812
breeders) by indirect ELISA, the seropositivity were 3.8% [16]. The combination therapies recommended by WHO for treatment of brucellosis are doxycycline plus rifampicin or doxycycline plus streptomycin [17]. Sudan was proved to be endemic with bovine brucellosis; Kuku Scheme was not an exception. There was no formal control strategy adopted to control the disease in cattle. The probability of the transmission of the disease to at risk population is very high; however, no data regarding human brucellosis in the scheme is available. This work aimed at providing data on human brucellosis among in contact individuals and the risk factors associated with the disease to highlight the seriousness of the problem and to draw the attention of the policy makers towards controlling the disease.
2. Materials and Methods
2.1 The Study Area
Kuku Scheme covers an area of about 2600 acre stretching out from the old riverain cultivation area on the Blue Nile banks, east of Khartoum North [18]. The project was established in 1963 by American aid to settle the semi nomadic tribes and to increase milk supply to the capital town by up grading the local cattle breeds. Currently the scheme is a part of East Nile locality one of the seven localities of Khartoum State [19].
2.2 Sample Collection and Testing:
A total of 176 out of 649 at risk were examined for brucellosis. Blood samples were collected using 5 cc disposable syringes. Case recording forms were used to collect data on the history and clinical symptoms. The blood samples were transferred to The National Health Laboratory for serological examination. Rose
Bengal Plate Test (RBT), was used as screening test as described by Alton et al. [20]. The positive sera were subject to Tube Agglutination Tests (TAT) as described by Meyer [21] and Competitive Enzyme-Linked Immunosorbent Assay (cELISA) tests using SVANOVIR®Brucella-Ab C-ELISA test kits. Actively infected persons were interviewed in depth and subjected to further clinical examination and treated with streptomycin and doxocycline for six weeks and followed up for 6 months for any relapse.
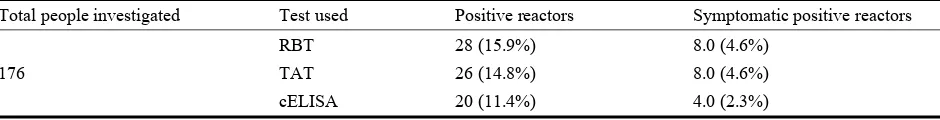
3. Results
A total of 176 sera were tested by using RBPT as screening test, the result is shown in Table 1 where a total of 28 (15.9%) serum samples reacted positive to the test. To confirm this result the positive sera were subject to TAT and c-Elisa. The seropositive samples were 26 (14.8%) and 20 (11.4%) based on TAT and c-Elisa respectively.
The positive sera of symptomatic individuals were 8 (4.6%), 8 (4.6%) and 4 (2.3%) in case of RBPT, TAT and c-Elisa respectively.
The study revealed that one individual who reacted positively to c-Elisa had symptoms of fever, headache, arthralgia, whereas another one suffered from fever, headache, arthralgia and night sweat and two individuals showed symptoms of fever, headache, arthralgia, night sweat and, fatigue as shown in Table 2.
4. Discussion
The result based on confirmatory c-Elisa and clinical signs indicated that the scheme is endemic with brucellosis and the infection rate was 2.3%. This infection occurred as a result of direct contacts with herds in the scheme. The infection rate among cattle
Table 1 Seroprevalence of human brucellosis Kuku Dairy Scheme.
Total people investigated Test used Positive reactors Symptomatic positive reactors
176
RBT 28 (15.9%) 8.0 (4.6%)
TAT 26 (14.8%) 8.0 (4.6%)
Seroprevalence of Human Brucellosis in Kuku Dairy Scheme, Khartoum State, Sudan 813
Table 2 Symptoms of individuals found serologically positive to c-Elisa.
Description (Symptoms) No infected Percent (%)
Fever, headache and arthralgia 1.0 0.6
Fever, headache, arthralgia and night sweating 1.0 0.6
Fever, headache, arthralgia, night sweat, fatigue 2.0 1.1
Total symptomatic people 4.0 2.3
population in the scheme was 24.9% based on c-Elisa [22].
The result obtained in this study is slightly less than the result of 12.7% reported in nomadic tribes in Southern Darfur-Sudan by Musa [15]. On the other hand the current result disagrees with the RBPT result obtained from Agricultural workers living in the coastal areas of Castellon, Spain [14] where all the participants reacted negative to RBPT in contrast to the current result where 15.9% of the participant reacted positive to the test. On the other hand the seroprevalence of 3.8% obtained from three nomadic communities of Chad by Schelling et al. [16] was much lower than that obtained in this study. Comparing the four-seroprevalence results of the agricultural workers: the current work, Darfur (nomads), Chad (nomads), Castellon, Spain and in Bari, Southern Italy. The prevalence rates obtained from Sudan are much alike in spite of the different production systems: sedentary in Kuku and nomadic in Darfur. The authors expect that the prevalence rates of the two nomadic communities in Darfur and Chad to be much alike than those of Kuku and Darfur. The two former communities are more similar alike with regard to geographical condition, pattern of life, type of cattle breed and animal husbandry practices, whereas the latter differ much in these aspects. The results of Spain and Italy differ largely from the results of Sudan and Chad this may be attributed to different factors including human behavior, and public health measures and control of the disease in animal. Unlike the case of Southern Saudi Arabia [11], the seroprevalence result of brucellosis in the general population in Dofar, Oman [12] is less than those in the agricultural workers.
5. Conclusions
The study proved that the scheme is endemic with brucellosis and that many cases passed undiagnosed. The recommendations were raising the awareness of in contact individuals, test the in contact population routinely, and formulation of a control strategy in animal population.
Acknowledgments
Prof. Musa Mohammed Khair from Faisal Professional Private Clinic was the Physician who examined the patients; Miss Zenab Fadlelmola who assisted in blood samples collection.
Ethical Considerations
This study carried out in The National Health Laboratory. The infected people were further clinically examined and treated in Faisal Professional Private Clinic.
References
[1] Caporale, V., Giovannini, A., Calistri, P., and Tittarelli, M. 2009. “An Approach to Developing Coordinated and Harmonised Actions for the Control of Brucellosis.” Presented at the 10th conference of the OIE Regional Commission for the Middle East Doha, Qatar, 26-29. [2] Ko, J., and Splitter, G. A. 2003. “Molecular
Host-pathogen Interaction in Brucellosis: Current Understanding and Future Approaches to Vaccine Development for Mice and Humans.” Clinical Microbiology Reviews 16 (1): 65-78.
[3] Elzer, P. H. 2002. “Brucellosis Vaccines for the 21st Century” In Proceedings of NIAA Annual Meeting, Louisiana State University Ag Center and School of Veterinary Medicine, Baton Rouge, LA.
Seroprevalence of Human Brucellosis in Kuku Dairy Scheme, Khartoum State, Sudan
814
[5] Gerald, E. M. 2001. “Brucella Infection.” eMedicine-Brucellosis: Article Medicine, Department of Veterinary Science, Jr, DO 111 Dalrymple Building, Baton Rouge, LA. Accessed April 8, 2014. ftp://ftp.cgiar.org/ilri/ICT/Theme%203/assam/consumer %20survey/eMedicine%20-%20CBRNE%20- %20Brucel losis%20%20Article%20by%20Gerald%20E%20Malone y,%20Jr,%20DO.htm.
[6] Collard, P. 1962. “Antibodies Against Brucellae in the Sera of Healthy Persons in Various Parts of Nigeria.” The WAMJ 89: 172-177.
[7] Alausa, K. O., and Awoseyi, A. 1976 “Brucellosis: the Situation in Western Nigeria.” Trop. geogr. Med. 28 (March): 54-59.
[8] Espinosa, B. J., Chacaltana, J., Mulder, M., Franco, M., P., Blazes, D. L., Gilman, R. H., Smits, H. L., and Hall, E. R. 2009. “Comparison of Culture Techniques at Different Stages of Brucellosis.” Am. J. Trop. Med. Hyg. 80 (April): 625-627.
[9] Young, E. J. 1995. “An Overview of Human Brucellosis.”
Clin. Infect. Dis. 21 (August): 283-290.
[10] Shapiro, D. S., and Wong, J. D. 1999. “Brucella.” In
Chief Manual of clinical microbiology 7th edition, edited by Murray, P. R. Washington.
[11] Robinson, A. 2003. “Guidelines for Coordinated Human and Animal Brucellosis Surveillance.” FAO Animal production and health Paper 156,Room. Accessed April 8, 2014. http://www.fao.org/3/a-y4723e.pdf.
[12] Scrimgeour, E. M., Mehta, F. R., and Suleiman, A. J. M. 1999. “Infectious and Tropical Diseases in Oman: A Review.” Am .J. Trop. Med. Hyg. 61 (6) Dec.: 920-925. [13] Monno, R., Fumarola, L., Trerotoli, P., Cavone, D.,
Giannelli, G., Rizzo, Ciceroni, C. L., and Musti, M. 2009. “Seroprevalence of Q Fever, Brucellosis and Leptospirosis in Farmers and Agricultural Workers in Bari, Southern Italy.” Ann. Agric. Environ. Med. 16 (2)
205-209.
[14] Villamarin-Vazquez, J. L., hiva-Nebot, and Arnedo-Pena, F. C. 2002. “Seroprevalence of Brucellosis in Agricultural Workers Living in the Coastal Areas of Castelon, Spain.” Salu Publica de Mexico 44 (2): 1-3. [15] Musa, M. T. 1995. “The Magnitude and the Problem of
Brucellosis in Darfur States and the Methods of Diagnosis and Control.” Ph.D. Thesis, Khartoum, Sudan. [16] Schelling, E., Diguimbaye, C., Daoud, S., Nicolet, J.,
Boerlin, P., Tanner, M., and Zinsstag, J. 2003. “Brucellosis and Q-fever Seroprevalence of Nomadic Pastoralists and Their Livestock in Chad.” J. Preventive Veterinary Medicine 61 (December): 279-293.
[17] Karabay, O., Sencan, I., Kayas, D., and Sahim, I. 2004. “Oflaxacin Plus Rifampicin Versus Doxycycline Plus Rifampicin in the Treatment of Brucellosis; A Randomized Clinical Trial.” Bio. Med. Central BMC Infectious Diseases 4:18. doi:10.1186/1471-2334-4-18. [18] Hadari, A. E., and Simpson, M. C. 1967. “The Production
and Marketing of Milk in Khartoum Province: An Economic Study of Traditional and Modern Methods.”
Research bulletin 7: 184.
[19] Angara, T-E. E. 1998. “Impact of Currency Devaluation on Dairy Production in Khartoum State.” M.Sc. Thesis, University of Khartoum, Khartoum Sudan.
[20] Alton, G. G., Jones, L. M., and Pietz, D. E. 1975. “Serological Methods.” In Laboratory Techniques in Brucellosis. Geneva: WHO, 64-124.
[21] Meyer, M. E. 1986. “Immune Response to Brucellae.” In
Manual of Clinical Laboratory Immunology, edited by Rose, N., Friedman, R. H., and Fahey, J. L. Washington, DC: American Society Microbiology, 385-387.
Journal of Life Sciences 8 (2014) 815-821 doi: 10.17265/1934-7391/2014.10.004
Detection of
Mycoplasma synoviae
Infection in Broiler
Breeder Farms of Morocco Using Serological Assays
and Real Time PCR
Nassik Saâdia1, Aboukhalid Rachid2, Azzam Falak2, Rahmatallah Naoufal1, Lahlou-Amine Idriss3, Fassi-Fihri Ouafaa4 and El Houadfi Mohammed1
1. Avian Pathology Unit, Department of Pathology and Veterinary Public Health, Agronomy and Veterinary Institute Hassan II, Rabat 10000, Morocco
2. Immunology and Biochemistry Laboratory, Faculty of Sciences Mohamed V-Agdal University, Rabat 10000, Morocco
3. University Mohammed V Souissi, Mohammed V Military Teaching Hospital, Research and Biosafety Level 3 Laboratory, Rabat 10000, Morocco
4. Microbiology Immunology and Infectious Diseases Unit, Department of Pathology and Veterinary Public Health, Agronomy and Veterinary Institute Hassan II, Rabat 10000, Morocco
Received: August 08, 2014 / Accepted: October 21, 2014 / Published: October 30, 2014.
Abstract: Mycoplasma synoviae (MS) is an economically important pathogenic agent of chickens, causing air sacculitis and synovitis. The diagnosis and monitoring of M. synoviae infection is usually made using serological assays, while confirmative diagnosis is made by isolation and identificationof the organism, because of the cross reaction between M. gallisepticum and M. synoviae. This study was conducted from 2011 to 2013 during which 11 broiler breeder flocks were sampled. These farms were located in all regions of morocco. The sampling was conducted as follows: Farms were visited on day one old chicks “day of importation from Europe”. 20 to 60 “chicks” were randomly sampled. At the age of 8, 16, 32 and 56 weeks, 60 blood samples and 60 tracheal swabs were collected at each sampling. The serological screening was performed using Rapid Slide Agglutination (RSA) according the OIE protocol and Indirect ELISA (IDEXX) according the manufacturer’s instructions. The molecular diagnosis was performed using a commercial kit of a duplex real time PCR (Life Biotechnology). The results revealed that one day old chicks were negative to MS by RSA and PCR, however they have a variable stock of maternal antibodies (Ig-Y) detected by iELISA. The seroprevalence found by RSA is variable and increase with the age (8thweek: 55%, 16th week: 91%, 32th and 56th week: 100%), the same profile was traced by PCR (8thweek: 36%, 16th week: 64%, 32th week: 82%, 56th week: 100%), however, all farms were positive by iELISA, from the first day to 56th weeks. These results show that MS infections are very frequent and very widespread among poultry breeder flocks, and showed a perfect agreement between serological tests and Real time PCR starting from 32th week of age.
Key words: Mycoplasma synoviae, Rapid Slide Agglutination, iELISA, PCR, broiler breeders flocks.
1. Introduction
Avian mycoplasmosis is caused by members of
Mycoplasmataceae family [1]. Mycoplasma
gallisepticum (MG) and Mycoplasma synoviae (MS) are considered the most pathogenic strains.
Corresponding author: Nassik Saâdia, Ph.D., research field: avian pathology. E-mail: [email protected].
Mycoplasma infections of avian species are both transmitted vertically, by egg transmission, and horizontally, by direct or indirect contact [2]. Currently, MG infections are rare except for multi-age egg production sites, whereasMS is considered one of the most frequent pathogenic avian mycoplasmas, because of its emergence in several countries [3, 4]. It is an important pathogenic agent of chickens, causing
D
Detection of Mycoplasma synoviae Infection in Broiler Breeder Farms of Morocco Using Serological Assays and Real Time PCR
816
air sacculitis and synovitis [5]. The economic losses in intensive production are caused by reduced growth rates, weight loss, condemnation at slaughter because of chronic respiratory disease in broilers and a suboptimal production of egg in layers [6].
Screening of poultry farms for pathogenic mycoplasma infections is usually accomplished with serological tests, especially Rapid Slide Agglutination (RSA) and enzyme-linked immunosorbent assay (ELISA), which can detect specific antibodies. However, serological tests may be insensitive or may give false-positive reactions. In fact, nonspecific reactions (false positive) were frequently observed by RSA, especially in flocks vaccinated with oil-emulsion vaccines (of tissue culture origin) against various agents [7]. Commercial ELISA kits are available and are often used to detect antibodies to MG and MS. ELISA detects basically Ig-Y class antibodies, whereas RSA detects IgM. In general, ELISA tests are slightly less sensitive but more specific than RSA [8]. Moreover, certain commercial indirect ELISA kits give false positive reactions with sera from chickens vaccinated with oil-emulsion vaccines, probably due to the presence of Serum Albumin in ELISA antigens [9].
Isolation and identification of mycoplasmas antigens is still the gold standard method for diagnosis and PCR is a rapid and sensitive test. Nevertheless, it is possible to find some nonspecific reactions with PCR, i.e., contamination with DNA resulting with false negative reactions due to inhibitors in clinical samples examined by PCR [10].
The aim of this study was to evaluate the prevalence of MS infection in broiler breeder flocks in Morocco at different ages and to compare serological tests and molecular diagnosis.
2. Materials and Methods
2.1 Sampling Procedure
The study was conducted from October 2011 to April 2013. Eleven broiler breeder flocks located in the
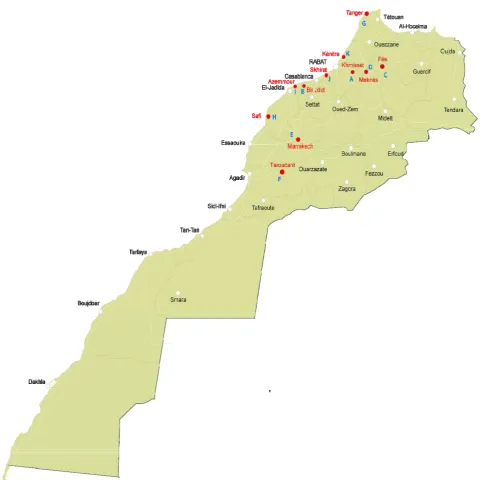
principal area of poultry production in Morocco were sampled (Fig. 1).
Serological screening and molecular diagnosis were conducted as follows:
First, 20 to 60 one day old chicks imported from Europe were sampled, before being placed in the farms. Then, every selected farm was visited at 8, 16, 32 and 56 weeks from introduction to collect 60 blood samples and 60 tracheal swabs. The sampling of the chickens was carried out from a single randomly selected house of each farm. During this investigation, all birds were apparently healthy and not vaccinated against MS; Birds were bled from the cutanea ulnae (wing) vein. Samples (blood and tracheal swabs) were transported under cold conditions for analysis in the Laboratory of Avian Pathology Unit at the Agronomy and veterinary Institute Hassan II in Rabat, Morocco.
After coagulation during 2 h at room temperature, sera were collected and sterile aliquots were maintained at 4 °C until use within the following 48 h. The tracheal swabs were stored at -20 °C until analysis.
2.2 Serology
Antibodies to MS were detected by RSA and Indirect ELISA (iELISA):
2.2.1 Rapid Slide Agglutination Test
Sera were tested according to the OIE protocol [11], using BIOVAC antigens©, MS (strain WVU-1853 colorful).
To avoid the false positive results caused by cross-reactions with other pathogenic mycoplasmas [7, 12] or non-specific reactions induced by some vaccines, the positive sera were diluted to 1/5 and inactivated at 56 °C during 30 min [11].
2.2.2 ELISA
Detection of Mycoplasma synoviae Infection in Broiler Breeder Farms of Morocco Using Serological Assays and Real Time PCR
817
Fig. 1 Map of Morocco showing farm’s localization.
Molecular Biology Analysis: DNA from five pooled tracheal swabs was extracted using the Pure Link® Genomic DNA Mini Kit (Invitrogen™ by Life technologies™, Foster City, CA) according to manufacturer’s instructions. Real time PCR reactions were conducted on a 7500 Fast Real Time PCR system (Applied Biosystems® by Life Technologies™, Foster City, CA) using VetMaxTM—Plus qPCR master mix with TaqMan Probe and primers designed by Life
technologies™ (Life Technologies™, Foster city, CA) according to the manufacture’s protocol.
3. Results
3.1 Serology
3.1.1 RSA
Detection of Mycoplasma synoviae Infection in Broiler Breeder Farms of Morocco Using Serological Assays and Real Time PCR
818
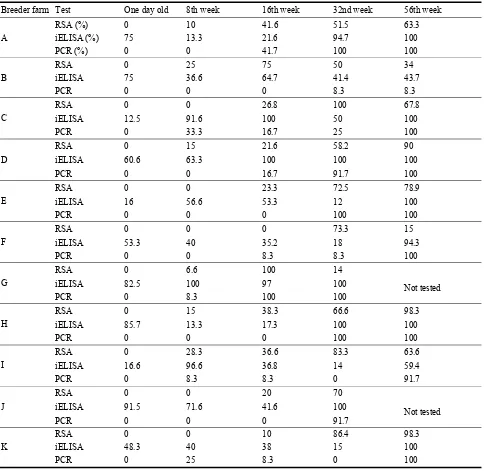
Table 1 Prevalence of Mycoplasma synoviae infection estimated by RSA, iELISA and real time PCR.
Breeder farm Test One day old 8th week 16th week 32nd week 56th week
A
RSA (%) 0 10 41.6 51.5 63.3
iELISA (%) 75 13.3 21.6 94.7 100
PCR (%) 0 0 41.7 100 100
B
RSA 0 25 75 50 34
iELISA 75 36.6 64.7 41.4 43.7
PCR 0 0 0 8.3 8.3
C
RSA 0 0 26.8 100 67.8
iELISA 12.5 91.6 100 50 100
PCR 0 33.3 16.7 25 100
D
RSA 0 15 21.6 58.2 90
iELISA 60.6 63.3 100 100 100
PCR 0 0 16.7 91.7 100
E
RSA 0 0 23.3 72.5 78.9
iELISA 16 56.6 53.3 12 100
PCR 0 0 0 100 100
F
RSA 0 0 0 73.3 15
iELISA 53.3 40 35.2 18 94.3
PCR 0 0 8.3 8.3 100
G
RSA 0 6.6 100 14
Not tested
iELISA 82.5 100 97 100
PCR 0 8.3 100 100
H
RSA 0 15 38.3 66.6 98.3
iELISA 85.7 13.3 17.3 100 100
PCR 0 0 0 100 100
I
RSA 0 28.3 36.6 83.3 63.6
iELISA 16.6 96.6 36.8 14 59.4
PCR 0 8.3 8.3 0 91.7
J
RSA 0 0 20 70
Not tested
iELISA 91.5 71.6 41.6 100
PCR 0 0 0 91.7
K
RSA 0 0 10 86.4 98.3
iELISA 48.3 40 38 15 100
PCR 0 25 8.3 0 100
RSA: Rapid Serum Agglutination
iELISA: Inderict Enzyme-Linked ImmunoSorbent Assay
farms became seropositive at this time. The infection rate was situated between 6.6% and 28.3%. At the 16th week, IgM class antibodies were detected in 10 farms. At the 32nd week, all farms became positive with a seroprevalence over 50%. The prevalence noted by RSA increased with the age and followed an exponential trend in 6 farms; however it decreased in 5 others (Table 1).
3.1.2 iELISA
Detection of Mycoplasma synoviae Infection in Broiler Breeder Farms of Morocco Using Serological Assays and Real Time PCR
819
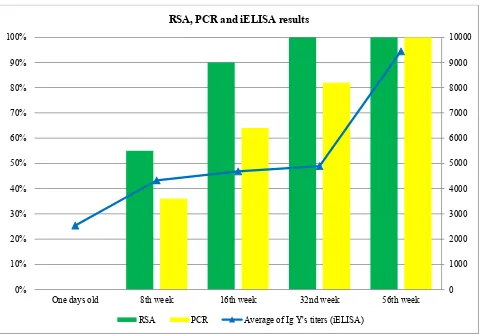
Fig. 2 Percentage of positive farms and antibody titers recorded by iELISA by age group.
3.1.3 Real Time PCR
The age of detection of MS infection by PCR was variable and increased following the age of birds. In fact, all samples of one day old chicks were negative to MS by real time PCR, but at 8 weeks of age 4 farms became positive and the infection rate was situated between 8.3% and 33.3%. At the 16th week the number of positive farms had increased to reach 7 farms, with a variable rate of infection (8.3% to 100%). At the 32nd week of age 9 farms became positive and at the end of this survey (56th week) all farms were positive by PCR, with rates of infection exceeding 90% except on farm with a low rate infection (8.3%) (Table 1).
3.2 Concordance Between RSA and PCR Results
The results obtained by RSA and PCR showed that the number of positives farm to MS increased with the
age of birds (Fig. 2). Despite the disagreement noted between RSA and real time PCR at 8 (6 disagreements), 16 (5 disagreements) and 32 weeks (2 disagreements), a perfect agreement was noted in the one day old chicks and at 56 weeks of age.
3.2.1 Discussion
RSA and iELISA used for serological screening in this study are most commonly used for the diagnosis of avian mycoplasmosis. They are generally recommended for monitoring flocks rather than testing individual birds [11] because of lack of specificity and/or sensitivity [7]. Results obtained show that one day old chicks were negative to MS by RSA, with a maternal stock of antibodies detected by iELISA. The disagreement between serological assays may be due to the fact that these serological techniques recognize two different immunoglobulin isotypes: IgM, which is detected by the RSA, appears within 2 to 3 d after
0 1000 2000 3000 4000 5000 6000 7000 8000 9000 10000
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
One days old 8th week 16th week 32nd week 56th week
RSA, PCR and iELISA results