www.elsevier.com / locate / bres
Research report
d
-, but not
m
- and
k
-, opioid receptor activation protects neocortical
neurons from glutamate-induced excitotoxic injury
a a,b a ,
*
Junhui Zhang , Gabriel G. Haddad
, Ying Xia
a
Department of Pediatrics, Yale University School of Medicine, 333 Cedar Street, LMP 3107, New Haven, CT 06520, USA
b
Department of Cellular and Molecular Physiology, Yale University School of Medicine, 333 Cedar Street, LMP 3107, New Haven, CT 06520, USA
Accepted 22 August 2000
Abstract
Recent observations from our laboratory have led us to hypothesize thatd-opioid receptors may play a role in neuronal protection against hypoxic / ischemic or glutamate excitotocity. To test our hypothesis in this work, we used two independent methods, i.e., ‘‘same field quantification’’ of morphologic criteria and a biochemical assay of lactate dehydrogenase (LDH) release (an index of cellular injury). We used neuronal cultures from rat neocortex and studied whether (1) glutamate induces neuronal injury as a function of age and (2) activation of opioid receptors (d, m and ksubtypes) protects neurons from glutamate-induced injury. Our results show that glutamate induced neuronal injury and cell death and this was dependent on glutamate concentration, exposure period and days in culture. At 4 days, glutamate (up to 10 mM, 4 h-exposure) did not cause apparent injury. After 8–10 days in culture, neurons exposed to a much lower dose of glutamate (100mM, 4 h) showed substantial neuronal injury as assessed by morphologic criteria (.65%, n523, P,0.01) and LDH release (n516, P,0.001). Activation of d-opioid receptors with 10 mM DADLE reduced glutamate-induced injury by almost half as assessed by the same criteria (morphologic criteria, n521, P,0.01; LDH release, n516, P,0.01). Naltrindole (10 mM), a d-opioid receptor antagonist, completely blocked the DADLE protective effect. Administration ofm- andk-opioid receptor agonists (DAMGO and U50488H respectively, 5–10 mM) did not induce appreciable neuroprotection. Also, m- or k-opioid receptor antagonists had no appreciable effect on the glutamate-induced injury. This study demonstrates that activation of neuronald-opioid receptors, but notm- and
k-opioid receptors, protect neocortical neurons from glutamate excitotoxicity. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Opioid receptors
Keywords: d-opioid receptors; Excitotoxicity; Hypoxia; Glutamate; Neurons; Protection
1. Introduction found that systemic administration of opioid receptor
agonists prolonged the survival period during hypoxia in Opioids are known to be inhibitory neurotransmitters mice in whole animal experiments. Furthermore, Bofetiado and their receptors, classified mainly as d-, m- or k- et al. [2] demonstrated that this protection is selectively subtypes, are widely distributed throughout the central blocked by d- but not by m- or k-opioid receptor antago-nervous system (CNS) [22,28,53,56]. These receptors and nists. Subsequently, several investigators have shown that their ligands have been shown to be important in a variety opioid receptor agonists induce cardioprotective effects of functions including pain modulation [25], cardiores- [45,49] byd-opioid receptor activation [46]. Finally, Chien piratory control [60] and neuronal activity [29]. In addi- et al. [4] reported that d-opioid agonists increased tissue tion, previous studies have suggested that opioid receptors preservation and survival time of organs before their use in play a role in increasing animal survival time during severe transplantation surgery. These studies, therefore, lead us to hypoxia [31,32]. For example, Mayfield and D’Alecy [33] believe that the opioid pathway is involved in tissue protection during hypoxia or ischemia and that this protec-tion is likely mediated viad-opioid receptors.
*Corresponding author. Tel.: 11-203-785-6101; fax: 1
1-203-737-Recently, we have shown that the turtle Pseudemys
6337.
E-mail address: [email protected] (Y. Xia). scripta elegans has a much higher density and binding
affinity of d-opioid receptors in the brain than the rat, k-opioid receptor agonist [36] and nor-binaltorphimine while m-opioid receptors and other membrane receptors / (nor-BNI), a selective k-opioid receptor antagonist [9]
3
channels have a much lower density in the turtle brain as were purchased from RBI (Natick, MA). [N-MePhe D
-4
compared to rat brain [52,54,56]. Clearly, the increased Pro ]-morphiceptin (PL017) was purchased from Penin-tolerance of the turtle to hypoxia [57] or to glutamate- sula Laboratory (Belmoont, CA). L-glutamic acid was induced injury [51] may not be linked to the presence of purchased from Sigma Chemical Co. (St Louis, MO).
3
thed-opioid receptors and these data provide only circum- Tritium-labeled DADLE ([ H]-DADLE) was purchased stantial evidence for a causal link. However, these results from New England Nuclear Co. (Boston, MA).
as well as the data from other laboratories detailed above
have certainly given us a rationale to hypothesize that 2.3. Preparation of neuronal cultures these receptors may be important in attenuating or
inhib-iting neuronal injury during stressful conditions, such as Primary neuronal cultures were done from the cortex of hypoxia, ischemia or increased micro-environmental gluta- embryonic day 16 and 17 rats as described previously [58]. mate. However, there is no direct evidence regarding the In brief, the animals were decapitated and cortical tissue role ofd-opioid receptors in neuronal injury and protection was collected under sterile conditions. The tissue was under hypoxic or ischemic conditions. dispersed using a 1-ml pipet and then passed through an 80 To test whether d-opioid receptors play a role in mm nylon mesh with a Teflon pestle. The cells were neuroprotection against glutamate-induced excitotoxicity in resuspended in a neuron-defined culture medium, serum-mammalian neurons, we performed the present study and free Neurobasal Medium (GIBCO, BRL, Grand Island, examined the effect of d-opioid receptor activation on NY), supplemented with B-27 (13), glutamine (0.5 mM), neurons exposed to glutamate. In addition, we studied the glutamate (25 mM) and combination of two antibiotics, effects ofm- andk-opioid receptors on the same neuronal penicillin (100 IU / ml) and streptomycin (100mg / ml). The model to determine whetherd-opioid receptors are unique cells were plated onto poly-D-lysine (100 mg / ml, Sigma,
6
and have a specific role in neuroprotection against ex- St. Louis, MO) coated 35 mm culture dishes at 1310 citotoxic injury. Since we [52,56] and others [48] have cells / ml / dish. Neurobasal medium and B-27 supplement shown that the forebrain, especially the neocortex and represent an optimized medium for sustaining the survival striatum, has the highest density of d-opioid receptors in of CNS neurons [3]. The medium supports long-term the rat brain, we used neocortical neurons in culture and survival and suppresses glial growth to ,2% of the total two independent methods to evaluate and quantitate neuro- cell population [29]. Cells were kept in a humidified nal injury in this study. We demonstrate that the activation atmosphere of 95% air and 5% CO at 378C. Half of the
2
of d-opioid receptors, but not the activation of m- and medium was replaced with fresh medium without gluta-k-opioid receptors, plays a major role in neuronal protec- mate every 3–4 days. Under our conditions, almost all tion against glutamate-induced injury. cells in the culture were typical neurons (Fig. 1).
2.4. Cell treatment
2. Materials and methods
For glutamate-induced neuronal injury, glutamate (0.1– 2.1. Animals
10 mM) was applied to neuronal cultures at 4, 8 or 10 days in vitro. After 4–24 h of glutamate exposure, neurons were Sprague–Dawley pregnant (embryonic day 16–17) rats
studied using morphologic criteria and an LDH assay (see were purchased from Charles River Laboratories
(Wil-below). To determine the effect of opioid receptor activa-mington, MA). All animal procedures were performed in
tion on glutamate-induced injury, DADLE (0.01–10mM), accordance with the guidelines of the Animal Care
Com-DAMGO (5–10 mM) or U50488H (5–10 mM) was co-mittee of Yale University School of Medicine, which is
administered with glutamate. To further confirm the spe-accredited by the American Association of Laboratory
cificity of the role of opioid receptor activation, opioid Animal Care.
receptor antagonists, Naltrindole (10 mM), b-FNA (10 mM), and nor-BNI (10 mM), were added onto cultures 2.2. Chemicals and regents
simultaneously with glutamate and opioid agonists. In
2 5 control experiments, dishes were treated using similar
[D-Ala ,D-Leu ]-enkephalinamide (DADLE), a selective
procedures as described above, but without the addition of d-opioid receptor agonist [12], Naltrindole, a highly
selec-2 glutamate, opioid agonists and antagonists.
tive d-opioid receptor antagonist [39–41], [D-Ala ,
N-4 5
MePhe , Gly -ol]-enkephalin (DAMGO), a selective m
-opioid receptor agonist [23], b-funaltrexamine (b-FNA), a 2.5. Morphologic studies selective m-opioid receptor antagonists [1], [2
and injured cells was counted before and after experiments. After subtracting the number of cells that were injured before each experiment, the number of injured cells was expressed as a percentage of the number of viable cells originally present. The quantification of cell injury was blinded. At least two fields were studied from each dish and the values from the same dish were averaged.
2.6. Lactate dehydrogenase assay
Since LDH release has been used as considered an index of cellular injury [27], we measured also LDH in neurons and in the medium using Sigma Lactate Dehydrogenase kit (Procedure No. 228-UV) and a Beckman DU-70 spec-trophotometer system. Culture medium was sampled and spun down to remove cells immediately after experiments. Once the medium sample was collected, neuronal cell collection was done using a scraper and mixture of Dulbecco’s Phosphate-Buffered Saline (PBS). Cortical neurons were then pipetted from the dish into a micro-centrifuge tube. The solution was then spun down and
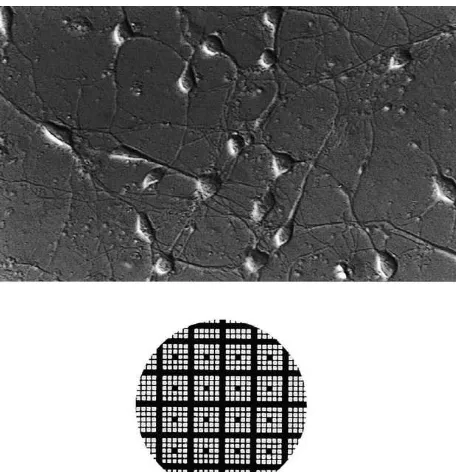
Fig. 1. Appearance of neocortical neurons in culture and a grid system vortexed to ensure homogeneity. Intracellular LDH was
for ‘‘same field quantification’’. Top, neocortical neurons in culture. Note then released into solution by ultrasonification of the that all cells are typical neurons with some having a pyramidal body and
sample for 1 min with ultrasonik (Barkmeyer Division,
long processes and others having more elliptical shapes. Bottom, a grid
2 Yucaipa, CA). Finally, the solution was centrifuged at
system delineating exact location of fields. Each square51 mm.
14 000 rpm with Eppendorf microcentrifuger for 4 min to remove cellular debris. Subsequently, a 50 ml of sample ‘‘same field ’’ method was developed in our laboratory and solution of either culture medium or neurons was added to used in this study for quantitative assessment of viable and a polystyrene cuvet containing 1 ml reagent (50 mmol / l injured neurons. In order to examine the same microscopic lactate, 7 mmol / l NAD in 0.05% sodium azide buffer pH field before and after experimental treatments, a grid 8.9). The cuvet was placed immediately into the spec-system for individual dishes was designed as shown in Fig. trophotometer, maintained at 258C. After stabilization for 1. The grid system with finely divided lines is drawn on a 30 s, absorbance at 340 nm was recorded at 30 s intervals transparent sheet that adheres to the bottom of the dish. for 2 min. The change in absorbency was then expressed in Before exposing to glutamate or other chemicals, mi- concentration units (U) per ml and then converted to crophotographs of cultured cells were taken using a phase- percent change as compare to control level in sister contrast microscope in order to establish a baseline viable / cultures.
injured cell count. First, each dish was placed under the
microscope and at least 2 fields, from each dish, were 2.7. Receptor binding chosen using a magnification of 103(310 with eyepiece).
Each field was randomly chosen except for over-crowded The receptor binding procedures were similar to those fields. Using a computerized system, print was then made described in our previous work [53]. In brief, culture from each field at 103 and at 323 magnification (310 dishes were collected after washing once with PBS. The with eyepiece). After the experimental treatment, previous- dishes were then incubated at room temperature for 60 min
3
ly examined culture dishes were re-examined and photo- with 4 nM [ H]-DADLE in 50 mM Tris–HCl buffer (pH micrographs were taken again. This was accomplished by 7.4), containing 100 nM NaCl, 40 mg / l bacitracin and 1 first locating the grid area using the 103 magnification mM PL017, which is a highly selectivem-ligand [21,8] and
3
Fig. 2. Age-related glutamate neurotoxicity in cultured neocortical neurons. Photomicrographs from the same fields were taken before (left, A and C) and after exposure (right, B and D) to glutamate for 4 h. Magnification 3203. (A) Control neurons at day 4; (B) 4-day cells treated with 2 mM glutamate for 4 h; (C) Control neurons at day 8; (D) 8-day cells treated with 100mM glutamate for 4 h. Injured neurons were illustrated by arrowheads. Arrowhead a, swelling. Arrowhead b, disrupted membrane. Arrowhead c, condensed soma. Arrowhead d, irregular cell body. Arrowhead e, disappearance of neurites. Arrowhead f, disappearance of cells. Note that there was no appreciable injury in 4-day neurons exposed to 2 mM glutamate, whereas more than most of cells were injured in 8-day culture exposed to only 100mM glutamate, suggesting that glutamate caused an age-dependent injury in neurons.
Radioactivity (CPM) was counted in a Packard beta statistical analysis with a non-paired, two-tailed Student’s counter. Non-specific binding was estimated using the t-test. Statistical significance was considered if the P-value same procedure, with excessive unlabeled DADLE in the was smaller than 0.05.
binding solution. The specifically-bound radioactive DADLE was determined by subtracting the non-specific
binding measurement from the total binding using only 3. Results
3
[ H]-DADLE.
3.1. Glutamate-induced neuronal injury is age-dependent 2.8. Statistics
Fig. 5. LDH activity in cultured neurons after exposure to glutamate at 4 and 8–10 days. LDH activity in neurons was expressed in percent of control. Results were expressed as the means6S.E.M. (n58 for all Fig. 3. Major difference in glutamate-induced neuronal injury at 4 days
groups). w, P,0.001 vs. control. Note that high concentration of versus 8–10 days. Cultured cortical neurons were exposed to glutamate
glutamate (up to 2 mM) had no apparent effect on LDH levels in 4-day for 4 h. Injury of neurons is expressed as a percentage of pre-treatment.
neurons, but a lower concentration of glutamate (100 mM) markedly Data were presented in means6S.E.M. (n58 for all groups).w, P,0.001
decreased LDH levels in 8- or 10-day neurons. vs. control (no glutamate). Note that there is no increase in neuronal
injury in 4 day cultures after exposure to 0.1–10 mM glutamate, while there was a significant increase in cell injury in 8 and 10 day cultures
exposed to only 100 mM glutamate, suggesting that glutamate-induced some cells disappearing. As shown in Fig. 4, there was neuronal injury greatly depends on age.
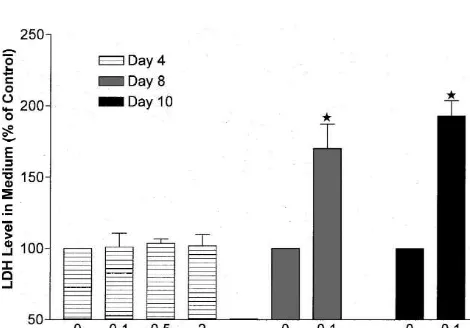
also a marked increase in LDH level in the medium of 8-or 10-day cultures after glutamate exposure, with a signifi-cant decrease in intracellular LDH activity (Fig. 5). injury (,5%) was detected (Figs. 2A, B and 3). Also, at
these concentrations of glutamate, LDH level did not
significantly change in the culture medium or intracellular- 3.2. d-opioid receptor agonist protects glutamate-ly (Figs. 4 and 5). In sharp contrast, glutamate at a induced neuronal injury
concentration as low as 100mM for 4 h induced significant
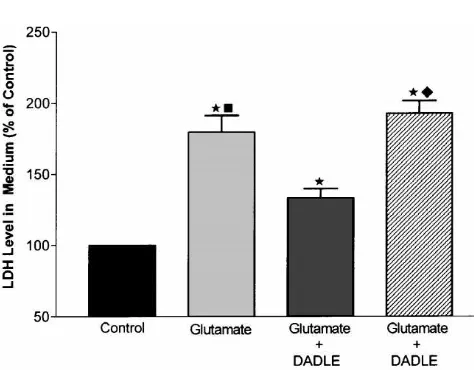
cell injury (.60%) in 8- or 10-day cortical neurons in Since the above experiments demonstrated that gluta-sister cultures (Figs. 2C, D and 3). Injured cells exhibited mate induced neuronal injury in neurons cultured for 8–10 neuronal swelling, neurite blebbing, and, in severe cases, days, we performed the rest of our studies at that age. neuronal membrane disruption and disintegration with After exposure to 100 mM glutamate for 4 h, more than 65% of the cell population appeared injured (n523, P, 0.01; Figs. 6A, B and 7), whereas only about 4% of neurons were injured if glutamate was not added (Fig. 7). A significant increase in medium LDH activity and a decrease in intracellular LDH activity were also observed when cultures were exposed to the same amount of glutamate (n516, P,0.01, Figs. 8 and 9). Application of DADLE at a concentration of 10 mM simultaneously with glutamate (100 mM) significantly reduced neuronal injury, on average, by almost half under the same test conditions (n521, P,0.05, Figs. 6C, D and 7). We further observed that increasing concentration of DADLE up to 50 mM could not further reduce neuronal injury (n54). With lower concentrations of DADLE (10–100 nM), only a slight protective effect was evident in some neuronal cultures (data not shown). Glutamate-induced LDH increase in the
Fig. 4. LDH levels in culture medium at different ages after glutamate medium was also significantly reduced by DADLE. As
exposure. LDH activity in the culture medium expressed in percent of shown in Fig. 8, LDH activity was almost 80% above control. Results were expressed as means6S.E.M. (n58 for all groups). control level in the glutamate alone group, but only about
w, P,0.001 vs. control. Note that in 4 day cultures exposed to glutamate
30% over control level in the glutamate plus DADLE
(up to 2 mM), LDH levels in the medium were similar to those in control,
group (n516, P,0.001, Fig. 8). As expected,
glutamate-while in 8 or 10 day cultures, LDH levels were significantly increased in
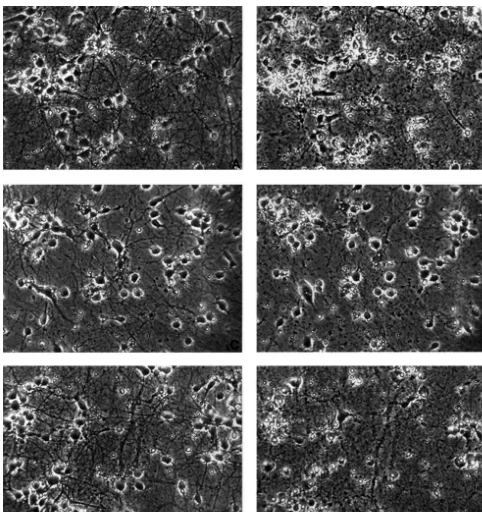
re-Fig. 6. Protective effect of DADLE against glutamate-induced neuronal injury. Photomicrographs of the same fields were taken before (left, (A), (C) and (E)) and after 4 h of cell treatment (right, (B), (D) and (F)) in 9-day cultures. Magnification 3203. (A) and (B) Glutamate induced injury. (A) Control (before treatment). (B) 100mM glutamate alone. (C) and (D) Neuroprotective effect of DADLE. (C) Control (before treatment). (D) 100mM glutamate plus 10mM DADLE. (E) and (F) Abolishment of DADLE neuroprotective effect by Naltrindole. (E) Control (before treatment). (F) 100mM glutamate plus 10 mM DADLE and 10 mM Naltrindole. Note that DADLE had a marked protection on glutamate-induced injury and this neuroprotection was completely blocked by Naltrindole at a concentration of 10mM.
versed by application of DADLE. LDH activity was in these cultured neurons. Receptor binding showed that significantly higher in neurons exposed to glutamate and d-opioid receptor expression was low at day 4 in culture DADLE as compared to glutamate only (n516, P,0.01, and this increased with age. On day 10,d-opioid receptor Fig. 9). density in neurons was 3-fold larger in magnitude than at 4
Fig. 9. Effect ofd-opioid receptor activation on LDH levels in 8–10 day Fig. 7. Effect of DADLE on glutamate-induced neuronal injury. The
neurons exposed to glutamate. LDH was assayed in the neurons after 4 h population of injured neurons was expressed as a percentage of the
of treatment. Data represent means6S.E.M. (n516 for control; n516 for control (before treatment). Data represent mean6S.E.M. (n513 for
glutamate; n514 for glutamate plus DADLE; n58 for glutamate plus control; n523 for glutamate; n521 for glutamate plus DADLE; n511 for
DADLE and Naltrindole). values obtained from 8 to 16 samples in six glutamate plus DADLE and Naltrindole). w, P,0.01 vs. control. ♦,
cultures. LDH activity in neurons was expressed in percent of the control. P,0.01 vs. glutamate plus DADLE. Note that DADLE (10mM) had a
w, P,0.01 vs. control.♦, P,0.05 vs. group of glutamate plus DADLE. significant neuroprotective effect on glutamate-induced neuronal injury
j, P,0.01 vs. group of glutamate plus DADLE. Note that LDH levels in and the protective effect of DADLE was completely blocked by 10mM
neurons are decreased with 100 mM glutamate and this decrease is Naltrindole.
reversed by 10mM DADLE. Also note that DADLE effect is completely blocked by 10mM Naltrindole.
3.3. d-receptor antagonist completely abolishes the neuroprotective effect by DADLE
To verify whether the protective effect of DADLE is mediated by d-opioid receptors, a d-opioid antagonist, Naltrindole (10 mM), was added simultaneously with glutamate (100 mM) and DADLE (10 mM). Microscopic analysis revealed that administering Naltrindole reversed the neuroprotective effect of DADLE. In neuronal cultures exposed to glutamate plus DADLE, as shown in Fig. 7, glutamate-injured neurons were reduced by almost half with existence of DADLE, while adding Naltrindole completely abolished this neuroprotection at the same time in sister cultures. In some cases, neuronal injury was even worse in Naltrindole-treated culture than glutamate only culture. On average, about 67% of neurons were injured in this group of glutamate plus DADLE and Naltrindole (n511, P,0.01, Figs. 6E, F and 7). This is similar to that of glutamate only group (Fig. 7).
Measurements of LDH release showed similar results. In
Fig. 8. Inhibition of glutamate-induced LDH release from neurons into
culture media with glutamate only, LDH activity was
media by activation ofd-opioid receptors in 8–10 day cultures. LDH was
79.4611.8% higher than control (n516, P,0.01; Fig. 8).
measured in culture medium after 4-h of cell treatment. Data represent
means6S.E.M. values (n516 for control; n516 for glutamate; n516 for This increased LDH level was markedly attenuated by
glutamate plus DADLE; n58 for glutamate plus DADLE and Naltrin- DADLE. Fig. 8 shows that the media containing both dole). LDH activity in culture medium was expressed in percent of the glutamate and DADLE had an LDH level of 33.166.7% control.w, P,0.01 vs. control.♦, P,0.05 vs. glutamate plus DADLE.
higher than control. As compared to glutamate only group,
j, P,0.01 vs. glutamate plus DADLE. Note that LDH is increased with
the increased LDH level was reduced by 58.3% (n516,
100 mM glutamate and this increase is reduced by 10 mM DADLE.
and Naltrindole showed a significantly higher activity level in the media, i.e., 192.568.8% of control (n58, P,0.01 when compared to the group of glutamate and DADLE; Fig. 8).
Intracellular LDH activity after glutamate exposure was significantly reduced by more than 20% (78.763.4% in the glutamate group vs. 100% in control, n516, P,0.01; Fig. 9). This decrease could be largely reversed by DADLE (n514, P,0.01) as shown in Fig. 9. Upon administration of Naltrindole, the effect of DADLE on LDH activity in neurons was completely abolished and reached a level of 78.46 2.9% of control (n58) which was very similar to that of glutamate alone group (P.0.05, Fig. 9).
3.4. Activation ofm- ork-opioid receptors has no appreciable effect on glutamate-induced neuronal injury
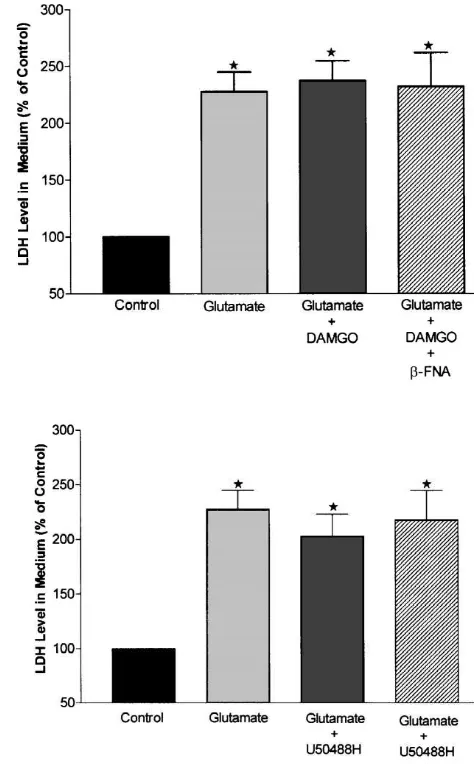
To determine whether m- ork-opioid receptors have an effect on glutamate-induced injury, we used DAMGO and U50488H to stimulate m- and k-opioid receptors respec-tively in exactly the same experimental conditions as in the studies of d-opioid receptors. LDH measurements showed that 100 mM glutamate induced a similar change in LDH release as shown above, i.e., a marked increase in LDH level in the culture medium (122.1619.3% over control, n58, P,0.01). This increase in LDH level was not affected by 5mM of DAMGO or U50488H. In the culture groups of glutamate plus DAMGO or U50488H, LDH activity increased by 133.4635.8% and 141.6633.1% respectively. There was no statistical difference between these groups and the glutamate only group (n58, P.0.05). Adding m- or k-opioid receptor antagonists (b-FNA or nor-BNI, 10mM) to opioid agonist groups had no effect on
Fig. 10. Effects ofm- andk-opioid receptor activation and inhibition on
LDH levels in the media (n58, P.0.05). To determine
glutamate-induced LDH release from neurons into media. Experiments
whether an increase in m- or k-opioid agonist
concen-were performed in the same way as in Fig. 8 except for application of
trations induces neuroprotection, we repeated the above
DAMGO, b-FNA (panel A), U50488H and nor-BNI (panel B). Data
experiments with 10 mM DAMGO and U50488H and represent means6S.E.M. values. LDH activity in culture medium was found similar results (Fig. 10). expressed in percent of the control. w, P,0.01 as compared to control (n58 for all groups). Note thatm- ork-opioid agonists and antagonists
The measurement of intracellular LDH activity further
had no appreciable effect on glutamate-induced neuronal injury.
demonstrated that m- ork-opioid receptors have no neuro-protection against glutamate-induced injury. In the group
of glutamate alone, LDH level in neurons decreased by ceptors, we limited our analysis to LDH measurement and 21.864.7% as compared to control level (n57, P,0.01). did not assess the neuronal injury with more time-consum-In glutamate plus 5 mM DAMGO group from sister ing morphological quantification.
cultures, LDH level in neurons showed a decrease by 28.266.3% as compared to control level (n57, P,0.01
when compared to control group, P.0.05 when compared 4. Discussion
to glutamate alone group). In the group of glutamate plus 5
is unique in protecting neurons from neurotoxicity of high [34,46]. Although dextrorphan, an opioid derivative, has glutamate exposure. been observed to protect against neuronal injury induced Since the outcome variables used are important for by brief exposure to glutamate [5] and to reduce neocorti-injury assessment of neurons, we have used, in this study, cal ischemic or hypoxic damage [16–18], it has been two independent methods. First, we used the method of shown to have little or no opioid action [11]. It is a ‘‘same field quantification’’ which has been developed non-competitive NMDA receptor antagonist [42], directly recently in our laboratory. Although it is very time-con- acting on NMDA receptors and not ond-opioid receptors suming, the major advantage of this technique is clearly [26]. Hence, it has been difficult to ascertain from previous the accuracy it provides in evaluating cellular injury since studies whether the neuronal protection against ischemia or the exact same field is compared before and after the hypoxia is directly related to thed-opioid receptor pathway intervention. This method therefore yields more consistent system and whether activation ofd-opioid receptors in the and reliable results than random field quantification as brain plays a role in increased survival of the animal. This neuronal survival may vary from area to area, even in the is the first study, indeed, to clearly show that opioid same culture dish. We also performed LDH assays. Unlike receptors and in particular the d-opioid receptor system, the morphologic results which focus on some fields in each substantially protect neurons against glutamate-induced dish, LDH is an injury estimate of the whole tissue culture injury since this effect was completely abolished by d -[30,38]. It is important to note that both LDH release and opioid receptor antagonists.
our morphological quantification have correlated well, as Our binding data show that d-opioid receptor density documented in Fig. 3 (morphology) and Fig. 4 (LDH). increases with neuronal development. This is very con-It is well known that glutamate is a mediator of neuronal sistent with what we have seen in in-vivo cortex and other death [7] in a number of pathophysiologic conditions. brain regions, i.e., very low density at birth and the density Glutamate neurotoxicity may contribute to the pathogene- increases during postnatal development with a peak level sis of human central neuronal loss induced by hypoxia, in adulthood [53,56]. However, this result may seem to ischemia, hypoglycemia, epilepsy, trauma and other insults contradict the data showing that neonatal neurons are more [6,43,44]. It would be very helpful therefore if we under- resistant to hypoxia or ischemia than mature neurons stand well enough the mechanisms underlying glutamate- [20,57] or data in this work showing that day 4 cultures, in induced injury to intervene pharmacologically and protect contrast to those at day 8–10, are more resistant to neurons from injury. One of the important clues for our glutamate excitotoxicity. We believe that the answer is not rationale to study the role of d-opioid receptors was our simple but may have to do with the following observa-observation in turtle central nervous system. Turtle neu- tions. First, immature neurons or early neuronal cultures rons are much more resistant to hypoxia and glutamate may have different mechanisms for glutamate or hypoxic excitotoxicity than rat neurons as demonstrated in our resistance. For example, intracellular calcium handling laboratory [57] and others [13,51]. Of major interest is that may be different [14,50]; glycogen stores may be larger recently, we have found thatd-opioid receptor density and and Na-dependent exchanger expression may be lower binding affinity are much higher in turtle than rat brain [24]; and expression of glutamate receptors and nitric [56]. In sharp contrast, the density of m-opioid receptors oxide synthase may be different. Second,d-opioid receptor [56] and other membrane receptors / channels such as expression may occur but may not have an effect unless
1
sulphonylurea receptors [52] and Na channel [54] was the ligand is present; hence the presence of receptors is not much lower in the turtle brain than rat brain. These data tantamount to protection from excitotoxicity. Lastly, it is lead us to speculate that d-opioid receptors may play a possible that they have lost what the newborn uses unique role in stress-resistance in turtle brains, although effectively to resist against neurotoxicity.
protec-[12] P.S. Eriksson, E. Hansson, L. Ronnback, Delta and kappa opiate
tive effect of d-opioid receptors. It is also possible that
receptors in primary astroglial cultures from rat cerebral cortex, d-opioid receptors modulated protein kinase C (PKC)
Neurochem. Res. 15 (1990) 1123–1126.
activity [59] may play a role in the neuroprotection. In [13] Z.C. Feng, M. Rosenthal, T.J. Sick, Suppression of evoked po-fact, there is evidence showing that the cardioprotective tentials with continued ion transport during anoxia in turtle brain,
effect of opioid is mediated via a PKC pathway [35] and Am. J. Physiol. 255 (3 pt 2) (1988) R478–R484.
21
[14] J.E. Friedman, G.G. Haddad, Major differences in Ca response to
that PKC activation inhibits glutamate-induced cytotoxicity i
21 anoxia between neonatal and adult rat CA1 neurons: role of Cao in a neuronal cell line [10]. In addition, modulation of 1
and Na , J Neuronsci. 13 (1993) 63–67.
21 o
voltage-dependent Ca channels may be involved in the [15] R.M. Fryer, A.K. Hsu, J.T. Eells, H. Nagase, G.J. Gross, Opioid-d-opioid receptor-mediated neuronal protection. Since induced second window of cardioprotection potential role of mito-there is evidence showing that DADLE modulates voltage- chondrial KATPchannels, Circ. Res. 84 (1999) 846–851.
21
[16] C.P. George, M.P. Goldberg, D.W. Choi, G.K. Steinberg,
Dex-dependent Ca currents [1]. We believe that further
tromethorphan reduces neocortical ischemic neuronal damage in
studies are needed to elucidate the molecular and cellular
vivo, Brain Res. 440 (1988) 375–379.
mechanisms underlyingd-opioid receptor-mediated
neuro-[17] M.P. Goldberg, P.C. Pham, D.W. Choi, Dextrorphan and
dex-protection. tromethorphan attenuate hypoxic injury in neuronal culture,
Neuro-sci. Lett. 80 (1987) 11–15.
[18] M.P. Goldberg, H. Monyer, D.W. Choi, Hypoxic neuronal injury in vitro depends on extracellular glutamine, Nerosci. Lett. 94 (1988)
Acknowledgements 52–57.
[19] G.G. Haddad, D.F. Donnelly, O2 deprivation induces a major
This work was supported by March of Dimes and NIH depolarization in brain stem neurons in the adult but not in the neonatal rat, J. Physiol. 429 (1990) 411–428.
(R01 HD-34852) grants to YX and NIH grants R01
HL-[20] G.G. Haddad, C. Jiang, O2 deprivation in the central nervous
39924 and P01 HD-32573 to GGH. The authors thank Ms.
system: on mechanisms of neuronal response, differential sensitivity
Ningyuan Chen for her technical assistance in neuronal
and injury, Prog. Neurobiol. 40 (1993) 277–318.
culture. [21] K.N. Hawkins, M. Morelli, K. Gulya, K.J. Chang, H.I. Yamamura,
3 3 4
Autoradiographic localization of [ H] [Mephe , D-pro
]morphicep-3
tin ([ H]PL017) to mu opioid receptors in rat brain, Eur. J. Pharmacol. 133 (1987) 351.
References [22] J.M. Hiller, L.Q. Fan, Laminar distribution of the multiple opioid
receptors in the human cerebral cortex, Neurochem. Res. 21 (1996) [1] C.G. Acosta, H.S. Lopez,dopioid receptor modulation of several 1333–1345.
21 21
voltage-dependent Ca( ) currents in rat sensory neurons, J. Neuro- [23] S.D. Hocherman, M. Randic, Reduction of NMDA-induced Ca sci. 19 (1999) 8337–8348. transients by a m-opioid receptor agonist in dorsal horn neurons, [2] D.M. Bofetiado, K.P. Mayfield, L.G. D’Alecy, Alkaloid d agonist Neuroreport 8 (1997) 3061–3065.
(1) 21
BW 373U86 increases hypoxia tolerance, Anesth. Analg. 82 (1996) [24] M. Juhaszova, M. Ruscak, Na -Ca exchange in the rat brain
1237–1241. during ontogeny, Gen. Physiol. Biophys. 10 (1991) 281–286.
[3] G.J. Brewer, J.R. Torricelli, E.K. Evege, P.J. Price, Optimized [25] R. Kanjhan, Opioids and pain, Clin. Exp. Pharmacol. Physiol. 22 survival of hippocampal neurons in B27-supplemented Neurobasal, (1995) 397.
a new serum-free medium combination, J. Neurosci. Res. 35 (1993) [26] H. Kato, G.K. Kanellopoulos, S. Matsuo, Y.J. Wu, M.F. Jacquin, C.Y.
567–576. Hsu, D.W. Choi, N.T. Kouchoukos, Protection of rat spinal cord
[4] S. Chien, P.R. Oeltgen, J.N. Diana, R.K. Salley, T.-P. Su, Extension from ischemia with dextrorphan and cyloheximide: effects on of tissue survival time in multigrain block preparation with a delta necrosis and apoptosis, J. Thoracic. Cardiovasc. Surg. 114 (1997)
2 5
opioid DADLE ([D-Ala , D-Leu ]-enkephalin), J. Thorac. Cardiov- 609–618.
asc. Surg. 107 (1994) 964–967. [27] J.Y. Koh, D.W. Choi, Quantitative determination of glutamate [5] D.W. Choi, Dextrorphan and dextromethorphan attenuate glutamate mediated cortical neuronal injury in cell culture by lactate
dehydro-neurotoxicity, Brain Res. 403 (1987) 333–336. genase efflux assay, J Neurosci. Meth. 20 (1987) 83–90. [6] D.W. Choi, Glutamate neurotoxicity and diseases of the nervous [28] A. Mansour, H. Khachaturian, M.E. Lewis, H. Akil, S.J. Watson,
system, Neuron 1 (1988) 623–634. Autoradiographic differentiation of mu, delta and kappa opioid [7] D.W. Choi, S.M. Rothman, The role of glutamate neurotoxicity in receptors in the rat forebrain and midbrain, J. Neurosci. 7 (1987)
hypoxia-ischemic neuronal death (Review), Annu. Rev. Neurosci. 2445–2464.
13 (1990) 171–182. [29] J. Manzanares, R.A. Durham, K.J. Lookingland, K.E. Moore, Delta-[8] J.A. Clark, L. Liu, M. Price, B. Merci, M. Edelson, G.W. Pasternak, Opioid receptor-mediated regulation of central dopaminergic
neu-Kappa opiate receptor multiplicity: evidence for two U50, 488- rons in the rat, Eur. J. Pharmacol. 249 (1993) 107–112.
sensitive kappa 1 subtypes and a novel kappa 3 subtype, J. [30] G.R. May, W.S. Rowand, J.G. McCormack, R.D. Sheridan, Neuro-Pharmacol. Exp. Ther. 251 (1989) 461–468. protective profile of lifarizine (RS-87476) in rat cerebrocortical [9] F.C. Dalman, K.L. O’Malley, Kappa-opioid tolerance and depen- neurons in culture, Br. J. Pharmacol. 114 (1995) 1365–1370.
dence in cultures of dopaminergic midbrain neurons, J. Neurosci. 19 [31] K.P. Mayfield, L.G. D’Alecy, Role of endogenous opioid receptors (1999) 5750–5757. in the acute adaptation to hypoxia, Brain Res. 582 (1992) 226–231. [10] J.B. Davis, P. Maher, Protein kinase C activation inhibits glutamate- [32] K.P. Mayfield, L.G. D’Alecy, Delta-1 opioid receptor dependence of induced cytotoxicity in a neuronal cell line, Brain Res. 652 (1994) acute hypoxic adaptation, J. Pharmacol. Exp. Ther. 268 (1994)
169–173. 74–77.
[11] C. Du, R. Hu, C.Y. Hsu, D.W. Choi, Dextrorphan reduces infarct [33] K.P. Mayfield, L.G. D’Alecy, Delta-1 opioid agonist acutely in-volume. Vascular injury, and brain edema after ischemic brain injury, creases hypoxic tolerance, J. Pharmacol. Exp. Ther. 268 (1994)
[34] T. Miki, J. Downey, Opioid receptors participate in ischemic receptor agonist, reduces infarct size via activation of Gi / o protein preconditioning in rabbits (Abstract), J. Mol. Cell Cardiol. 28 (1996) and KATPchannels, Am. J. Physiol. 274 (1998) H909–H914. A187. [48] A. Tempel, R.S. Zukin, Neuroanatomical patterns of them,d, andk
[35] T. Miki, M. Cohen, J. Downey, Opioid receptor contributes to opioid receptors of rat brain as determined by quantitative in vitro ischemic preconditioning through protein kinase C activation in autoradiography, Proc. Nalt. Acad. Sci. USA 84 (1987) 4308–4312. rabbits, Mol. Cell Biochem. 186 (1998) 3–12. [49] A. Tsuchida, T. Miura, M. Tanno, Y. Nozawa, H. Kita, K. [36] W. Muller, S. Hallermann, D. Swandulla, Opioidergic modulation of Shimamoto, Time window for the contribution of the delta-opioid
1
voltage-activated K currents in magnocellular neurons of the receptor to cardioprotection by ischemic preconditioning in the rat supraoptic nucleus in rat, J. Neurophysiol. 81 (1999) 1617–1625. heart, Cardiovasc. Drugs Ther. 12 (1998) 365–373.
[37] C. Nyakas, B. Buwalda, P.G.M. Luiten, Hypoxia and brain develop- [50] A. Verkhratsky, E.C. Toescu, Calcium and neuronal ageing (Re-ment, Progr. Neurobiol. 49 (1996) 1–51. view), Trends Neurosci. 21 (1998) 2–7.
[38] M. Okamoto, S. Mori, M. Ichimura, H. Endo, Chondroitin sulfate [51] A.M. Wilson, A.R. Kriegestein, Turtle cortical neurons survive proteoglycans protect cultured rat’s cortical and hippocampal neu- glutamate exposures that are lethal to mammalian neurons, Brain rons from delayed cell death induced by excitatory amino acids, Res. 540 (1991) 297–301.
Neurosci. Lett. 172 (1994) 51–54. [52] Y. Xia, G.G. Haddad, Major differences in CNS sulfonylurea [39] P.S. Portoghese, M. Sultana, A.E. Takemori, Naltrindole, a highly receptor distribution between the rat (newborn, adult) and turtle, J.
selective and potent non-peptidedopioid receptor antagonist, Eur. J. Comp. Neurol. 314 (1991) 278–289.
Pharmacol. 146 (1988) 185–186. [53] Y. Xia, G.G. Haddad, Ontogeny and distribution of opioid receptors [40] P. S Portoghese, M. Sultana, H. Nagase, A.E. Takemori, Application in the rat brainstem, Brain Res. 549 (1991) 181–193.
of the message–address concept in the design of highly potent and [54] Y. Xia, G.G. Haddad, Neuroanatomical distribution and binding selective non-peptidedopioid receptor antagonists, J. Med. Chem. properties of saxitoxin sites in the rat and turtle CNS, J.Comp.
31 (1988) 281–282. Neurol. 330 (1993) 363–380.
1 [41] P.S. Portoghese, M. Sultana, A.E. Takemori, Design of pep- [55] Y. Xia, G.G. Haddad, Effect of prolonged O deprivation on Na2
tidomimetic d opioid receptor antagonists using the message–ad- channels: differential regulation in adult versus fetal rat brains, dress concept, J. Med. Chem. 33 (1990) 1714–1720. Neuroscience 94 (1999) 1231–1243.
[42] C.K. Rokkas, L.R. Helfrich Jr, D.C. Lobner, D.W. Choi, N.T. [56] Y. Xia, G.G. Haddad, Major differences ind-opioid receptor density Kouchoukos, Dextrorphan inhibits the release of excitatory amino and binding affinity between turtle and rat brains, Soc. Neurosci. acids during spinal cord ischemia, Ann. Thoracic Surg. 58 (1994) Abstr. 25 (1999) 579.
312–319. [57] Y. Xia, C. Jiang, G.G. Haddad, Oxidative and glycolytic pathways in [43] S.M. Rothmen, J.W. Olney, Glutamate and pathophysiology of rat (newborn, adult) and turtle: Role in anoxia, Am. J. Physiol. 262
hypoxic–ischemic brain damage, Ann. Neurol. 19 (1986) 105–111. (1992) R595–R603.
[44] S.M. Rothman, J.W. Olney, Excitotoxicity and the NMDA receptor, [58] H. Xiang, D.W. Hochman, H. Saya, T. Fujiwara, P.A. Schwartzkroin, Trends Neurosci. 10 (1987) 299–302. R.S. Morrison, Evidence for p53-mediated modulation of neuronal [45] J.J. Schultz, A.K. Hsu, G.J. Gross, Morphine mimics the cardio- viability, J. Neurosci. 16 (1996) 6753–6765.
protective effect of ischemic preconditioning via a glibenclamide- [59] S.H. Yoon, W. Jin, R.J. Spencer, H.H. Loh, S.A. Thayer,
Desensiti-21
sensitive mechanism in the rat heart, Circ. Res. 78 (1996) 1100– zation of delta-opioid-induced mobilization of Ca stores in
1104. NG108-15 cells, Brain Res. 802 (1998) 9–18.