Dilinoleoylphosphatidylcholine protects human low density
lipoproteins against oxidation
Khursheed P. Navder
a,b, Enrique Baraona
a, Charles S. Lieber
a,*
aAlcohol Research and Treatment Center,Bronx Veterans Affairs Medical Center and Mount Sinai School of Medicine,New York,
NY 10468, USA
bNutrition and Food Science in Urban Public Health Program,Hunter College of C.U.N.Y.,New York,NY 10010, USA
Received 20 February 1999; received in revised form 5 October 1999; accepted 3 November 1999
Abstract
LDL oxidation may promote atherosclerosis. We found that polyenyphosphatidylcholine (PPC), a mixture of polyunsaturated phospholipids extracted from soybeans, has antioxidant effects in in vivo models of oxidative stress. To assess whether components of PPC affect the in vitro oxidizability of LDL, plasma from 15 healthy volunteers was incubated with 10 mM of
either dilinoleoyl-, palmitoyl-linoleoyl-, linoleoyl-palmitoyl- or distearoyl-phosphatidylcholine as well as 10 mM and 1 mM a-tocopherol. LDL oxidation was initiated with 5 mM Cu2+ sulfate and monitored by conjugated diene production, or with
2,2%-azobis (2-amidinopropane) dihydrochloride, a free radical generator, and monitored by O
2 consumption. After addition of
Cu2+, the lag phase (indicative of resistance of LDL to oxidation) was longer (140% of controls;PB0.001) for LDL incubated
with dilinoleoyl-, but not with the other phosphatidylcholine species. This effect was similar to that of 1 mMa-tocopherol (135%).
After addition of 2,2%-azobis (2-amidinopropane) dihydrochloride, the inhibition time (also reflecting the antioxidant content of LDL) was prolonged (PB0.001) for a-tocopherol (206%) and dilinoleoyl-(188%), but not for distearoyl-phosphatidyl-choline.
Thus, dilinoleoyl-phosphatidylcholine (the main component of PPC) protects against LDL oxidation, a possible mechanism for its reported anti-atherosclerosis effects. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Phospholipids; Anti-oxidants; Low-density lipoproteins; Atherosclerosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
Growing evidence in human subjects indicates that oxidative modifications of plasma low density lipo-proteins (LDL) enhance their atherogenic properties [1]. Peroxidation of LDL-lipids may increase atherogenicity by damaging the apoprotein B-100, thus reducing the normal catabolism through the regulated LDL receptor pathway and enhancing cholesterol uptake via an un-regulated ‘scavenger’ pathway. The latter leads to cholesterol overloading of the macrophages in the arte-rial intima, engendering the ‘foam cells’, a key compo-nent of the atherosclerotic plaques [2].
Phosphatidylcholine (PC) extracted from soybeans retards the development of atherosclerosis in several
animal models [3 – 8], but the mechanism remains spec-ulative. More recently, a more purified polyenylphos-phatidylcholine mixture (PPC), containing 94 – 96% phosphatidylcholines, 40 – 52% of which is dilinoleoyl phosphatidylcholine (DLPC), and 23 – 24% palmitoyl-linoleoyl phosphatidylcholine (PLPC), prevented the development of cirrhosis in alcohol-fed baboons [9].
It is generally believed that polyunsaturation of fatty acids favors their lipoperoxidation, because fatty acids with multiple double-bonds are much more vulnerable than saturated or monounsaturated ones to free radical attack. Indeed, a diet rich in polyunsaturated fatty acids increased the susceptibility of LDL to oxidation, compared with a diet high in monounsaturated and saturated fatty acids [10 – 14]. Surprisingly, however, recent evidence revealed striking ‘antioxidant’ effects of PPC in vivo [15] and in cultured hepatoma cell models of oxidative stress [16]. These effects were associated with normalization of peroxidation products, such as
* Corresponding author. Present address: Alcohol Research Cen-ter, Veterans Affairs Medical CenCen-ter, 130 West Kingsbridge Road, Bronx, NY 10468, USA. Tel.: +1-718-579-1646; fax: + 1-718-733-6257.
K.P.Na6der et al./Atherosclerosis152 (2000) 89 – 95 90
F2-isoprostane or 4-hydroxynonenal, and glutathione [15], raising the possibility that a component of this phospholipid mixture may act as an antioxidant. More-over, chronic administration of 50% of calories as ethanol (corresponding to the average consumption of alcoholics) to baboons resulted in increased LDL-hy-droperoxides, electronegativity of apo B100 and vulner-ability of LDL to be oxidized (oxidizvulner-ability), alterations which were markedly attenuated by supplementation of the alcohol-containing diet with PPC [17]. Therefore, the purpose of the present study was to determine whether any of the main phosphatidylcholine species of PPC possesses antioxidant properties and affects the in vitro oxidizability of human LDL.
2. Methods
2.1. Materials
1,2,Dilinoleoyl phosphatidylcholine (DLPC),
1,palmitoyl-2,linoleoyl phosphatidylcholine (PLPC) and 1,2,distearoyl phosphatidylcholine (DSPC) were pur-chased from Avanti Polar Lipids (Alabaster, AL).
1,Linoleoyl-2,palmitoyl phosphatidylcholine (LPPC)
was obtained from Sigma (St Louis, MO) and a
-toco-pherol (vitamin E) from Aldrich (Milwaukee, WI).
2.2. Subjects
A total of 15 healthy volunteers (11 females and four
males) participated in this study. Their age (mean9
S.E.M.) was 37.397.5 years (range 21 – 65), their body
weight was 59.593.4 kg, and the body mass index
(calculated by dividing the weight in kilograms by the
square of height in meters) was 22.590.99. Plasma
total cholesterol was 4.990.3 mmol/l (189.63 mg/dl),
and plasma triacylglycerols were 1.1890.3 mmol/l
(104.43 mg/dl). All subjects were in good physical con-dition at the time of study and, in particular, none of them was obese or hyperlipidemic. The study was ap-proved by the institutional Human Research
Commit-tee and all subjects gave informed consent to
participate in the study. Blood was collected in EDTA-containing tubes and centrifuged at 1200×gfor 20 min to separate the plasma.
2.3. Incorporation of phospholipids and6itamin E into plasma
The phospholipid species and the vitamin E were dissolved in absolute ethanol, dried under nitrogen, and incorporated into the plasma by incubation at 37°C for 3 h [17] to produce final concentrations of either 10mM
DLPC or 10 mM PLPC in plasma. Their effects were
compared with those of 10mM LPPC, 10mM DSPC (a
saturated phospholipid), and with either 10 mM or 1
mM a-tocopherol, a recognized antioxidant. In each
subject, the plasma from which the control LDL was isolated, was incubated for 3 h at 37°C as for the plasma incubated with test phospholipids to allow for any consumption of endogenous LDL antioxidants during this treatment.
2.4. LDL isolation from plasma incubated with either phospholipids or 6itamin E
LDL were isolated by a rapid technique [18]. Plasma (1 ml) was adjusted to a density of 1.21 g/ml with solid KBr in Quick-Seal polyallomer tubes (Beckman
Instru-ments, Palo Alto, CA) and spun at 260 000×g for 45
min, without braking, in a vertical rotor. The locations of the lipoproteins were determined by staining plasma with Fat Red 7B (Helena Laboratories, Beaumont, TX). LDL, which appear as a distinct band, were removed through the side of the tube with a needle and syringe. The purity of the fraction was confirmed by its migration on agarose gel electrophoresis [19]. The iso-lated LDL were dialyzed overnight with two to three changes of phosphate buffer (pH 7.4), containing 150 mM NaCl (PBS), in the dark and at 4°C to remove KBr and EDTA. All buffers were deoxygenated by nitrogen bubbling before use, and the dialysis was performed in filled stoppered bottles at 4°C. LDL protein was measured by the method of Lowry [20].
2.5. LDL oxidation
Oxidation was performed within 48 h of LDL isola-tion. Oxidation kinetics of LDL were determined in vitro, using two methods described below.
2.5.1. Copper oxidation method
Treated and control LDL (100 mg protein/ml) were
incubated with 5 mM Cu2+ sulfate in 2 ml of PBS
buffer (pH 7.4) at 37°C for 8 h, by monitoring every 5 min the absorbance at 234 nm of conjugate dienes [21]. The plot of absorbance against time produces three phases: (a) a lag phase, (b) a propagation phase and (c) a decomposition phase. The lag time (which indicates the resistance of LDL to oxidation) was measured as the intercept between the baseline and the tangent of the absorbance curve during the propagation phase. The lag time in normal subjects has a reported range from 33 to 138 min [22], similar to the values found by us (Section 3). Although part of this variation may be due to ex vivo LDL oxidation (despite precautions), a valid comparison is against the subject’s own serum treated the same way as the samples exposed to the phospholipids, because the inter-assay coefficient of
variation (CV=4.36%) and the intra-subject one
The rate of diene production (which depends on the amount and type of oxidizable substrates) was calcu-lated from the slope of the absorbance curve during the propagation phase. The maximum amount of conju-gated dienes (at the interception between the propaga-tion and decomposipropaga-tion phases) was calculated by using the extinction coefficient for conjugated dienes at 234 nm [21].
2.5.2. Thermal decomposition of 2,2%-azobis
(2-amidinopropane)dihydrochloride (AAPH)
LDL (100mg protein) was incubated in PBS to which
AAPH was added, at a final concentration of 10 mM. AAPH is a water-soluble diazo compound that has the advantage of a constant yield of lipid hydroperoxides (whereas copper-induced oxidation depends on the ini-tial release of free radicals). The LDL oxidation by AAPH was monitored by the oxygen consumption measured at 41°C with a Clark’s electrode [12]. Two phases with different rates of oxygen consumption were identified: (a) the inhibition and (b) the peroxidation
phases with low and high rates of O2 consumption,
respectively, The length of the first phase (inhibition time, expressed in minutes) depends on the antioxidant content of LDL, reflecting the consumption of antioxi-dants as they react with free radicals. The rate of the steeper phase (peroxidation rate, expressed as nAtoms of O2consumed per minute) is an index of the
suscepti-bility of LDL to oxidation once all antioxidants have been consumed.
2.6. Statistics
All results are expressed as means9S.E.M. The sig-nificance of the differences was assessed by parametric or non-parametric (Kruskal – Wallis on Ranks) one-way ANOVA, as appropriate, using SAS-PROC GLM pro-cedure with SAS software (version 6.12; SAS Institute, Cary, NC). When effects from the ANOVA were sig-nificant, treatment means were compared by the Tukey’s multiple comparison test. A probability of
B0.05 was considered to be significant.
3. Results
During Cu2+-mediated oxidation, the lag times of
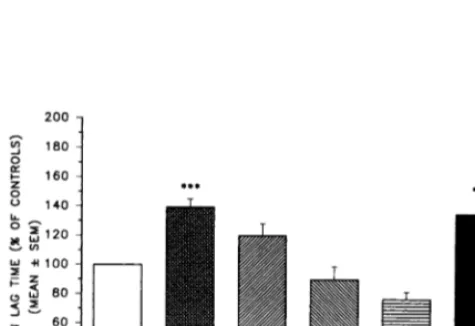
control LDL were very variable, with a range from 38 to 172 min. Therefore, comparisons were made as percent of the corresponding controls (Fig. 1). The lag time was prolonged when LDL were incubated with DLPC (139.595.7% of controls;PB0.001), whereas it was not significantly affected by incubation with PLPC
(120.198.2%), the other main component of PPC (the
soybean’s lecithin extract). The effect of DLPC was not
reproduced by DSPC (76.795.1% of controls), a
phos-pholipid with a saturated fatty acid of the same carbon chain length as linoleate bound to the carbon 1 and 2 of the glycerol. Similarly, a phospholipid with an unsatu-rated fatty acid in position 1 and a satuunsatu-rated one in position 2, such as LPPC, did not reproduce the effect of DLPC (90.199.0% of controls). The effect of 10mM
DLPC was similar to that of 1 mM a-tocopherol
(135.497.2% of controls), whereas an equivalent
con-centration (10 mM) of a-tocopherol showed no effect
(98.097.2% of controls).
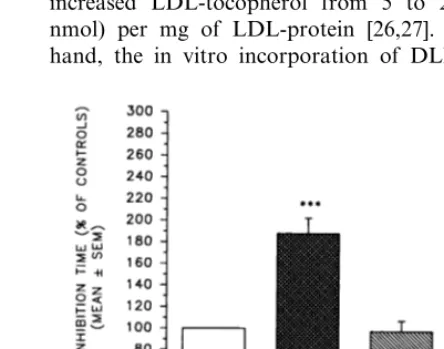
During LDL oxidation by AAPH, the inhibition time
was prolonged (PB0.001) after incubation of LDL
with either DLPC or 1 mMa-tocopherol, but not with
DSPC (Table 1). When compared to the corresponding
controls, the values with DLPC and a-tocopherol were
188 and 206% of the controls, respectively (Fig. 2). By contrast, the incubation of LDL with the various
phospholipids, as well as with a-tocopherol, did not
change the rate of diene production or the maximum amount of dienes produced (Table 2). Similarly, the oxidation rate by the AAPH method also was not significantly affected by these agents (Table 1).
4. Discussion
Our results indicate that DLPC significantly pro-tected human LDL against in vitro oxidation produced by either copper or AAPH. DLPC is the major compo-nent of PPC, a soybean derived phosphatidylcholine mixture. PPC has been reported to prevent
atheroscle-Fig. 1. Effects of various phosphatidylcholines anda-tocopherol on
the lag time during copper-mediated LDL oxidation, assessed by the conjugated diene method and expressed as per cent of the corre-sponding controls. Values are mean9S.E.M. (n=5 – 15). a-TOC, a-tocopherol; DLPC, 1,2,dilinoleoyl phosphatidylcholine; DSPC,
K.P.Na6der et al./Atherosclerosis152 (2000) 89 – 95 92
Table 1
Effects of phosphatidylcholines anda-tocopherol during 2,2%-azobis (2-amidinopropane) dihydrochloride-mediated LDL oxidation measured by the oxygen consumption methoda
Control, +DLPC (10mM), +DSPC (10 Pb
Inhibition time (min) 5.9090.34c 3.2190.23 6.0790.48c B0.001
172922 154947 149925 NS
220926 Oxidation rate (nAtoms/min per mg protein)
aValues are mean9S.E.M.
a-TOC,a-tocopherol; DLPC, 1,2,dilinoleoyl phosphatidylcholine; DSPC, 1,2,distearoyl phosphatidylcholine.
bOverallP-value from one-way ANOVA; NS, not significant (P]0.05). cSignificantly different from control.
rosis in animal models. This effect was found to depend upon the binding of unsaturated fatty acids (linoleate) to both positions 1 and 2 of the glycerol, a structure differing from that of mammalian phospholipids, in which a single unsaturated fatty acid is bound, and only to position 2 [8].
The incorporation of DLPC increased the resistance of LDL against lipoperoxidation. As previously re-ported for a-tocopherol [23,24], this resistance is
mani-fested by a prolongation of the time needed for the consumption of antioxidants present in LDL, as they react with free radicals prior to reaching maximal rates of lipoperoxidation. Similar results were obtained using two different sources of free radicals, namely oxidation by copper and thermal decomposition of AAPH, and two types of measurements, namely diene conjugates and O2 consumption. Whereas the release of free
radi-cals occurs predominantly at early times after copper addition, their production remains constant with AAPH [12], which probably accelerates the consump-tion of antioxidants. Thus, despite their quantitative differences, both the lag and the inhibition times (ob-served with these methods) depend on the antioxidant content of the LDL. Once all antioxidants were con-sumed, neither the rate of LDL oxidation nor the amount of dienes produced were changed by incubation
with DLPC, other phosphatidylcholines or a
-toco-pherol. This indicates that the amount of substrate available for oxidation was not significantly altered by the additions.
It appears paradoxical that DLPC displays antioxi-dant properties despite being rich in unsaturated fatty acids, main substrates for lipoperoxidation. However, the possibility that this phospholipid could act as an antioxidant was suggested because of the results ob-tained after in vivo administration of a DLPC-rich mixture (PPC) to baboons fed alcohol [15] and to rats treated with CCl4 [25] as well as after addition of the
component phospholipids to cultured hepatoma cells undergoing an oxidative stress [16]. The possibility that the decrease in lipoperoxidation indexes could have been secondary to improvement of other cell functions is excluded in this in vitro study, which reveals a direct antioxidant effect of DLPC. The exact mechanism by
which this phospholipid acts as an antioxidant is un-known, but appears to be specific for DLPC, since its antioxidant property was not shared by phospholipids containing either saturated fatty acids (of the same chain length as linoleate) in both positions 1 and 2 or a single linoleate bound to either position 1 or 2 of the glycerol.
The antioxidant capacity of DLPC for LDL appears
to be much greater than that of a-tocopherol: the
prolongation of either the lag or the inhibition phases
by 10 mM DLPC in plasma was similar to that
pro-duced by 1mM a-tocopherol, whereas 10 mM a
-toco-pherol had no detectable effect. This difference is not likely to be due to a more efficient uptake of DLPC
than a-tocopherol into LDL, because the uptake of
a-tocopherol [26,27] was greater than that of DLPC
[28], under conditions similar to ours. Incubation of
plasma with 1 mM a-tocopherol at 37°C for 3 h
increased LDL-tocopherol from 5 to 20 mg (12 – 46
nmol) per mg of LDL-protein [26,27]. On the other hand, the in vitro incorporation of DLPC into LDL
Fig. 2. Comparison of the effects of polyunsaturated (dilinoleoyl-) and saturated (distearoyl-) phosphatidylcholines anda-tocopherol on
the inhibition time during 2,2%-azobis (2-amidinopropane) dihy-drochloride (AAPH)-mediated LDL oxidation, assessed by oxygen consumption and expressed as percent of the corresponding controls. Values are mean9S.E.M. (n=5 – 9).a-TOC,a-tocopherol; DLPC,
P
.
Na
6
der
et
al
.
/
Atherosclerosis
152
(2000)
89
–
95
93
Table 2
Effects of phosphatidylcholines anda-tocopherol on production of conjugated dienes during Cu2+mediated oxidation of LDLa
+LPPC (10mM), +DSPC (10mM), +a-TOC (10 +a-TOC (1 mM), Pb
+DLPC (10
Control,n=15 +PLPC (10mM),
mM),n=15 n=15 n=7 n=5 mM),n=6 n=7
0.6090.10 0.5790.06 0.5090.09 0.6690.09 NS 0.6390.10
Rate of diene production (nmol/min) 0.6090.10 0.5190.08
364955 387961 320999 NS
332962
340944 338949 336948
Maximal amount of dienes (nmol/mg protein)
aValues are mean9S.E.M.
a-TOC,a-tocopherol; DLPC, 1,2,dilinoleoyl phosphatidylcholine; DSPC, 1,2,distearoyl phosphatidylcholine; LPPC, 1,linoleoyl-2,palmitoyl phosphatidylcholine;
PLPC, 1,palmitoyl-2,linoleoyl phosphatidylcholine.
K.P.Na6der et al./Atherosclerosis152 (2000) 89 – 95 94
was studied by Zierenberg et al. [28], who found that 5 – 14% of 1 mg labeled DLPC was incorporated into
human LDL. This corresponds to 1 – 2.8 mg (2.3 – 3.6
nmol) per mg of LDL-protein. In addition, 50% of
the DLPC incorporation occurred in HDL [28]. It has been suggested that the protective role of HDL on atherogenesis could be mediated, at least in part, by preventing the generation of oxidized LDL [29]. Intra-venous administration of doubly labeled DLPC has shown that this lecithin species is incorporated in toto and unaltered into the cell membranes [29]. The in vitro incorporation of DLPC into LDL was similar to that after intravenous administration of the dose [28].
A DLPC-containing mixture extracted from soy-beans retards the development of atherosclerosis in
hypercholesterolemic rabbits [2], baboons [3,4],
Japanese quails [5], minipigs and rats [6].
Supplementa-tion with a-tocopherol provides some protection
against coronary artery disease [30 – 32]. By analogy, the findings of the present study suggest that the pre-ventive effect of DLPC on atherosclerosis is mediated, at least in part, by its protective effect on LDL oxida-tion. Thus, because of its antioxidant capacity and its apparent lack of toxicity [8], DLPC is thus a good
candidate to be used for the prevention of
atherosclerosis.
Acknowledgements
This research was supported by NIH grant AA11115, the Department of Veterans Affairs and the Kings-bridge Research Foundation. The expert technical as-sistance of Lilia Shoichet is gratefully acknowledged.
References
[1] Steinberg D, Parthasarthy S, Carew TE, Khoo JC, Witzum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. New Engl J Med 1989;320:915 – 24.
[2] Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Rad Biol Med 1996;20:707 – 27.
[3] Adams CWM, Abdulla YH, Bayliss OB, Morgan RS. Modifica-tion of aortic atheroma and fatty liver in cholesterol-fed rabbits by intravenous injection of saturated and polyunsaturated lecithins. J Pathol Bacterol 1967;94:73 – 6.
[4] Howard AN, Patelski J, Bowyer DE, Gresham GA. Atheroscle-rosis induced in hypercholesterolaemic baboons by immunologi-cal injury; and the effects of intravenous polyunsaturated phosphatidylcholine. Atherosclerosis 1971;14:17 – 29.
[5] Howard AN, Patelski J. Mechanism of anti-atherosclerotic ac-tion of intravenous polyunsaturated phosphatidyl choline. Scand J Clin Lab Invest 1974;114:64 – 5.
[6] Stafford WW, Day CE. Regression of atherosclerosis effected by intravenous phospholipid. Artery 1975;1:106 – 14.
[7] Samochowiec L, Kadlubowska D, Ro´zewicka L. Investigations in experimental atherosclerosis. Part 1. The effects of
phos-phatidylcholine (EPL) on experimental atherosclerosis in white rats. Atherosclerosis 1976;23:305 – 17.
[8] Galli C. Polyenoyl phosphatidylcholine and lipid metabolism in experimental and clinical atherosclerosis: an introduction to pharmacology of PPC. In: Ricci G, editor. Therapeutic selectiv-ity and risk/benefit assessment of hypolipidemic drugs. New York: Raven Press, 1982:237 – 44.
[9] Lieber CS, Robins S, Li J, DeCarli LM, Mak KM, Fasulo JM, et al. Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterology 1994;106:152 – 9.
[10] Parathasarathy S, Khoo JC, Miller E, Barnett J, Witzum JC, Steinberg D. Low density lipoprotein rich in oleic acid is pro-tected against oxidative modification: implications for dietary prevention of atherosclerosis. Proc Natl Acad Sci USA 1990;87:3894 – 8.
[11] Berry E, Eisenberg S, Haratz D, Friedlander Y, Norman Y, Kaufmann N, et al. Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins: The Jerusalem Nutrition Study — high MUFAs versus high PUFAs. Am J Clin Nutr 1991;53:899 – 907.
[12] Bonanome A, Pagnan A, Biffanti S, Opportuno A, Sorgato F, Dorella M, et al. Effect of dietary monounsaturated and polyun-saturated fatty acids on the susceptibility of plasma low-density lipoproteins to oxidative modification. Arterioscler Thromb 1992;12:529 – 33.
[13] Reaven P, Parathasarathy S, Grasse BJ, Miller E, Steinberg D, Witzum JL. Effects of oleate-rich and linoleate-rich diets on the susceptibility of low density lipoproteins to oxidative modifica-tion in mildly hypercholesterolemic subjects. J Clin Invest 1993;91:668 – 76.
[14] Abbey M, Belling GB, Noakes M, Hirata F, Nestel PJ. Oxida-tion of low-density lipoproteins: intraindividual variability and the effect of dietary linoleate supplementation. Am J Clin Nutr 1993;57:391 – 8.
[15] Lieber CS, Leo MA, Aleynik SI, Aleynik MK, DeCarli LM. Polyenyl-phosphatidylcholine decreases alcohol-induced oxida-tive stress in the baboon. Alcohol Clin Exp Res 1997;21:375 – 9. [16] Aleynik SI, Leo MA, Takeshige U, Aleynik MK, Lieber CS. Dilinoleoylphosphatidylcholine is the active antioxidant of polyenylphosphatidylcholine. J Invest Med 1999;47:507 – 12. [17] Navder KP, Baraona E, Leo MA, Lieber CS. Oxidation of LDL
in baboons is increased by alcohol and attenuated by polyenylphosphatidylcholine. J Lipid Res 1999;40:983 – 7. [18] Chung BH, Segrest JP, Ray MJ. Single vertical spin gradient
ultracentrifugation. Methods Enzymol 1986;128:181 – 209. [19] Noble RP. Electrophoretic separation of plasma lipoproteins in
agarose gel. J Lipid Res 1968;9:693 – 700.
[20] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265 – 75.
[21] Puhl H, Waeg G, Esterbauer H. Methods to determine oxidation of low-density lipoproteins. Methods Enzymol 1994;233:425 – 41. [22] Esterbauer H, Wa¨g G, Puhl H. Lipid peroxidation and its role in
atherosclerosis. Br Med Bull 1993;49:566 – 76.
[23] Esterbauer H, Dieber-Rotheneder M, Striegl G, Waeg G. Role of vitamin E in preventing the oxidation of low density lipo-protein. Am J Clin Nutr 1991;53:314S – 21S.
[24] Esterbauer H, Puhl H, Dieber-Rotheneder M, Waeg G, Rabl H. Effect of antioxidants on oxidative modification of LDL. Ann Med 1991;23:574 – 81.
[25] Aleynik SI, Leo MA, Ma X, Aleynik MK, Lieber CS. Polyenylphosphatidylcholine prevents carbon tetrachloride-in-duced lipid peroxidation while it attenuates liver fibrosis. J Hepatol 1997;27:554 – 61.
[27] Suzukawa M, Abbey M, Clifton P, Nestel PJ. Effects of supple-menting with vitamin E on the uptake of low-density lipoprotein and the stimulation of cholesteryl ester formation in macrophages. Atherosclerosis 1994;32:1325 – 32.
[28] Zierenberg O, Odenthal J, Betzing H. Incorporation of polyenylphosphatidylcholine into serum lipoproteins after oral or intravenous administration. Atherosclerosis 1979;34:259 – 76. [29] Parathasarathy S, Barnett J, Fong LG. High-density lipoprotein
inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta 1990;1044:275 – 83.
[30] LeKim D, Betzing H, Stoffel W. Incorporation of complete phospholipid molecules into cellular membranes of rat liver after uptake from blood serum. Hoppe-Seyler’s Z Physiol Chem 1972;353:949 – 64.
[31] Rimm E, Stampfer M, Ascherio A, Giovannucci E, Colditz G, Willett W. Vitamin E consumption and the risk of coronary heart disease in men. New Engl J Med 1993;328:1450 – 6. [32] Stampfer M, Hennekins C, Manson J, Colditz G, Rosner B,
Willett W. Vitamin E consumption and the risk of coronary disease in women. New Engl J Med 1993;328:1444 – 9.