E
ach day, thousands of uniquely differen-tiated root border cells are synthesized and programmed to separate from the root tips (the region at the apex housing the api-cal meristem and root cap) of higher plants1,2. Although they are attached to the root periphery by a water soluble polysaccharide matrix, the middle lamellae of these cells become solubi-lized by the action of pectolytic enzymes in the cell wall3. By definition, border cells are those cells that disperse into suspension within sec-onds when root tips are placed into water (Fig. 1). These cells originate from root cap meri-stematic cells, which give rise to cell layers that progressively differentiate through specialized stages before they finally separate from the cap periphery4. Thus, by the time an individual bor-der cell is delivered to its ultimate destination external to the root, it has already served several functions, such as mucilage secretion, the sens-ing of gravity and other environmental signals. How many of those functions continue to oper-ate in border cells is unknown. However, the border cells of most species are viable after they detach from the root (Fig. 1), and in culture the cells can divide and differentiate into organized tissue (Fig. 1). Upon separation from the cap, metabolic activity in border cells increases and gene expression undergoes a global switch, such that mRNA and protein profiles are grossly dis-tinct from those of progenitor cells in the root cap5. Border cells then produce specific metab-olites, such as anthocyanins and antibiotics6 (Fig. 1), and enzymes including a Rhizobium -induced peroxidase7and a low-pH galactosidase (C. Jian and M.C. Hawes, unpublished; Fig. 1). We call these unusual cells (previously termed ‘sloughed root cap cells’) root ‘border’ cells toemphasize that they constitute a biotic bound-ary layer between the root surface and the soil. In the past, border cells largely have been overlooked as a structural and functional component of root systems. One reason for this oversight is the notion that the cells are a mori-bund by-product of root-cap turnover. This pre-sumption has prevailed in recent years in spite
of the fact that L. Knudson8demonstrated that it was incorrect in 1919 (Refs 1,2). Although species in a few families, such as the Compositae, have border cells that die before detachment, in the majority of species exam-ined, border cell populations are .90% viable5. Border cells of maize and pea grown hydro-ponically can remain viable for more than three months8, and cells of maize have been reported to survive for a week or more in field soil9. A second reason for the lack of attention paid to border cells is that the cells disperse so effi-ciently in response to free water that even the gentlest washing of root systems to remove soil before experimental manipulation and exami-nation removes all border cells1. However, in the absence of free water the cells cling so tena-ciously to the root periphery that their presence is difficult to distinguish even by experienced investigators.
Regulation of border cell production The number of border cells that can be pro-duced daily by a given root is conserved at the family level, and can range from a dozen for tobacco to .10 000 for cotton and pine. The process of border cell production for tap roots, lateral and branch roots appears to be similar. Therefore, for complex root systems with hun-dreds of branches, millions of border cells would be deposited into the soil daily if their production were a constitutively expressed process, which was assumed to be the case for many years10. However, recent studies have established that border cell production in species such as cereals, legumes and cotton, is a tightly regulated process that is controlled by endogenous and environmental signals. In the laboratory, radicles synthesize a set number of border cells within 24 h of emergence. As they accumulate on the cap periphery, border cells of pea release an extracellular suppressor that specifically inhibits mitosis in the root-cap meristem, without affecting mitosis in the adjacent apical meristem that gives rise to root growth11. Root-cap turnover ceases and no new border cells are made, but the set of ~4000 cells that has been made remains appressed to the periphery of the cap as the root elongates. If border cells are removed, or if the extracellular suppressor is diluted by dipping the root into water, cell division in the root cap meristem resumes within 5 min, and remains high for 2 h. New cells can be col-lected from the cap periphery within 1 h after removal of the old ones; after 24 h a new set of 4000 cells is complete and no more cells are made. This process, which can be synchro-nized experimentally, has been exploited to identify two genes, psugt1and rcpme1, which play a role at both ends of border cell devel-opment – cell division in the cap meristem and cell separation at the cap periphery, respec-tively3,12. When the expression of either gene in transgenic roots is inhibited by antisense mRNA mutagenesis, border cell development is blocked. Such mutants are being used under controlled conditions to dissect the molecular and cellular mechanisms by which border cell development is regulated.
How border cell development is regulated in soil is unknown. Because border cells do not detach readily except when they are actually placed into water, a root growing through con-ditions where the tip never experiences a flush of water, could, theoretically, be expected to retain the same set of border cells throughout its lifetime. Renewed border cell production in response to any exposure to free water, such as during rain or irrigation, would be a logical prediction. However, when roots are grown in a wet semisolid matrix, such as water agar, where border cells can be seen to be re-moved continuously when viewed microscopi-cally, the results are not always found to be
The role of root border cells
in plant defense
Martha C. Hawes, Uvini Gunawardena, Susan Miyasaka
and Xiaowen Zhao
The survival of a plant depends upon the capacity of root tips to sense and move towards water and other nutrients in the soil. Perhaps because of the root tip’s vital role in plant health, it is ensheathed by large populations of detached somatic cells – root ‘border’ cells – which have the ability to engineer the chemical and physical properties of the external environment. Of particular significance, is the production by border cells of specific chemicals that can dramatically alter the behavior of populations of soilborne microflora. Molecular approaches are being used to identify and manipulate the expression of plant genes that control the production and the specialized properties of border cells in transgenic plants. Such plants can be used to test the hypothesis that these unusual cells act as a phalanx of biological ‘goalies’, which neutralize dangers to newly generated root tissue as the root tip makes its way through soil.
consistent with such straightforward predic-tions. Under these conditions, the extracellu-lar suppressor, which inhibits cell division in the root cap meristem, presumably would be diluted continuously or would be removed by physical forces as the tip elongates, thus a con-stant induction of mitosis with a continuous production of border cells would be expected. In some cases, continuous production does occur, such that the entire root is ensheathed in layers of detached border cells. In other instances, sometimes in adjacent roots in a sin-gle Petri dish, border cell production occurs only intermittently or not at all, suggesting that localized conditions around individual roots might influence the process1. Recent studies have confirmed that environmental signals can override the control of border cell devel-opment using endogenous signals13. For example, when pea is exposed to increased carbon dioxide levels, the normal control of border cell production is overridden, such that instead of producing the usual set of 4000 cells, twice as many cells are made (Fig. 2). By contrast, border cell development in alfalfa is impervious to increased carbon dioxide. The regulation of this process at multiple levels, with species-dependent variation in responses to external signals, is one of the properties that led us to suggest that border cells can act as cellular ‘goalies’ (players assigned to protect the goal in various sports; Webster’s Unabridged Dictionary) to ward off threats to the apical meristem.
Proposed functions of root border cells The root cap provides physical protection for the apical meristem; the border cells and their associated mucilage were long presumed to provide lubrication for the passage of the root cap. To date, the hypothesis that border cells lubricate the root tip is unsupported by ex-perimental data2,14,15. The fact that plants turn border cell production on and off without hampering their ability to penetrate solid media, and that a few species do not appear to undergo border cell production at all argues against such a ‘housekeeping’ function1,2 . We have proposed that border cells serve plants by enabling roots to define their own ecology. The best characterized properties of border cells involve their specific recognition and responsiveness to soilborne microflora1,2. In a genotype-dependent manner, border cells can: • Attract zoospores (Fig. 1).
• Synthesize defensive structures in response to fungal attack16.
• Repel or bind pathogenic bacteria (Fig. 1). • Control growth and gene expression in
symbiotic bacteria (Fig. 1).
Chemotactic attraction to border cells of soil-grown plants has been shown to facilitate root infection by pathogenic bacteria, and such recognition might create ‘biased rhizospheres’
by dictating which populations can colonize plant root systems17. By modulating the prop-erties of the external environment
surround-ing the advancsurround-ing root tip, the regulated production of border cells could, directly or indirectly, influence virtually all of the physi-cal and chemiphysi-cal parameters (i.e. pH, nutrient solubility or soil structure) of the root– soil ecosystem. Here we focus on the direct role that border cells might play in defense. The recently discovered cellular responses of border cells to nematodes, aluminum and
fungi suggest novel mechanisms by which border cells might function to protect the root tip from biotic and abiotic stress.
Border cells attract and immobilize nematodes
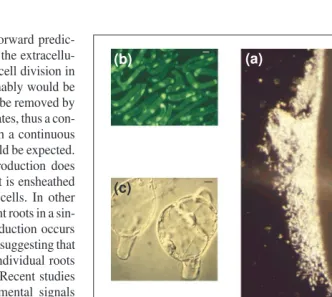
In Greek mythology, the Sirens’ beautiful songs tempted sailors to linger on their island, and thus distracted them forever from com-pleting their intended journeys. Recent studies suggest that border cells might carry out a similar distraction to pathogenic nematodes on their way to infect root tips (X. Zhao and M.C. Hawes, unpublished). When a pea root, whose border cells have been removed, is placed onto water agar containing a lawn of root knot nema-todes, no sign of recognition is evident at the root tip (Fig. 3). By contrast, when border cells are present nematodes rapidly accumulate around the root tip periphery (Fig. 3). When Fig. 1.Properties of root border cells. (a) Border cells have dispersed away from the cotton root tip after immersion in water for 30 sec, showing one days’ accumulation of border cells (~10 000) surrounding the tip (scale bar 51 mm). (b) The viability of detached border cells is revealed by the accumulation of a vital stain, fluorescein diacetate (scale bar 510 mm). (c) Cell division in border cells – induced by incubation in tissue culture medium containing plant hormones28
(scale bar 510 mm). (d) Expression of the red-pigmented antibiotic shikonin is localized in root border cells of Lithospermum erythrorhizon6
(scale bar 5 1 mm). (e) Expression of a low-pH galactosidase is specific to root border cells of pea (C. Jian and M.C. Hawes, unpublished) (scale bar 5 1 mm). (f) Border cells of cotton specifically attract zoospores of Pythium dissotocum, which germinate, penetrate and kill the cells within 2 min (Ref. 29) (scale bar 510 mm). (g) Genotype-specific binding of Agrobacterium tumefaciens
by border cells is correlated with susceptibility to infection19
(scale bar 510 mm). (h) When pea roots are incubated on a lawn of Rhizobium leguminosarumexpressing a nod-lacZ reporter gene on solidified culture medium (pH 7.0), intensenodgene expression is detected in bacte-ria present at the root tip in the region where border cells are present30,31
(scale bar 51 mm).
(b)
(c)
(d)
(a) (h)
(f) (g)
(e)
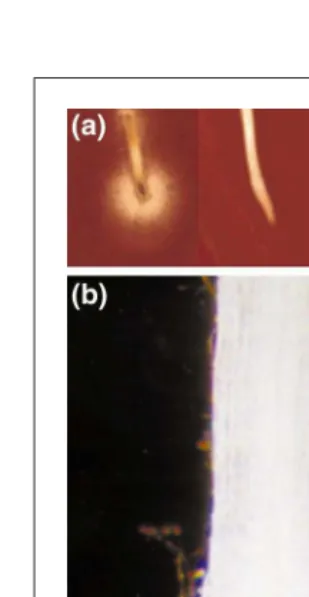
these roots are lifted from the surface, clumps of border cells left behind reveal high popu-lations of actively motile nematodes (Fig. 3). However, within 30 min, the motility of the nematodes ceases and the worms appear straight and inert, as they do when dead (Fig. 3). When the immobilization phenomenon is mea-sured using quantitative assays, a .99% loss of mobility develops in response to a single
heat-stable, polar fraction of the root exudates (sol-uble extracellular material). This immobiliza-tion effect is reversible – within a few hours to a few days (depending on conditions of the assay) the nematodes resume full mobility. If a similar process occurs in the soil, by the time that the nematodes resume motility, a root tip growing at a rate of 1 mm per hour would be long past being in danger of pen-etration. This phenomenon might account, in part, for the fact that the normal site of infection by nematodes is behind the root tip, just past the region where border cells are released18.
Mucilage production can repel bacteria and decrease sensitivity to aluminum Border cells of legumes and cereals produce a mucilage layer in response to co-cultivation with pathogenic bacteria, but not in response to E. coli19. This layer appears to repel the bac-teria (Fig. 4), but its mechanism of action is unknown. Recent studies have revealed that co-cultivation of border cells with aluminum results in the production of a mucilage layer similar to that which occurs in response to pathogenic bacteria (S. Miyasaka and M.C. Hawes, unpublished). Within 2 h of exposure to aluminum, a layer of mucilage around indi-vidual border cells increases in a dosage-dependent manner, from being barely detectable (Fig. 4), to being as wide (Fig. 4) or wider than the cell’s diameter (Fig. 4). Interestingly, the development of the layer is correlated with a near-total cessation of alu-minum-induced border cell death. For the first 2 h of exposure to aluminum, a dosage-depend-ent linear-rate of border cell death proceeds. After 2 h, when the mucilage layer is in place, the rate of cell death drops precipitously, such that the viability of the cell population remains largely static for the next 20 h (S. Miyasaka and M.C. Hawes, unpublished). The results
are consistent with the hypothesis that border cells have the capacity to synthesize an in-ducible extracellular structure that interferes with the ability of aluminum to cause further cellular damage. The mechanism by which this occurs is unknown, but one possibility is that the charged aluminum molecule becomes immobilized by binding to the polysaccharide layer20. If this is the case, the capacity of thou-sands of detached border cells to immobilize aluminum ions could have a profound effect on the ability of aluminium to damage the root tip, the primary site of aluminum’s toxic effects21.
Border cells act as a decoy for fungal infection
When pea seedlings are inoculated uniformly with spores of pathogenic fungi, nearly all develop visible lesions in the region just behind the root tip within 24 h (U. Gunawardena and M.C. Hawes, unpublished). A small propor-tion of seedlings also develop a visible lesion at the root tip. When excised and placed onto culture medium, fungal mycelium emerges from these infected tips, confirming that the root tip is not inherently resistant to infection (Fig. 5). In the majority of seedlings (.90%), the root tip remains white, and to the naked eye appears to be infection free. However, microscopic observation reveals that the tip is actually covered in fungal hyphae (Fig. 5). Fig. 2.Stimulation of border cell production by carbon dioxide. (a) In an ambient atmosphere,
roots of germinating seedlings produce ~4000 border cells in 24 h, cell production then ceases unless the existing cells are removed. (b) In atmospheres containing increased carbon dioxide, border cell production does not level off, and more than twice as many cells accumulate13
.
Fig. 3.Attraction and immobilization of the root knot nematode by root border cells. (a) When a pea root is placed onto water agar inoculated with nematodes, nematodes are rapidly attracted to sur-round the border cells (right), but do not surround roots whose border cells have been removed (left). (b). When roots are lifted from the surface, the active motility of nematodes can be detected within clumps of border cells left behind. (c) After 30 min, motility within the clumps of border cells ceases, and the nematodes are rigid and inert.
Fig. 4. Development of a mucilage layer by individual border cells. (a) A bor-der cell of oats incubated for 2 h with
When the tip is placed into water and agitated gently, this ‘mantle’ detaches and can be seen microscopically to consist of thousands of digested border cells held together by actively growing fungal mycelium2. Even under extreme conditions, which include high concentrations of spores and heat-shock temperatures, the root tip remains sterile for days (Fig. 5), indistin-guishable from uninoculated controls (Fig. 5) in spite of having been covered by a sheath of necrogenic fungus. In these plants, root growth and development are comparable to that of the controls, indicating that the apical meristems are fully functional. In the 1950s, running backs (American football players) were outfitted with
tear-away jerseys that were designed to detach when grabbed by would-be tacklers, leaving the runner free to keep moving down the field. A similar mechanism, mediated by border cell detachment, could, in part, account for the observation that root tips generally escape infection and colonization in the field2.
Model
Taken together, the observed responses of bor-der cells to aluminum, nematodes and patho-genic fungi are consistent with a two-step model in which the root tip is protected by an unusual and complex process. First, the root cap meristem actively produces a ‘front’ consisting of thousands of border cells that can engineer the environment to render a threat temporarily harmless. Elongating cells gener-ated by the apical meristem then push the root tip into and through the newly ‘engineered’ environment. The particular mechanism by which the threat is rendered harmless by the border cells might vary and could be constitu-tive (i.e. the secretion of a chemical that anes-thetizes nematodes) or inducible in response to a particular signal (i.e. the production of a mucilage layer that immobilizes aluminum). It is important to note again that only in the presence of a flush of free water, such as after rain or irrigation, would the en masserelease
of border cell populations occur. In the absence of water, existing border cells remain appressed to the root periphery and are carried along as the root grows. It is also only in the presence of free water that microorganisms or toxins, such as aluminum, constitute a threat to plants, because only then can movement and uptake occur.
Reasons for protecting the tip in a different way
The root tip is essential to plant health This costly defensive process might be needed because of the multifaceted role played by tis-sues within the root tip. One of the most-cited functional distinctions between animals and plants is that animals can move and plants can-not. Whereas animals can respond to signals to avoid danger and seek nutrients, it is said that plants must rely on internal signals to
generate chemical responses in situto synthe-size their own food and protect themselves from stresses. This simple dogma does not take into account that complex root systems, with each root tip elongating at a rate of 1 mm per hour, can traverse .6 m of soil in a day22. As in animals, this movement occurs as a result of specific signals perceived by root cells and transduced into behavioral responses via common gene expression systems. Plant roots move towards nutrients and water, and away from toxins, such as aluminum (Fig. 6), by sensing signals that notify them of the presence of such stimuli. Anyone who has ever battled to keep willow (Salix) roots out of their septic tanks will appreciate the effec-tiveness of roots’ directed-movement capac-ities. Signals generated by the root as a result of the detection of toxins or the uptake of nutrients, are transmitted to the aboveground parts of the plant. This communication forms the basis, not only for the daily metab-olism of the plant and its ultimate reproduc-tive activities, but also for the overall size and architecture of the whole plant23.
The primary site for perception of under-ground signals is the root tip23. Cells within the terminal few millimeters of the root respond to light, electricity, water, touch, ions and other soluble molecules, to generate impulses that direct the rate, direction and magnitude of root movement. Just like animals, a capacity to move away from danger and towards water and other required elements is a crucial component of plant life. So perhaps a more important distinction between plants and ani-mals is the mechanism by which movement is generated: whereas animals can get up and walk, plants can move only by the generation and elongation of new cells by root meristems. Fig. 5.Mantle production in response to
pathogenic fungi (U. Gunawardena et al., unpublished). When roots are inoculated with spores of Nectria haematococca, most root tips remain white and appear to be uninfected, but a few seedlings develop visible lesions at the tip. When such infected tips are plated onto culture medium, abundant fungal growth emerges (a, left). Most root tips remain white and appear to be uninfected, but when examined microscopically, are seen to be covered in a sheath or ‘mantle’ of fungal hyphae (b). When these roots are placed into water and agitated gently, the mantle of fungus falls away from the root tip. When the tip is excised and placed onto culture medium, no fungal growth emerges (a, center), indicating that the tips are sterile like the tips of the uninoculated control seedlings (a, right).
Fig. 6.Directed movement of roots in response to exposure to aluminum. Pea seedlings with 1-cm-long roots were placed onto medium containing no alu-minum (left), 100 mM (center) or 200 mM aluminum (right). Arrows indicate the position of the root tip at the time that the roots were placed onto the medium. In response to aluminum, roots im-mediately began to grow away from the plate at a 458 angle (S. Miyasaka and M.C. Hawes, unpublished).
This newly synthesized tissue comprises the same terminal few millimeters of tissue that detects and responds to environmental stimuli to condition movement toward nutrients and away from danger. Not surprisingly, such newly synthesized tissue is also especially vulnerable to abiotic and biotic disease. For humans, this is the functional equivalent of having to rely on newborn babies to explore and search a predator-filled wilderness for supplies to send back to base camp. This bio-logical dilemma might help to account for the fact that in most species the vulnerable but essential root meristems are surrounded by an army of border cell ‘foot soldiers’2.
Other defensive strategies might not work for the tip
We have proposed that a fail-safe defense mechanism is needed to protect the root tip because of its importance to the overall health of the plant. However, it is well established that plants already have complex defense pathways that are activated rapidly in response to external signals. Within moments of recog-nition of biotic and abiotic stresses, plants can alter their surface structures, generate toxic moieties, and activate the expression of genes for the synthesis of dozens of antimicrobial proteins and other metabolites24. Why would an elaborate alternative method involving the separation of masses of detached cells be needed by plants that already are well equipped to defend their cells? One expla-nation is that defense responses in plants often involve the so-called ‘hypersensitive reac-tion’, in which the rapid necrosis of a few plant cells is thought to inhibit the spread of a pathogen25. In most tissues, including the region behind the root tip where most root–microbe relationships are initiated, the sacrifice of a few cells offers containment of the infection with little or no deleterious effect on plant health. However, in the root tip, there is no margin for the loss of a few cells in the interest of containing infection when those few cells comprise the apical meristem. Indeed, experiments have confirmed that even a minor necrotic reaction in the terminal 2 mm of the root tip is correlated with the rapid ces-sation of growth of that root (U. Gunawardena and M.C. Hawes, unpublished).
The activation of defense pathways in response to biotic and abiotic signals does not always result in hypersensitive cell death26. The production of antimicrobial chemicals can occur systemically, without any assoc-iation with cell death. However, even in the absence of cell death the activation of defense pathways is a costly metabolic process. As with other such responses (e.g. heat shock), plants cope with this cost by down-regulating other pathways. One activ-ity that is known to be virtually shut down in
response to the activation of defense path-ways is the expression of genes needed for cell division27. In most tissues, this would not present a problem, but in a meristem it does. In a meristem that plays a central role in allowing the root system to avoid potentially lethal stresses, a transient inhibition of cell division during exposure to pathogens or toxins could be deadly.
Future perspectives
Our model is congruent with the known biol-ogy of root infection: nearly all pathogenic and symbiotic relationships are initiated not
at the tip, but in the region of elongation, just behind the tip where the production of border cells does not occur. We suspect that the main reason that the effect of border cells on root infection has never been examined, is its effectiveness: so few pathogens and sym-bionts have been reported to initiate infection or colonization at the root tip that there has been little reason to focus on its role in infec-tion. The availability of plants with altered border cell production means that the most obvious prediction of our model can now be tested. If the presence of border cells is the reason root tips are largely impervious to infection, then inhibiting their production and/or release will result in a change in the site and/or nature of infection. If this is the case, the study of how border cells can defend the tip so successfully might elucidate novel defense strategies that can be applied to tissues that do not come equipped with their own cellular ‘goalies’. It might not be feasible to program other tissues to produce and separate populations of border cells. However, genetic engineering approaches can be used to harness the cellular and extra-cellular mechanisms by which border cells neutralize dangerous molecules or organisms and express them in tissues that are not as well protected as the root tip.
Acknowledgements
The authors thank Merritt R. Nelson for the ‘tear-away jersey’ model for border cell function, Hans D. VanEtten for critical read-ing of the manuscript, and the Dept of Energy, Division of Energy Biosciences and United States Dept of Agriculture for funds support-ing basic research on the functions of root border cells.
References
1Hawes, M.C. and Brigham, L.B. (1992) Impact of root border cells on microbial populations in the rhizosphere. Adv. Plant Pathol. 8, 119–148
2Hawes, M.C. et al.(1998) Function of root border cells in plant health: pioneers in the rhizosphere. Annu. Rev. Phytopathol.36, 311–327
3Wen, F. et al.(1999) Effect of pectin methylesterase gene expression on pea root development. Plant Cell11, 1129–1140
4Feldman, L.J. (1984) Development and dynamics of the root apical meristem. Am. J. Bot.7, 1308–1314
5Brigham, L.A. et al.(1995) Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiol.109, 457–463
6Brigham, L.A. et al.(1999) Cell-specific production and antimicrobial activity of naphthoquinones in roots of Lithospermum erythrothizon. Plant Physiol.119, 417–428
7Cook, D. et al.(1995) Transient induction of a peroxidase gene in Medicago truncatulaprecedes infection by Rhizobium meliloti. Plant Cell7, 43–55
8Knudson, L. (1919) Viability of detached root cap cells. Am. J. Bot. 6, 309–310
9Vermeer, J. and McCully, M.E. (1982) The rhizosphere in Zea: new insight into its structure and development. Planta156, 45–61
10 Clowes, F.A.L. (1994) Origin of the epidermis in root meristem. New Phytol.127, 335–347
11 Brigham, L.A. et al.(1998) Meristem-specific suppression of mitosis and a global switch in gene expression in the root cap of pea by endogenous signals. Plant Physiol. 118, 1223–1231
12 Woo, H.H. et al. (1999) Meristem-localized inducible expression of a
UDP-glycosyltransferase gene is essential for plant growth and development in pea and alfalfa. Plant Cell11, 2303–2315
13 Zhao, X. et al.(2000) Stimulation of border cell production in response to increased CO2 levels.
Plant Physiol. 122, 181–188
14 Rogers, H.T. et al.(1942) The source and phosphatase activity of exoenzyme systems of corn and tomato roots. Soil Sci. 54, 353–365
15 Guinel, F.C. and McCully, M.E. (1986) Some water-related physical properties of maize root cap mucilage. Plant Cell Environ. 9, 657–666
16 Sherwood, R. (1987) Papilla formation in corn root cap cells and leaves inoculated with
Colletotrichum graminicola. Phytopathology 77, 930–934
17 Hawes, M.C. and Smith, L. (1988) Requirement for chemotaxis in pathogenicity of Agrobacterium tumefacienson roots of soil-grown pea plants.
J. Bacteriol. 171, 5668–5671
18 Prot, J.C. (1980) Migration of plant-parasitic nematodes towards plant roots. Revue Nematol.
3, 305–318
19 Hawes, M.C. and Pueppke, S.G. (1987) Correlation between binding of Agrobacterium tumefaciensby root cap cells and susceptibility of plants to crown gall. Plant Cell Rep. 6, 287–290
20Horst, W.J. et al. (1982) Mucilage protects root meristem from aluminum injury.
Z. Pflanzenphysiol. Bd.105, 435–444
21Ryan, P.R. et al. (1995) Characterisation of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta196,
103–110
22Lynch, J. and van Beem, J.J. (1993) Growth and architecture of seedling roots of common bean genotypes. Crop Sci. 33, 1253–1257
23Aiken, R.M. and Smucker, A.J.M. (1996) Root system regulation of whole plant growth. Annu. Rev. Phytopathol.34, 325–346
24 Somssich, I.E. and Hahlbrock, K. (1998) Pathogen defence in plants – a paradigm of biological complexity. Trends Plant Sci. 3, 86–90
25Kamoun, S. et al.(1999) Resistance to oomycetes: a general role for the hypersensitive response? Trends Plant Sci.4, 196–200
26 Grant, M. and Mansfield, J. (1999) Early events in host–pathogen interactions. Curr. Opin. Plant Biol. 2, 312–319
27 Logemann, E. et al.(1995) Gene activation by UV light, fungal elicitor or fungal infection in
Petroselinum crispumis correlated with repression of cell cycle-related genes. Plant J.8, 865–876
28 Hawes, M.C. and Pueppke, S.G. (1986) Sloughed peripheral root cap cells: yield from different species and callus formation from single cells.
Am. J. Bot. 73, 1466–1473
29 Goldberg, N. et al.(1989) Specific attraction to and infection of cotton root cap cells by zoospores of Pythium dissotocum. Can. J. Bot.
67, 1760 –1767
30 Zhu, Y. et al. (1997) Release of nodulation gene inducing chemicals from root border cells. Plant Physiol. 115, 1691–1698
31Peters, N.K. and Long, S.R. (1988) Alfalfa root exudates and compounds which promote or inhibit induction of Rhizobium meliloti
nodulation genes. Plant Physiol.88, 396–400
Errata
The review article by Richard A. Dixon and Christopher L. Steele (1999) Flavonoids and isoflavonoids – a gold mine for meta-bolic engineering. Trends Plant Sci. 4, 394–400 in the October 1999 issue of Trends in Plant Science contained two errors.
In Figure 2, dihydroflavonol (not flavanone) is acted on by flavonol synthase (FLS) to form flavonol. In Figure 4, the struc-ture of the 2-hydroxyisoflavanone intermediate was depicted incorrectly. Corrected versions of these portions of the figures are printed below.
2-Hydroxyisoflavanone
O OH
OH HO
O H (OH)
FLS
O 2
OH
HO
O (R1) Flavanone
O
OH
HO
O OH
OH
Dihydroflavonol O
OH
HO
O OH
OH
Flavonol
F3βH
Fig. 4. Fig. 2.
Erratum
In the December 1999 issue of Trends in Plant Science, we published a research news article by Albrecht G. von Arnim (1999) Phytochrome in the limelight.
Trends Plant Sci. 4, 465–466. In Figure 1, top left it should read phyAr and not phyBr. A corrected version of this portion of the figure is printed left.
We apologize to the authors and to our readers for these errors. A corrected ver-sion of these articles can be viewed in HTML format at plants.trends.com
phyBr phyBfr phyAfr
phyAr
Light
Martha C. Hawes*, Uvini Gunawardena and Xiaowen Zhao are at the University of Arizona, Dept of Plant Pathology, Tucson, AZ 85721, USA;
Susan Miyasaka is at the University of Hawaii, 461 W. Lanikaula St, Hilo, HI 96720, USA.
*Author for correspondence (tel 11 520 621 5490; fax 11 20 621 9290;
e-mail [email protected]).
Fig. 1.
PII:S1360-1385(00)1573-9
PII:S1360-1385(00)1572-7