Comparison of the phytotoxic activity of the phytotoxin destruxin
B and four natural analogs
M.S.C. Pedras *, C.J. Biesenthal, I.L. Zaharia
Department of Chemistry,Uni6ersity of Saskatchewan,110 Science Place,Saskatoon,Sask., Canada S7N5C9
Received 1 December 1999; received in revised form 6 March 2000; accepted 6 March 2000
Abstract
A quantitative bioassay utilizing staining of plant cell suspension cultures of Sinapis alba was employed to establish a structure-phytotoxic activity correlation among destruxin B, homodestruxin B, and desmethyldestruxin B, toxins produced by
Alternaria brassicae (Berk.) Sacc., the causative agent of Alternaria blackspot of brassicas. In addition, the phytotoxicity of destruxin B, homodestruxin B, and their respective metabolites hydroxydestruxin B and hydroxyhomodestruxin B were tested on resistant and susceptible plant species utilizing in planta leaf assays and leaf uptake of toxin solutions. Overall, the results obtained from punctured leaf and cell staining assays indicated that homodestruxin B (EC503×10−4M) was the most toxic of the five
compounds, followed by destruxin B (EC505×10−4M), and desmethyldestruxin B (EC505×10−4M). On the other hand, the
hydroxylated destruxins (hydroxydestruxin B EC505×10−4 M) were significantly less phytotoxic than the parent toxins.
© 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Alternaria blackspot;Alternaria brassicae; Desmethyldestruxin B; Destruxin B; Homodestruxin B; Host-selective; Hydroxydestruxin B; Hydroxyhomodestruxin B; Phytotoxicity; Phytotoxins
www.elsevier.com/locate/plantsci
1. Introduction
We have been evaluating the role of host-selec-tive toxins as part of a research program aimed at understanding the chemical and biochemical basis of plant disease resistance [1]. Host-selective toxins are, by definition, fungal or bacterial secondary metabolites toxic to plants that host the pathogen but have lower phytotoxicity on non-host plants [2]. The phytopathogen Alternaria brassicae (Berk.) Sacc., causative agent of Alternaria blackspot disease, is one of the most destructive fungal pathogens [3] of the economically impor-tant oilseeds rapeseed and canola (Brassica napus
and Brassica rapa) and brown mustard (Brassica juncea). Because both destruxin B and homode-struxin B are toxins produced both in vitro [4,5] and in planta [6] by A. brassicae (Berk.) Sacc., it was of great interest to establish their effect and fate on resistant and susceptible plant tissues. To-wards this end, the metabolism of the host-selec-tive toxins destruxin B (1) and homodestruxin B (2) by plants resistant and susceptible to Al-ternaria blackspot was recently investigated using synthetic radiolabeled toxins [7]. It was established that those toxins were transformed into the respec-tive hydroxydestruxin B (3) and hydroxyhomode-struxin B (4). Importantly, the rate of the metabolic transformation correlated with the plant’s disease resistance, i.e. significantly faster rates were observed for plants resistant to A. brassicae. Furthermore, preliminary bioassay data indicated that both hydroxydestruxins 3 and 4
were less toxic (caused smaller lesions on leaves) to disease susceptible species such as canola than
Abbre6iations:BAP, 6-Benzylaminopurine; 2,4-D,
2,4-Dichlorophe-noxyacetic acid; EC, Effective concentration; HPLC, High pressure liquid chromatography; HRMS, High resolution mass spectrometry; NAA, Naphthaleneacetic acid; NMR, Nuclear magnetic resonance; TLC, Thin layer chromatography.
* Corresponding author. Tel.: +1-306-9664772; fax: + 1-306-9664730.
E-mail address:[email protected] (M.S.C. Pedras).
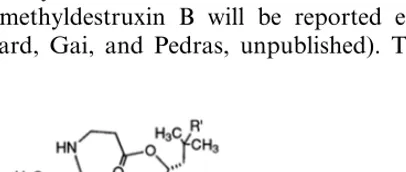
destruxin B (1) and homodestruxin B (2) [7]. Sub-sequently, to determine the usefulness and poten-tial application of these metabolic studies, it was essential to establish the phytotoxicity of destrux-ins 1–4 (Fig. 1). Although the phytotoxic activity of destruxin B and homodestruxin B was previ-ously evaluated, there is no quantitative evaluation of their toxicity to the disease resistant species Sinapis alba.
Consequently, we have developed a quantitative bioassay utilizing the staining of plant cell suspen-sion cultures of S. alba to establish a structure-phytotoxic activity correlation among destruxin B (1), homodestruxin B (2), and desmethyldestruxin B (5) (Fig. 1), a presumed toxin produced also by A. brassicae. Next, we have compared the phyto-toxicity of destruxin B (1) and hydroxydestruxin B (3) in cell suspension cultures of resistant and susceptible plant species. Finally, destruxin B (1), homodestruxin B (2), hydroxydestruxin B (3), and hydroxyhomodestruxin B (4) (Fig. 1), were tested on resistant and susceptible plant species utilizing leaf assays of whole plants and leaf uptake of toxin solutions.
2. Materials and methods
2.1. Preparation of solutions of destruxins for bioassays
All chemicals were purchased from Sigma – Aldrich Canada Ltd., Oakville, Ont. All solvents were HPLC grade. Destruxin B, [8] hydroxyde-struxin B and hydroxyhomodehydroxyde-struxin B [7] were synthesized and purified as previously reported; the syntheses of homodestruxin B and desmethyldestruxin B will be reported elsewhere (Ward, Gai, and Pedras, unpublished). The
spec-troscopic data, including NMR and HRMS, and physical data obtained for the synthetic de-struxins were identical in all respects to the natu-rally occurring compounds isolated either from cultures of A. brassicae (destruxin B [9], homode-struxin B and desmethyldehomode-struxin B [4]) or from leaves of S. alba incubated with destruxin B or homodestruxin B (hydroxydestruxin B and hy-droxyhomodestruxin B [7]). The purity of each compound was determined by TLC, HPLC, and HRMS to be higher than 99%. Stock solutions of destruxins (1×10−2 M) in acetonitrile were
utilized to prepare lower concentrations for bioassays.
2.2. Plant material
Plants were grown in a growth chamber, with 16 h light (fluorescent and incandescent, 450 – 530 mmol s−1 m−2)/8 h dark, at 2492°C.
Three different species of known resistance toA. brassicae were employed in the bioassays:S. alba, cv. Ochre (resistant), B. napus, cv. Westar (susceptible) and B. juncea, cv. Cutlass (suscepti-ble).
2.3. Preparation of cell suspension cultures
Cell cultures of three different species of known resistance to A. brassicae (blackspot) were ob-tained from protoplasts prepared by a modifica-tion of previously reported work [10], as described below. S. alba and B. juncea seeds were surface sterilized with 70% (v/v) ethanol for 1 min, 80% (v/v) commercial bleach for 20 min, and then were rinsed three times with sterile water.B.napusseeds were surface sterilized similarly but were kept in 80% (v/v) commercial bleach for 30 min. The sterilized seeds were germinated on MS (Sigma) medium (2.2 g/l MS medium, 20 g/l sucrose, 7.5 g/l agar) in the dark for 5 days at 2090.5°C. The hypocotyls were sliced into 0.5 – 1 mm pieces and incubated in plasmolysis medium for 30 min. After removal of plasmolysis medium, an enzyme solu-tion of 1% Cellulysin and 0.1% Macerase (Cal-biochem-Behring) in K3 medium [10] containing 0.4 M glucose was added to hypocotyls of S. alba and B. juncea, followed by incubation at 259
0.5°C, in the dark for 12 and 8 h, respectively; B. napus was incubated in 0.5% cellulysin and 0.05% macerase for 16 – 18 h.
The enzyme treated hypocotyls, i.e. protoplasts, were filtered through a nylon mesh of 100 mm to
eliminate large pieces of plant tissue. To the filtrate an equal volume of CPW 16 solution [10] was added and mixed with the enzyme-protoplast solution. A layer of the W5 medium [10] was carefully added to the top of the CPW 16/ enzyme-protoplast mixture, keeping the layers distinct. This mixture was then centrifuged in a swing-out rotor at 100 g for 20 min at 2590.5°C. Viable protoplasts with intact cell membranes remained between the two layers and were removed with a Pasteur pipette. The protoplast suspension was diluted with W5 medium and was centrifuged at 70 g for 10 min 2590.5°C. The pellet was rinsed again with W5 medium and centrifuged at 70 g for 10 min 2590.5°C. The pelleted protoplasts were then diluted to a density of 2×104/ml with 8p
medium (casamino acid and coconut water were omitted from 8p medium, otherwise prepared as previously by Vamling and Glimelius [10]) con-taining growth factors to a concentration of 2 – 5×104/ml and incubated in complete darkness at
2590.5°C. S. alba and B. napus were initially cultured in medium containing the following growth factors, 1.0 mg/l of 2,4-D, 0.1 mg/l of NAA and 0.1 mg/l of BAP then reduced to quar-ter strength afquar-ter 7 days;B. junceawas cultured in medium containing 1.0 mg/l NAA and 0.4 mg/l BAP continuously [11] with no growth factor re-duction in four-well Nunclon plates with no shak-ing (first cell division observed after 24 h). The cell cultures were used for phytotoxicity assays (100
ml/well) after 2 weeks of incubation.
2.4. Punctured leaf assay
Three toxin concentrations (1×10−4 M, 5×
10−5 M and 2×10−5 M) in 2% (v/v) aqueous
acetonitrile were prepared by a serial dilution. Leaves were scratched on the left side with the tip of a glass pipette, and punctured on the right side with a needle; 10ml droplets (six droplets
per leaf) were applied on scratched and punctured sites. Control leaves were treated similarly employ-ing 2% aqueous acetonitrile instead of toxin solu-tion. Plants were incubated in a growth chamber 16 h light (fluorescent and incandescent, 450 – 530 mmol s−1 m−2)/8 h dark, at 2492°C and
the diameter of the lesions was measured after 7 days.
2.5. Leaf uptake assay
Two toxin concentrations (2×10−5M and 1×
10−5 M) in 2% (v/v) aqueous acetonitrile were
prepared by a serial dilution. Leaves were cut at the base of their petiole and each leaf immediately placed in a 1.5-ml Eppendorf tube containing the phytotoxin solution (1 ml per tube per leaf). After the phytotoxin solution was taken up, an aqueous solution of BAP (1×10−5M) was added to each
tube and leaves were incubated (16-h fluorescent light, 25 – 60 mmol s−1 m−2/8-h dark at 209
0.5°C) for 7 days. Control leaves were treated similarly.
2.6. Cell staining assay
This bioassay was adapted from previously pub-lished reports [12,13]. Three toxin concentrations (5×10−4M, 5×10−5 M, and 1×10−5 M) were
prepared by a serial dilution using 8p culture medium [10] containing growth factors at a quar-ter concentration, except for B. juncea where the original growth factors concentration was used. The final concentration of acetonitrile in the cell culture medium was 5%. Experiments were carried out in triplicate in four-well Nunclon plates, with each well containing 500ml of toxin solution at the
various concentrations and 100 ml of 2-week-old
cell cultures prepared as described above. The plates were incubated without shaking in complete darkness at 2590.5°C for 10 days. The cell viabil-ity was determined after adding 10 ml of 0.1%
(w/v) phenosafranin (Sigma – Aldrich Canada) to each well and slightly shaking plates, and counting cells directly in the four-well plates using a Hund Wilovert S inverted microscope (100×). Dead cells stained red or pink and could be clearly distinguished from non-stained live cells. The per-cent viability was determined, by counting random fields of view for each replicate (at least 300 cells).
2.7. Data analysis
Table 1
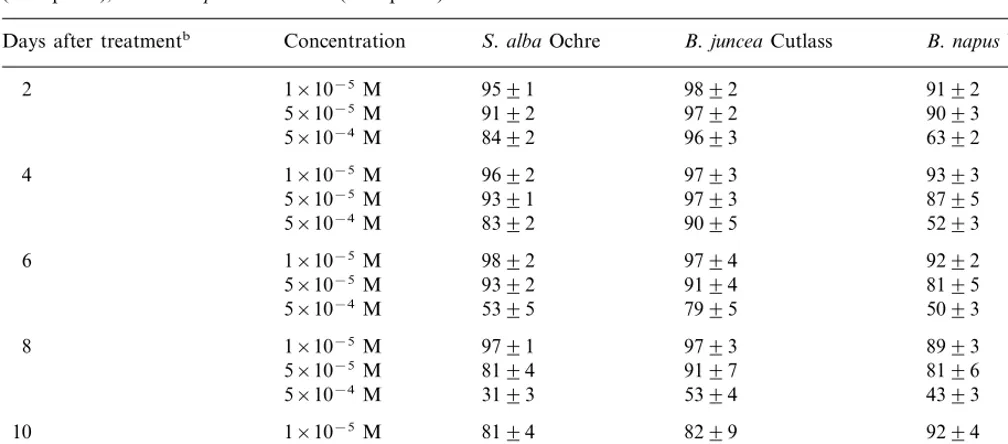
Effect of destruxin B on percent viabilityaof 2-week-old cell
cultures ofS.albacv. Ochre (resistant)
Days after treatmentb
Concentration
4
2 6 8 10
7591 6892
Controlc 8592 6591 6892
1×10−5M 8191 7292 6792 6591 5594
7792
5×10−5M 7091 6392 5494 4394
6292 3695 2193
7192 1592 5×10−4M
aResults are the means of at least four independent
experi-ments; mean9standard error.
bTime in culture following addition of destruxin B. cControl cell cultures were incubated in medium containing
5% acetonitrile.
3. Results and discussion
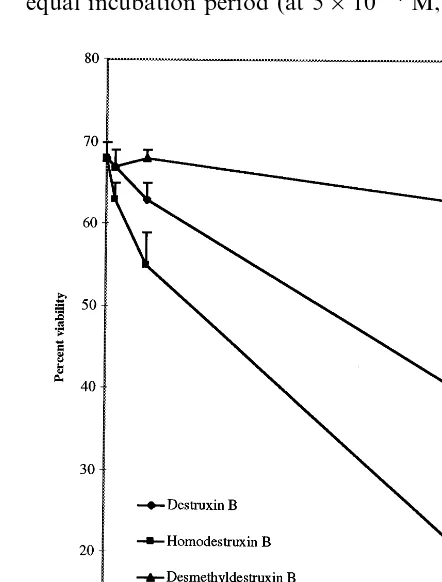
To establish a structure-activity relationship among the toxins produced by the phytopatho-genic fungus A. brassicae, the phytotoxicity of destruxin B (1), homodestruxin B (2), and desmethyldestruxin B (5) was compared utilizing cell suspension cultures ofS. albacv. Ochre (resis-tant). The results shown in Tables 1 – 3 indicate that homodestruxin B (2) is the most phytotoxic of the three compounds, whereas desmethyldestruxin B (5) is the least phytotoxic. As shown in Table 1, destruxin B (1), showed intermediate phytotoxicity toS. alba, decreasing cell viability from 65% (con-trol) to 21% after 8 days of incubation (at 5×
10−4 M, Table 1). Moreover, while
homo-destruxin B decreased the cell viability of Ochre (resistant) from 65 to 5% after 8 days of incuba-tion (at 5×10−4 M, Table 2), desmethyldestruxin
B decreased cell viability to only 44% after an equal incubation period (at 5×10−4M, Table 3). Table 2
Effect of homodestruxin B on percent viabilityaof 2-week-old
cell cultures ofS.albacv. Ochre (resistant)
Concentration Days after treatmentb
2 4 6 8 10
7591 6892 8592
Controlc 6892 6591
5393
1×10−5M 7691 6892 6392 4597
6295 7591 5594
5×10−5M 4394 3895
5×10−4M 6296 2692 1593 592 0
aResults are the means of at least three independent
exper-iments; mean9standard error.
bTime in culture following addition of homodestruxin B. cControl cell cultures were incubated in medium containing
5% acetonitrile.
Fig. 2. Effect of destruxin B (1), homodestruxin B (2), and desmethyldestruxin B (5) on percent viability of 2-week-old cell cultures of S.alba cv. Ochre (resistant), after 6 days of incubation; results are the means of six independent experi-ments9standard error (to simplify graph only positive error bars are shown); control cells were incubated in medium containing 5% acetonitrile.
Table 3
Effect of desmethyldestruxin B on percent viabilitya of
2-week-old cell cultures ofS.albacv. Ochre (resistant)
Concentration Days after treatmentb
2 4 6 8 10
6591 6892 7591
8592 6892 Controlc
7791
1×10−5M 7592 6792 6491 5693
5×10−5M 7892 7392 6891 6391 5394
7591
5×10−4M 6892 6293 4495 3398
aResults are the means of at least three independent
exper-iments; mean9standard error.
bTime in culture following addition of desmethyldestruxin
B.
cControl cell cultures were incubated in medium containing
Table 4
Effect of destruxin B on the relative viabilitya of 2-week-old cell cultures ofS.albacv. Ochre (resistant),B.junceacv. Cutlass
(susceptible), and B.napus cv. Westar (susceptible)
S.albaOchre
Days after treatmentb Concentration B.junceaCutlass B.napus Westar
9591
2 1×10−5M 9892 9192
9192 9792
5×10−5M 9093
8492 9693 6392 5×10−4M
9692
4 1×10−5M 9793 9393
5×10−5M 9391 9793 8795
8392 9095
5×10−4M 5293
9892
6 1×10−5M 9794 9292
9392 9194
5×10−5M 8195
5×10−4M 5395 7995 5093
9791
8 1×10−5M 9793 8993
5×10−5M 8194 9197 8196
5×10−4M 3193 5394 4393
8194
10 1×10−5M 8299 9294
6394 8298 8195 5×10−5M
2292 3793 3291 5×10−4M
aValues represent the ratios of percentage of viable cells (means of at least three separate experiments) incubated with destruxin
B over percentage of viable control cells incubated in medium containing 5% acetonitrile (% viable cells treated with destruxin B/% untreated cells).
bTime in culture following addition of destruxin B.
The higher toxicity of homodestruxin B is clearly shown in Fig. 2. After 6 days of incubation at any of the concentrations, desmethyldestruxin B (5) did not appear to affect significantly the cell viabil-ity of Ochre (resistant), while destruxin B at 5×
10−5 M decreased the cell viability from 65 to
36%, and homodestruxin B at similar concentra-tion, decreased cell viability from 65 to 15%. The EC of each toxin, defined as the concentration at which the viability of toxin treated cells is 50% of the viability of untreated cells (control cells) after 6 days of incubation, also indicated that homode-struxin B (EC50 3×10−
4 M) is the most toxic of
the destruxins, followed by destruxin B (EC505×
10−4 M), and desmethyldestruxin B (EC
505×
10−4 M). These results indicated that
demethylation of the valyl amino acid residue of destruxin B, to give the slightly more polar com-pound desmethyldestruxin B, led to a significant decrease in phytotoxicity, whereas introduction of a methylene group in the same valyl amino acid residue of destruxin B, to give the slightly less polar compound homodestruxin B, led to a signifi-cant increase in phytotoxicity. To the best of our knowledge this is the first time a quantitative assay is utilized to establish a structure-activity
correla-tion of these toxins to plant tissues of a disease resistant species. Interestingly, due to the produc-tion of a variety of destruxins by different fungal species and genera, their biological activity to very different organisms has been investigated. For ex-ample, destruxin B and desmethyldestruxin B showed similar insecticidal activity to the tobacco budworm (Heliothis 6irescens) [14], while
homode-struxin B was a more potent inducer of human erythropoietin than destruxin B [15].
Next, the toxicity of destruxin B (1) towardsS. alba cv. Ochre (resistant), B. napus cv. Westar (susceptible), and B. juncea cv. Cutlass (suscepti-ble) was compared and the results are shown in Table 4. Up to 4 days of incubation, cell suspen-sion cultures of cv. Westar were the most sensitive to destruxin B, in direct correlation with plant disease susceptibility. Nonetheless, after 6 days of incubation destruxin B at the highest concentra-tion (5×10−4 M) decreased the viability of cell
viabil-ity of cells of B. juncea cv. Kranti treated with destruxin B (2×10−4 M) for 4 days was
deter-mined to be 39% by flow cytometric analysis [16]; however, considering that the viability of those cells was determined utilizing a different assay and cultivar, no comparisons can be established.
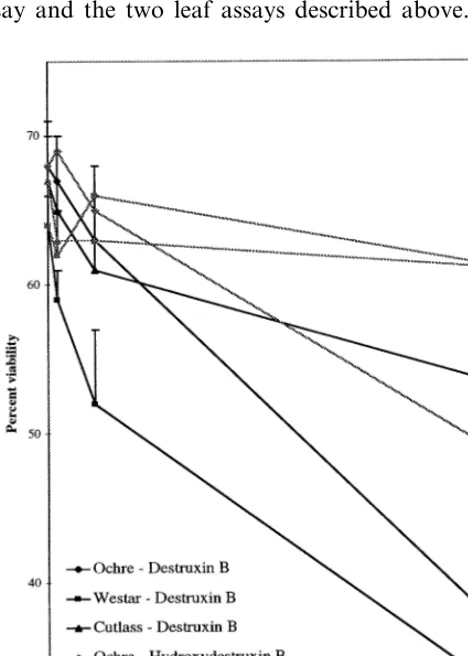
To establish if the hydroxylation of destruxin B and homodestruxin B by plants resistant and sus-ceptible to A. brassicae represented in fact a metabolic detoxification, it was crucial to deter-mine the phytotoxicity of hydroxydestruxin B and hydroxyhomodestruxin B towards those plants [7]. Thus, the phytotoxicity of destruxin B (1) and hydroxydestruxin B (3) towards S. albacv. Ochre, B.napus cv. Westar, andB.junceacv. Cutlass was evaluated utilizing the cell suspension culture as-say and the two leaf asas-says described above. The
results of the cell suspension culture assays are shown in Fig. 3; after 6 days of incubation, de-struxin B was slightly more toxic to cells of Westar (EC504.5×10−
4M) than to those of Ochre
(resis-tant) (EC50 5×10−4 M), and cells of Cutlass
(EC505×10−4M) were much less affected.
Im-portantly, this assay allowed a clear distinction between the toxicity of destruxin B and hydrox-ydestruxin B. That is, destruxin B was relatively more toxic to each of the three cultivars than hydroxydestruxin B (EC505×10−4 M); in fact,
as shown in Fig. 3, the viability of cell cultures of the susceptible cultivars Westar and Cutlass treated with hydroxydestruxin B was similar to those of untreated cell cultures.
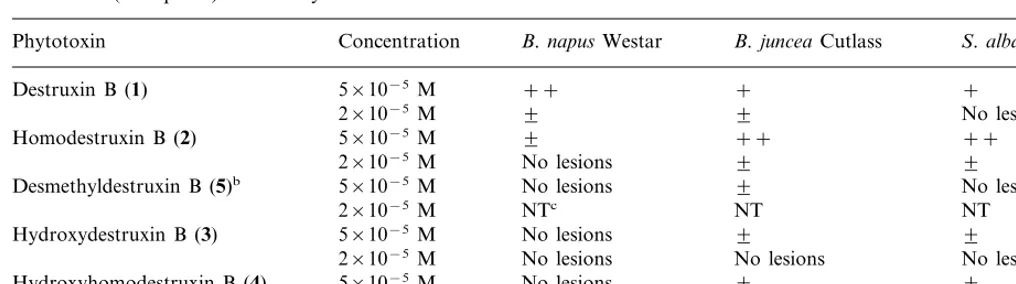
Punctured/scratched leaf assays were carried out as described in the materials and methods with the cultivars Ochre (resistant), Cutlass (susceptible), and Westar (susceptible) and the five destruxins. There were no significant differences between le-sions caused by toxins observed on the scratched side of the leaf and lesions observed on the punc-tured side. Although these assays may allow only qualitative comparisons, the results shown in Table 5 are consistent with the cell suspension assays (Tables 1 – 4). That is, both destruxin B and homodestruxin B appeared to cause larger lesions than desmethyldestruxin B, consistent with the lower phytotoxicity observed for the latter in cell culture assay (Table 3). As well, hydroxydestruxin B and hydroxyhomodestruxin B caused very small to no detectable lesions on the leaves of the three cultivars, indicating a lower toxicity than the par-ent toxins. In addition, leaf uptake assays were carried out with the cultivars Ochre (resistant), Cutlass (susceptible), and Westar (susceptible) and the compounds destruxin B, homodestruxin B, hydroxydestruxin B and hydroxyhomodestruxin B. The results of leaf uptake assay indicated that Ochre (resistant) leaves were strongly affected by both destruxin B and homodestruxin B (at both concentrations); after 2 days of incubation, leaves showed yellow lesions spread over the whole lam-ina, and after 7 days appeared highly dehydrated. Both hydroxylated derivatives (hydroxydestruxin B and hydroxyhomodestruxin B) caused signifi-cantly less damage than the parent toxins, at the highest concentration, and had no effect at the lowest concentration. Both destruxin B and ho-modestruxin B caused yellow lesions spread at the edges of the leaves of Cutlass but not as intense as
Fig. 3. Effect of destruxin B and hydroxydestruxin B on percent viability of 2-week-old cell cultures of S. alba cv. Ochre (resistant), B. napus cv. Westar (susceptible), and B.
Table 5
Results of bioassays carried out with destruxin B, homodestruxin B, hydroxydestruxin B, hydroxyhomodestruxin B, and desmethyldestruxin B applied to punctured leavesaofS.albacv. Ochre (resistant),B.napuscv. Westar (susceptible), andB.juncea
cv. Cutlass (susceptible) after 7 days of incubation
B.napusWestar
Phytotoxin Concentration B.junceaCutlass S.albaOchre
++
Destruxin B (1) 5×10−5M + +
2×10−5M 9 9 No lesions
9 ++ ++
Homodestruxin B (2) 5×10−5M
No lesions 9
2×10−5M 9
No lesions 9
Desmethyldestruxin B (5)b 5×10−5M No lesions
NTc NT
2×10−5M NT
5×10−5M
Hydroxydestruxin B (3) No lesions 9 9
No lesions No lesions
2×10−5M No lesions
5×10−5M
Hydroxyhomodestruxin B (4) No lesions 9 9
2×10−5M No lesions No lesions No lesions
aThree-week-old plants punctured with needle and 10ml droplet of compound (in acetonitrile-H
2O) applied with micropipette;
leaves scored after 1 week; × =lesion diameter 1–2 mm; + =lesion diameter 3–5 mm; ++ =lesion diameter 6–8 mm;
+++ =lesion diameter]9 mm.
bCaused slight (9) lesions at 2×10−4M. cNot tested.
in Ochre (resistant), while hydroxydestruxin B and hydroxyhomodestruxin B did not appear to cause obvious damage. Westar appeared to be much less affected than either Ochre or Cutlass, with ho-modestruxin B causing some lesions, while the other four compounds did not cause obvious le-sions. Overall, the leaf uptake assays did not indi-cate a direct correlation between the plant disease resistance and the toxicity of destruxin B or ho-modestruxin B.
It is pertinent to contrast our bioassay results of destruxin B (quantitative plant assays of other destruxins have not been reported to date) with plant assays previously carried out by other groups. Similar to our findings with cell suspen-sion cultures, pollen germination (and pollen tube growth) and leaf assays [17] utilizing a variety of different species, found Westar more sensitive to destruxin B than Ochre. On the other hand, based on leaf scratch or puncture assays (concentration range from 4×10−4 M to 2×10−5 M) it was
reported that B. juncea cv. Lethbridge was more sensitive to destruxin B thanB. napuscv. Altex (S. alba not examined) [5]. Somewhat different data was reported for tests conducted on leaves of 30 plant species, including S. alba, B. juncea, and B. napus (cvs. not reported); while destruxin B (2×
10−4 M) appeared to affect similarly leaves of S.
alba andB. juncea, leaves ofB. napus appeared to be less sensitive [18]. These reports [5,16 – 18] and
also our leaf puncture/scratch and leaf uptake assays indicate that it is important to utilize less subjective and also more quantitative assays to evaluate phytotoxicity and be able to make con-clusions regarding the selective activity of fungal or bacterial metabolites. The cell suspension cul-ture assays we report here indicate that a better correlation between the disease resistance of each cultivar and the toxicity level of each compound can be achieved utilizing appropriate experimental conditions. These assays, however, have two dis-advantages, i.e. they are technically demanding and time consuming and therefore more difficult to carry out with large number of species than the much simpler leaf assays.
Finally, it is worth noting that the effects caused by phytotoxins depend highly on the experimental conditions and on the mechanism of action of each particular compound. It is well-known that the toxic concentration ranges can be very wide (10−3– 10−10 M), two extreme examples being
HC-toxin, which is inhibitory of maize root growth but does not kill cells or protoplasts, and victorine, highly toxic to oat cells at very low concentrations (10−10 M) [19]. The ED
50 of
Acknowledgements
The synthesis of destruxins was carried out by Y. Gai, R. Lasmy and D.E. Ward. This work was supported by a Strategic Research Grant from the Natural Sciences and Engineering Research Coun-cil of Canada to MSCP.
References
[1] M.S.C. Pedras, Towards an understanding and control of plant fungal diseases in Brassicaceae, Recent Re-search Dev. Agricultural Food Chem. 2 (1998) 513 – 532. [2] A. Graniti, Phytotoxins and their involvement in plant
disease. Introduction, Experientia 47 (1991) 751 – 756. [3] G.S. Saharan, Disease resistance, in: K.S. Labana, S.S.
Banga, S.K. Banga (Eds.), Breeding Oilseed Brassicas, Monographs on Theoretical and Applied Genetics, vol. 19, Springer-Verlag, Berlin, 1993, pp. 181 – 205. [4] W.A. Ayer, L.M. Pen˜a-Rodriguez, Metabolites produced
by Alternaria brassicae the blackspot pathogen of canola. Part 1, the phytotoxic components, J. Nat. Prod. 50 (1987) 400 – 407.
[5] P.S. Bains, J.P. Tewari, Purification, chemical characteri-sation and host-specificity of the toxin produced by
Alternaria brassicae, Physiol. Mol. Plant Pathol. 30 (1987) 259 – 271.
[6] L. Buchwaldt, J.S. Jensen, HPLC purification of destrux-ins produced by Alternaria brassicae in culture and leaves of Brassica napus, Phytochemistry 30 (1991) 2311 – 2316.
[7] M.S.C. Pedras, I.L. Zaharia, Y. Gai, K.C. Smith, D.E. Ward, Metabolism of the host-selective toxins destruxin B and homodestruxin B: probing a plant disease resis-tance trait, Organic Lett. 1 (1999) 1655 – 1658.
[8] D.E. Ward, R. Lazny, M.S.C. Pedras, Synthesis of the host-selective phytotoxin destruxin B. Avoiding dike-topiperazine formation from an N-methyl amino acid dipeptide by use of the Boc-Hydrazide derivative, Tetra-hedron Lett. 38 (1997) 339 – 342.
[9] M.S.C. Pedras, K.C. Smith, Sinalexin, a phytoalexin from white mustard elicited by destruxin B and Al
-ternaria brassicae, Phytochemistry 46 (1997) 833 – 837.
[10] K. Vamling, K. Glimelius, Regeneration of plants from protoplasts of oilseed Brassica crops, in: Y.P.S. Bajaj (Ed.), Legumes and Oilseed Crops I. Biotechnology in Agriculture and Forestry, vol. 10, Springer-Verlag, Berlin, 1990, pp. 385 – 417.
[11] H.-M. Kao, G. Se´guin-Swartz, Study of factors affecting the culture of Brassica napus L. and B. juncea Coss. mesophyll protoplasts, Plant Cell Tissue Organ Culture 10 (1987) 79 – 90.
[12] J.M. Widholm, The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells, Stain Technol. 47 (1972) 189 – 194.
[13] S. Li, G.L. Hartman, J.M. Widholm, Vital staining of soybean suspension cultured cells and a seedling stem-cutting assay to evaluate phytotoxicity of Fusarium solaniculture filtrates, Phytopathology 87 (1997) S58. [14] S. Gupta, D.W. Roberts, J.A.A. Renwick, Insecticidal
cyclodepsipeptides from Metarhizium anisopaliae, J. Chem. Soc. Perkin Trans. I (1989) 2347 – 2357.
[15] P. Cai, D. Smith, B. Katz, C. Pearce, D. Venables, D. Houck, Destruxin-A4 chlorohydrin, a novel destruxin from fungus OS-F68576: isolation, structure determina-tion, and biological activity as an inducer of erythropoi-etin, J. Nat. Prod. 61 (1998) 290 – 293.
[16] T.R. Sharma, J.P. Tewari, Flow cytometric analysis of
Brassica juncea cell and pollen cultures treated with destruxin B, a toxin produced by Alternaria brassicae, Physiol. Mol. Plant Pathol. 48 (1996) 379 – 387. [17] K.R. Shivanna, V.K. Sawhney, Pollen selection for
al-ternaria resistance in oilseed brassicas: responses of pol-len grains and leaves to a toxin ofA.brassicae, Theor. Appl. Genet. 86 (1993) 339 – 344.
[18] L. Buchwaldt, H. Green, Phytotoxicity of destruxin B and its possible role in the pathogenesis of Alternaria brassicae, Plant Pathol. 41 (1992) 55 – 63.
[19] J.D. Walton, Host-selective toxins, agents of compatibil-ity, Plant Cell 8 (1996) 1723 – 1733.