Summary Beech leaves were sampled at the end of a pro-longed hot dry period at a tree decline site in the Black Forest, Germany to investigate the potential role of flavanols in defense mechanisms against environmental stress. Green and yellow-ing leaves were harvested from the uppermost canopy of trees that were more than 200 years old and 30 m high. Yellowing leaves had a 7.4-fold higher concentration of total flavanols than green leaves. Green leaves contained flavanol inclusions, but during yellowing the inclusions disintegrated and the cells became filled with flavanols. Abscisic acid (ABA) stimulated the release of flavanols from intravacuolar inclusions of leaf petioles and flower pedicels. In addition, ABA caused flavanols to leach from the trichomes of beech galls. The antioxidative potential of leaf extracts, as estimated by indoleacetic acid (IAA) oxidation, was significantly higher in yellowing leaves than in green leaves. In vitro experiments revealed that (+)-cate-chin promoted growth of beech tissue.
Keywords: abscisic acid, catechins, histology, leaf yellowing, tree decline.
Introduction
Photooxidants induce yellowing in coniferous needles and accumulation of stilbenes (Rosemann et al. 1991) and tannin (Pääkkönen et al. 1995). Similarly, UV radiation results in an accumulation of phenolic metabolites in suspension cultures of beech (Jungblut et al. 1994). Zielke and Sonnbichler (1990) found a novel biphenyl compound that accumulated several fold in fungus-infected leaves of the upper crown of tall beech trees. Based on the observation of Richter et al. (1996) that the accumulation of (+)-catechin in declining trees is positively correlated with needle loss and the altitude of the tree site, we hypothesized that air pollution causes an increase in active oxygen radicals such as superoxide and hydroxyl radicals (Smirnoff 1993) that result in changes in tree growth processes and an increase in the concentrations of phenols (Walton and Sondheimer 1968) and abscisic acid (ABA) (Eze et al. 1986). Abscisic acid acts as a signalling component in tree systems (Hetherington and Quatrano 1991).
If polyphenols increase in response to stress, one function of proanthocyanidins, the condensation products of flavanols, might be to complex with macromolecules (Haslam 1994). Furthermore, the antioxidative properties of soluble low mo-lecular weight o-dihydroxyphenols (Stonier and Yang 1973,
Volpert et al. 1995) could be associated with a capacity to scavenge free oxygen radicals (Torel et al. 1986, Bors et al. 1990).
Based on the finding that yellowing leaves of young under-story beech trees contain more flavanols than do green leaves, it has been suggested that flavanols are involved in defence mechanisms against environmental stress (Feucht et al. 1994). Because the uppermost crowns of tall beech trees are exposed to more sunlight and air pollution than understory trees, they may divert more carbon toward flavanol synthesis than under-story trees. The purpose of this study, therefore, was (i) to quantify the flavanols of leaves from the uppermost crown of beech trees, and (ii) to evaluate possible metabolic roles of flavanols in maintaining cellular viability.
Material and methods
Study sites and sampling
Two sample beech (Fagus sylvatica L.) trees were selected from a mixed hardwood--conifer stand in a nature reserve forest area in the Northern Black Forest, Germany, 30 km east of Strassburg at 730 m elevation. This area is exposed to west-wind air pollution. The soil is a well-drained, deep, fine sandy loam derived from red sandstone. During July and August 1994 and 1995, extended periods of high intensity illumination were recorded in the region. The sample trees were both 200 years old and approximately 30--33 m tall. The two trees, 25 m apart, differed in leaf coloration; one tree had green leaves and the other tree had yellowing leaves. Leaves were sampled randomly toward the end of August from four branches (2 m long) and pooled to give one composite sample per branch. The branches had been cut from the uppermost crown at a height of 30 m above the ground. For histological studies, leaves from the same branches were sampled. Addi-tionally, sun-exposed leaves from 20-year-old understory trees were sampled at a second study site characterized by a 4-ha tract of healthy looking and vigorously growing beech trees. The understory trees were located in a wind-protected stand with an eastern exposure in a valley at 480 m elevation, 15 km away from the first site.
Histochemical localization of flavanols
Small leaf pieces (3 × 3 mm) and segments of leaf petioles and female flower pedicels, 3 mm in length, were fixed in 2.5%
Role of flavanols in yellowing beech trees of the Black Forest
W. FEUCHT, D. TREUTTER and E. CHRIST
Technical University of Munich, Faculty of Agriculture and Horticulture, D-85354 Freising-Weihenstephan, Germany
Received February 21, 1996
glutaraldehyde. Dehydration, dealcoholization, and embed-ding in Historesin (Reichert-Jung, Vienna, Austria) was carried out according to standard procedures. Semi-thin sections (5 µm) were cut with a Leitz sliding microtome, and stained with p-dimethylaminocinnamaldehyde (DMACA) (Gutmann and Feucht 1991). This reagent is highly selective for flavanols, yielding a bluish color. At low flavanol concentra-tions the stain has a violet tinge. A total of 100 leaf cross sections per tree were prepared for examination by light mi-croscopy. The pedicel sections from female flowers were sec-tioned longitudinally; a total of 40 sections were prepared per treatment.
Tissue culture
Three-mm segments of leaf petioles and female flower ped-icels were grown for 3 weeks in test tubes in a 16-h photope-riod (Gro Lux Lamps 60 µmol m--2 s--1) on MS-medium (Murashige and Skoog 1962) containing 0.8 µM benzyladen-ine (BA) and 57 µM indoleacetic acid (IAA) in the absence and presence of 68 µM (+)-catechin or 20 µM ABA. After 3 weeks of cultivation the cultures were analyzed histochemi-cally.
Identification of flavanols by HPLC--CRD
Freeze-dried and powdered leaf tissue samples (100 g dry weight) were thoroughly extracted with acetone (80% v/v in water) and analyzed by high-performance liquid chromatogra-phy (HPLC) coupled with a post-column reaction detection (CRD) technique, described by Treutter (1989) and Treutter et al. (1994), and modified by Feucht et al. (1994). A major advantage of the CRD technique is the use of DMACA which selectively stains all monomeric and oligomeric flavanols pre-sent in the plant extract.
Total flavanols in the extracts were determined colorimetri-cally after staining with DMACA (Feucht et al. 1994). To estimate total soluble proanthocyanidins, acetone extracts were boiled for 20 min with butanol/HCl (5/1, v/v). Because insoluble proanthocyanidins bound tightly to the acetone pow-der, 20 mg of dried acetone powder was boiled for 1 h in butanol/HCl to yield anthocyanidins. The resulting red colora-tion was measured spectrophotometrically at 550 nm and cal-culated as cyanidin.
Four independent samples from each tree were analyzed and paired t-tests were applied to evaluate differences between green and yellowing leaves.
Determination of total antioxidant properties of leaf extracts
We used the peroxidative IAA degradation system developed by Volpert and Elstner (1993) to compare the antioxidative properties of crude methanolic extracts. The assay mixture (1 ml) contained: 270 µl 150 mM citrate-phosphate buffer, pH 5.6; 100 µl 0.3 mM IAA dissolved in 10 mM NaOH; 130 µl (corresponding to 0.3 U) of horse radish peroxidase (HRP, Sigma, St. Louis, MO) in buffer; and 500 µl of diluted leaf extract (10 µl of the methanolic solution diluted with 990 µl of buffer). Trace amounts of methanol did not influence the reac-tion. Ten µl of undiluted methanolic extract was equivalent to
1 mg dry weight of leaf tissue. Each assay was run with three independent samples from both the green-leaved tree and the tree with yellowing leaves.
Leaching experiments
To study the leaching of flavanols, 100 mg of hairy galls was submerged for 3, 6 and 9 days in distilled water (controls) or in 40 mM ABA. After the leaching period, the incubation media were dried under vacuum at 40 °C, dissolved in metha-nol and then analyzed for flavametha-nols (Feucht et al. 1994). The flavanols were calculated as (+)-catechin. Each experiment was repeated twice.
Results and discussion
Determination of flavanols
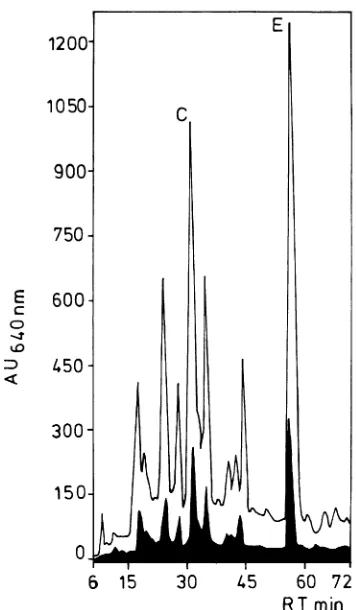
Figure 1 shows HPLC profiles of flavanols in extracts from green and yellowing leaves collected from trees at the air-pol-luted site. The concentration of total flavanols in yellowing leaves was 7.4-fold greater than in green leaves (28.3 versus 3.8 mg gDW--1 ) (Table 1). The means of the four replicates were
significant at P < 0.001. In both green and yellowing leaves, the major compounds were (+)-catechin (C) and epicatechin
(E). None of the other 19 flavanolic compounds detected in the beech leaves have been identified previously. They are prob-ably di- and trimeric proanthocyanidins. Insoluble proanthocy-anidins were present in all samples.
Frequency of flavanol inclusions in green and yellowing leaves
There were few flavanol inclusions in green leaves and none in yellowing leaves collected from trees at the air-polluted site
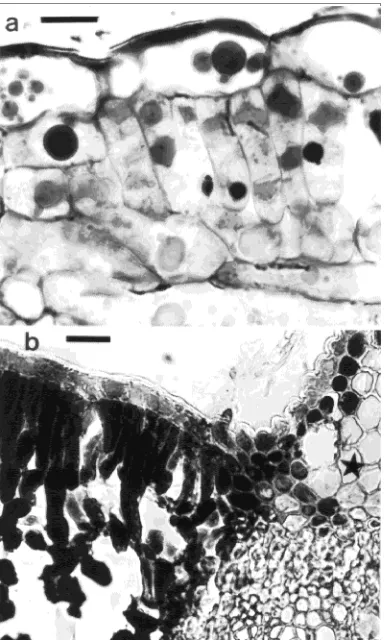
(Figure 2); however, green leaves collected from understory trees at an unpolluted site contained a high frequency of inclusions, especially in the bundle sheaths of the veins. Al-though the epidermal cells of the yellowing leaves were devoid of flavanol inclusions, they presented a diffuse faint stain for flavanols.
Staining with DMACA showed that most flavanols in the green leaves from the unpolluted site were deposited in inclu-sions in both the epidermis and mesophyll (Figure 3a). During yellowing, the inclusions disintegrated and both the palisade cells and spongy parenchyma cells became entirely filled with large amounts of flavanols (Figure 3b, left side). The lumen of the upper epidermis also became completely filled with flavanols, but the staining intensity was less pronounced than for the palisade cells and spongy parenchyma cells. The roun-dish parenchyma cells of the upper vein stained heavily for flavanols, whereas the underlying cells were distorted and contained clumped plasmic material with moderate staining intensity. Palisade cells located around a lesion were abnor-mally rounded (Figure 3, asterisk denotes a lesion). The small cells located beneath the epidermis contained abundant flavanols.
Table 1. Flavanol concentrations (mggDW--1) of green and yellowing
leaves sampled in the uppermost crown at a height of 30 m. Peak number refers to the elution peaks of the HPLC profile shown in Figure 1. Mean values of four independent replicates (± SE).
Peak no. Yellowing leaves Green leaves
1 0.47 ± 0.26 0.33 ± 0.20 2 1.89 ± 0.19 0.60 ± 0.35 3 1.97 ± 0.22 0.25 ± 0.08
4a 4.13 ± 0.43
--4 (Catechin) 3.06 ± 0.35 0.67 ± 0.22 5 0.53 ± 0.00 0.37 ± 0.27 6 1.26 ± 0.10 0.15 ± 0.02 7 2.05 ± 0.22 0.19 ± 0.04 8 3.06 ± 0.32 0.32 ± 0.03 9 (Epicatechin) 4.98 ± 0.51 0.53 ± 0.15 10 0.33 ± 0.12 0.03 ± 0.01 11 1.25 ± 0.07 0.10 ± 0.06 12 0.72 ± 0.08 0.05 ± 0.02
13 0.23 ± 0.03 0.03
14 0.57 ± 0.19 0.04
15 0.28 ± 0.04 0.00
15a 0.17 ± 0.02 0.01
16 0.38 ± 0.05 0.03
17 0.16 ± 0.01 0.03
18 0.48 ± 0.16 0.04
19 0.31 ± 0.03 0.02
Figure 2. Number of cells with flavanol inclusions (mean ± SE, n = 4 leaves) for green and yellowing leaves at the polluted site, and for green leaves at the unpolluted site (B. Sheath = bundle sheath cells).
Effect of ABA on flavanol inclusions
To determine whether ABA was involved in the disintegration of the flavanol inclusions during leaf yellowing, we studied the effect of ABA on flavanol inclusions in in vitro cultivated leaf petioles and female flower pedicels. Longitudinal sections of control flower pedicels (Figure 4b) were characterized by a high frequency of ball-shaped flavanol inclusions between 2 and 10 µm in diameter and located in the phloem. On addition of 40 µM ABA (Figure 4c), the inclusions disintegrated and flavanols moved to the cell periphery and accumulated along the walls of narrow and elongated phloem cells.
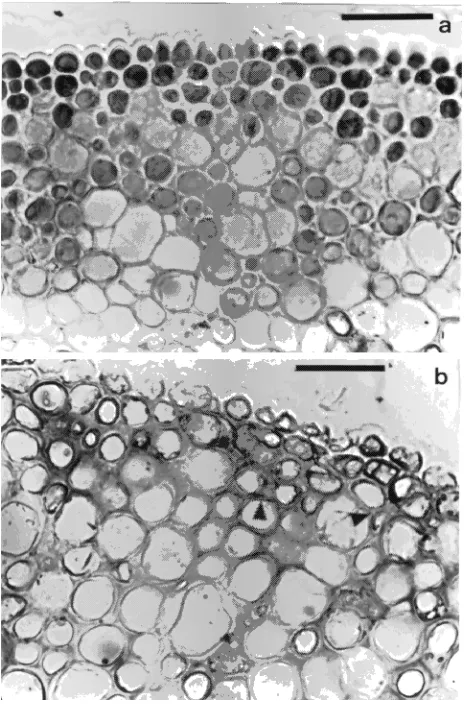
A similar response to ABA was observed in transverse sections of leaf petioles grown in vitro. In the controls, the flavanols were located in inclusions in the lumen of epidermal and one or two subepidermal layers, with the frequency of occurrence of the inclusions decreasing progressively in the underlying cortex (Figure 5a). On addition of ABA, the inclu-sions disintegrated and the flavanols moved toward the cell periphery and occasionally into the cell walls (arrowheads Figure 5b). Movement of flavanols into the cell walls was prevalent in subepidermal layers, whereas flavanols did not penetrate the epidermal cell walls at all.
Leaching of flavanols from gall trichomes
Galls on beech leaves induced by the insect Hartigiola annuli-pes (Hartig) contain abundant flavanols in the lumen of the thick-walled hair trichomes that cover the galls as a dense felt. During October, when the entire galls abscise easily from the leaves, they were collected and subjected to leaching experi-ments (Figures 6A and 6B). Leaching of flavanols was greatly increased in the presence of 40 µM ABA. The leaching pattern differed slightly between galls collected in early October (A) and those collected at the end of October (B), although after nine days, in the presence of ABA, leaching of flavanols from the galls was negligible, irrespective of collection date.
Antioxidative properties of leaf extracts
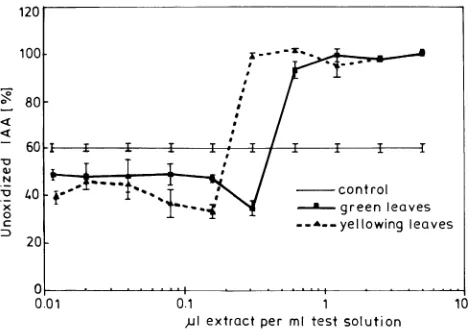
The peroxidase-catalyzed IAA-oxidation test (Volpert and Elstner 1993) revealed a significantly higher antioxidative potential in methanolic extracts of yellowing leaves than of
green leaves (Figure 7). Stepwise dilution of the methanolic extracts indicated a threshold value at which there was an abrupt, steep fall in their reducing power, resulting in a 65%
Figure 5. Transverse sections of leaf petioles cultivated in vitro. (a) Control explants with abundant flavanol inclusions in the cell lumen and non-staining cell walls, especially in the epidermis and in the first subepidermal layer; and (b) explants cultivated on ABA showing disintegration of flavanol inclusions and movement of flavanols to the cell periphery and into the cell walls (arrowheads) (bar = 50 µm).
decrease in IAA. This threshold value was higher for green leaves than for yellowing leaves.
Growth promotion of flower pedicels by (+)-catechin
Compared with the controls (Figure 4b), addition of 68 µM (+)-catechin to in vitro cultivated flower pedicels enhanced growth of both the cortex and phellogen, (as shown by toluid-ine staining in Figure 4a) resulting in an increase in pedicel diameter. In the phellogen (P), (+)-catechin enhanced pericli-nal cell division, which resulted in well-ordered radial cell lineages. The outermost phellem cells, however, were radially elongated. The (+)-catechin-induced stimulation of cell divi-sion in the cortical parenchyma (Figure 4a) resulted in the formation of isodiametric cells that gave the extracambial tissue a callus-like appearance (arrowheads).
In conclusion, we observed that, in green leaves, flavanols were confined to inclusions; however, during leaf yellowing the inclusions disintegrated and cells became filled with flavanols. Disintegration of inclusions was mimicked experi-mentally by applying ABA. Several studies have shown that both ABA synthesis and solute leaching are stimulated during long-term environmental stress (Rich 1964, Trippi and Thi-mann 1983, Lethiec et al. 1995). We obtained evidence that flavanols are involved in a variety of defence reactions of yellowing beech leaves. Thus, (+)-catechin has the potential both to stabilize IAA and to promote growth (Feucht and Treutter 1995). The antioxidative potential of yellowing leaves, as determined by inhibition of IAA oxidation (Volpert and Elstner 1993), was greater than that of green leaves. Based on HPLC profiles, we postulate that nearly 20 flavanols con-tributed to this antioxidative potential, in addition to a number of other antioxidative compounds. We conclude that flavanols are involved in the defence mechanisms of beech against environmental stress; however, leaf yellowing and the in-creased abundance of flavanols is only one aspect of the complex phenomenon of beech decline.
References
Bors, W., W. Heller, C. Michel and M. Saran. 1990. Radical chemistry of flavonoid antioxidants. In Antioxidants in Therapy and Preventive Medicine. Eds. I. Emerit, L. Packer and C. Auclair. Plenum Press, New York, pp 165--170.
Eze, J.M.O., S. Mayak, J.E. Thompson and E.B. Dumbroff. 1986. Senescence in cut carnation flowers: Temporal and physiological relationships among water status, ethylene, abscisic acid and mem-brane permeability. Physiol. Plant. 68:323--328.
Feucht, W. and D. Treutter. 1995. Catechin effects on growth related processes in cultivated calli of Prunus avium. Gartenbauwissen-schaft 60:7--11.
Feucht, W., D. Treutter and E. Christ. 1994. Accumulation of flavanols in yellowing beech leaves from forest decline sites. Tree Physiol. 14:403--412.
Gutmann, M. and W. Feucht. 1991. A new method for selective localization of flavan-3-ols in plant tissues involving glycol-methacrylate embedding and microwave irradiation. Histochemis-try 96:83--86.
Haslam, E. 1994. Plant polyphenols----a case of biochemical co-evolu-tion. In Int. Symp. Natural Phenols in Plant Resistance, Weihen-stephan. Eds. M. Geibel, D. Treutter and W. Feucht. Acta Hortic. 381:722--737.
Hetherington, A.M. and R.S. Quatrano. 1991. Mechanisms of action of abscisic acid at the cellular level. New Phytol. 119:9--32. Jungblut, T.P., W. Heller, N. Hertkorn, J. Lintelmann and H.
Sander-mann, Jr. 1994. Isolation and identification of induced secondary metabolites from cell suspension cultures of Fagus sylvatica. In Int. Symp. Natural Phenols in Plant Resistance, Weihenstephan. Eds. M. Geibel, D. Treutter and W. Feucht. Acta Hortic. 381:470--473. Lethiec, D., M. Dixon and J.P. Garrec. 1995. Distribution and
vari-ations of potassium and calcium in different cross sections of Picea abies (L.) Karst. needles and Fagus sylvatica (L.) leaves exposed to ozone and mild water stress. Ann. Sci. For. 52:411--422.
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473--497. Figure 6. Effect of incubation with ABA for 3, 6 or 9 days on leaching
of flavanols from trichomes of beech galls calculated as (+)-catechin in mggDW--1 (mean ± SE, n = 4). The galls were collected at the beginning
(A) and end (B) of October.
Figure 7. Antioxidative potential of extracts from green and yellowing leaves as measured by IAA oxidation. Bars indicate ± SE of the means,
Pääkkönen, E., T. Holopainen and L. Kärenlampi. 1995. Ageing-re-lated anatomical and ultrastructural changes in leaves of birch (Betula pendula Roth.) clones as affected by low ozone exposure. Ann. Bot. 75:285--294.
Rich, S. 1964. Ozone damage to plants. Annu. Rev. Phytopathol. 2:253--266.
Richter, C.M., U. Eis and A. Wild. 1996. Phenolic compounds as a tool of bioindication for novel forest decline at numerous spruce tree sites in Germany. Z. Naturforsch. 51C:53--58.
Rosemann, D., W. Heller and H. Sandermann, Jr. 1991. Biochemical plant responses to ozone. II. Induction of stilbene biosynthesis in Scots pine (Pinus sylvestris L.) seedlings. Plant Physiol. 97:1280--1286.
Smirnoff, N. 1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 125:27--58. Stonier, T. and H. Yang. 1973. Studies on auxin protectors. XI.
Inhibi-tion of peroxidase catalyzed oxidaInhibi-tion of glutathione by auxin protectors and o-dihydroxyphenols. Plant Physiol. 51:391--395. Torel, J., J. Cillard and P. Cillard. 1986. Antioxidant activity of
fla-vonoids and reactivity with peroxy radical. Phytochemistry 25:383--385.
Treutter, D. 1989. Chemical reaction detection of catechins and proan-thocyanidins with 4-dimethylaminocinnamaldehyde. J. Chroma-togr. 467:185--193.
Treutter, D., C. Santos-Buelga, M. Gutmann and H. Kolodziej. 1994. Identification of flavan-3-ols and procyanidins by high performance liquid chromatography and chemical reaction detection. J. Chroma-togr. 667A:290--297.
Trippi, V. and K.V. Thimann. 1983. The exudation of solutes during senescence of oat leaves. Physiol. Plant. 58:21--28.
Volpert, R. and E.F. Elstner. 1993. Biochemical activities of propolis extracts. I. Standardization and antioxidative properties of ethanolic and aqueous derivatives. Z. Naturforsch. 48C:851--857.
Volpert, R., W. Osswald and E.F. Elstner. 1995. Effects of cinnamic acid derivates on indole acetic acid oxidation by peroxidase. Phyto-chemistry 38:19--22.
Walton, D.C. and E. Sondheimer. 1968. Effects of abscisin II on phenylalanine ammonia-lyase activity in excised bean axes. Plant Physiol. 43:467--469.