Supplementary Materials.
Table I.
a) Internuclear Distances (Å) and b) Angles (degrees) for the optimized
structures of the three energetically most stable N(1)-H/N(5)-H (K15),
N(2)-H/N(5)-H (K25) and cis-enolic/N(1)-H (cE1) neutral allopurinol tautomers.
Table II.
Corrected Wave Number (
, cm
-1), Intensity (I, KM/mol) and
Vibrational Normal Modes Assignment for each absorption corresponding to the
theoretical IR vibrational spectra of a) N(1)-H/N(5)-H (K15), and b)
N(2)-H/N(5)-H (K25) and cis-enolic/N(1)-N(2)-H/N(5)-H (cE1) neutral allopurinol tautomers. * means out
of the molecular plane vibrational modes at this wave number;
* means and
combined vibrational modes.
Figure 1.
Total Electronic Charge Density contour maps (0.1-0.3 e/Å
3range,
changes in 0.05 units) at the optimized molecular plane for the three
energetically most stable N(1)-H/N(5)-H (K15), N(2)-H/N(5)-H (K25) and
cis-enolic/N(1)-H (cE1) allopurinol tautomers.
Figure 2.
3-D Molecular Electrostatic Potential isosurfaces (-30 kcal/mol) for
the N(1)-H/N(5)-H (K15), N(2)-H/N(5)-H (K25) and cis-enolic/N(1)-H (cE1)
neutral allopurinol tautomers.
Group
K15
K25
cE1
N1-N2
1.372
1.365
1.374
N2-C3
1.343
1.364
1.338
C3-C9
1.425
1.398
1.432
C9-C4
1.450
1.454
1.415
C4-N5
1.443
1.427
1.340
N5-C6
1.376
1.388
1.363
C6-N7
1.316
1.307
1.341
N7-C8
1.373
1.384
1.353
C8-C9
1.416
1.439
1.426
N1-C8
1.366
1.353
1.369
C4-O
1.235
1.237
1.356
N5-H
1.025
1.024
--O-H
--
--
0.988
C6-H
1.097
1.097
1.097
C3-H
1.091
1.090
1.091
N1-H
1.021
--
1.020
N2-H
--
1.021
Group
K15
K25
cE1
N1-N2-C3
105.16
115.38
105.81
N2-C3-C9
111.30
104.91
110.98
C3-C9-C8
104.93
105.13
104.99
C9-C8-N1
105.78
111.69
105.53
C8-N1-N2
112.82
102.88
112.68
C8-C9-C4
119.02
120.94
114.09
C9-C4-N5
109.65
109.48
121.31
C4-N5-C6
125.91
125.43
117.68
N5-C6-N7
124.96
125.83
128.29
C6-N7-C8
112.11
113.36
111.93
N7-C8-C9
128.35
124.95
126.71
N1-C8-N7
125.87
123.35
127.77
C3-C9-C4
136.05
133.92
140.92
N5-C4-O
120.16
121.13
118.28
C9-C4-O
130.19
129.39
120.41
C4-N5-H
114.64
115.12
--C4-O-H
--
--
105.22
C6-N5-H
119.45
119.45
--N5-C6-H
115.92
114.97
115.32
N7-C6-H
119.12
119.20
116.39
N2-C3-H
119.78
122.78
119.60
C9-C3-H
128.92
132.30
129.43
C8-N1-H
127.43
--
127.65
C3-N2-H
--
126.81
N2-N1-H
119.75
--
119.66
N1-N2-H
--
117.81
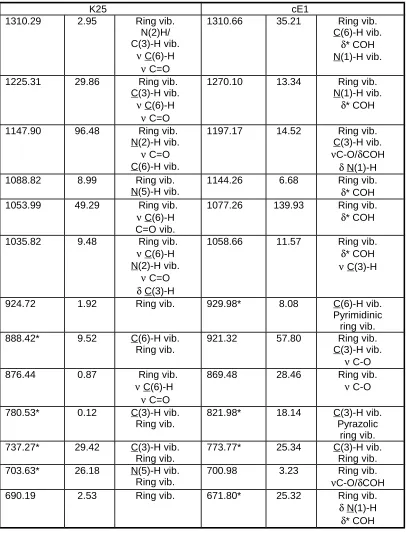
I Assignment
I Assignment3561.74 98.76 N(1)-H 923.16 40.07 Ring vib.
C(3)-H vib.
3497.38 61.51 N(5)-H 886.79* 7.16 C(6)-H vib.
Pyrimidinic ring vib.
3206.65 0.78 C(3)-H 873.86 13.84 Ring vib.
3133.62 7.94 C(6)-H 834.42* 19.60 C(3)-H vib.
Ring vib.
1747.00 597.0
6 Ring vib. C=O N(5)-H vib.
751.77* 15.99 C(3)-H vib.
Ring vib. C=O vib.
1589.29 125.6
1 C(6)-H vib.Ring vib.
C=O
703.10* 54.46 N(5)-H vib.
Ring vib.
C=O
1546.98 70.13 Ring vib.
N(1)-H vib.
C=O
678.85 9.49 Ring vib.
1502.57 10.84 Ring vib.
C(3)-H vib. N(5)-H vib.
C=O
653.19* 3.46 N(5)-H vib.
Ring vib.
1425.52 12.44 Ring vib.
N-H vib. C(3)-H vib.
C=O
628.35* 48.02 N(5)-H vib.
Ring vib.
C=O
1398.13 3.56 Ring vib.
N(5)-H vib.
C=O
585.73 9.62 Ring vib.
1387.24 24.31 Ring vib.
N(5)-H vib. 532.34* 70.51 N(1)-H vib.N(5)-H vib. Ring vib.
1352.55 6.08 Ring vib.
C(6)-H vib.
C=O
517.08 3.78 Ring vib.
1286.12 2.68 Ring vib.
N(1)-H vib. C=O vib.
491.27 1.44 Pyrimidinic
ring vib.
1209.03 34.64 Ring vib.
C(3)-H vib.
C=O
476.05* 15.99 N(1)-H vib.
Ring vib.
1190.30 14.00 Ring vib.
C(3)-H vib.
C=O
300.53 2.04 Ring vib.
1104.93 6.54 Ring vib.
N(5)-H vib.
C=O
251.00* 1.01 Ring vib.
K25 cE1
I Assignment
I Assignment3562.08 124.21 N(2)-H 3571.37 65.77 OH
N(1)-H
3501.56 56.40 N(5)-H 3568.59 115.59 N(1)-H
OH
3216.28 1.41 C(3)-H 3204.47 1.05 C(3)-H
3124.77 12.78 C(6)-H vib. 3133.37 18.44 C(6)-H
1735.96 558.78 Ring vib.
N(5)-H vib. C=O vib.
C(6)-H
1628.95 162.28 Ring vib.
* COH
N(1)-H
1609.11 144.59 Ring vib.
C-H/N-Hvib.
C=O
1575.11 227.17 Ring vib.
C-O N(1)-H vib.
1556.49 18.62 Ring vib.
C(6)-H
N(5)-H
C=O
1475.93 67.59 Ring vib.
C(6)-H vib.
C-O/COH
1470.16 43.07 Ring vib.
C(6)-H
N(5)-H
C=O
N(2)-H C(3)-H vib.
1451.56 82.54 rings
C(3)-H vib.
C-O C(6)-H/ N(1)-H vib.
1449.14 6.95 Ring vib.
N(2)-H vib.
C(6)-H C(3)-H vib.
C=O
1421.48 26.69 Ring vib.
N(1)-H/ C(6)-H vib.
C-O/COH
1399.41 24.94 Ring vib.
N(5)-H vib.
C=O
1369.24 95.70 Ring vib.
C(6)-H vib. N(1)-H vib.
C-O/COH
1381.96 6.04 Ring vib.
N(5)-H vib. C(6)-H C=O
1352.85 55.14 Ring vib.
C(6)-H vib. N(1)-H vib.
C-O/COH
1354.89 11.54 rings
C(6)-H N(2)-H/ C(3)-H vib.
C=O
1325.35 31.65 Ring vib.
* COH
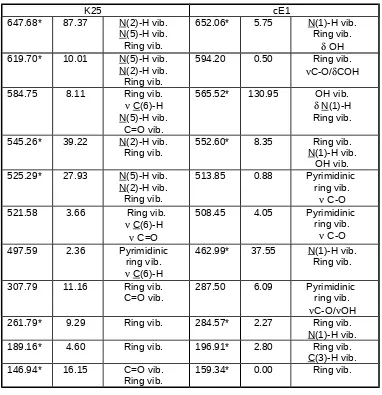
K25 cE1
1310.29 2.95 Ring vib.
N(2)H/ C(3)-H vib.
C(6)-H
C=O
1310.66 35.21 Ring vib.
C(6)-H vib.
* COH N(1)-H vib.
1225.31 29.86 Ring vib.
C(3)-H vib.
C(6)-H
C=O
1270.10 13.34 Ring vib.
N(1)-H vib.
* COH
1147.90 96.48 Ring vib.
N(2)-H vib.
C=O C(6)-H vib.
1197.17 14.52 Ring vib.
C(3)-H vib.
C-O/COH
N(1)-H
1088.82 8.99 Ring vib.
N(5)-H vib. 1144.26 6.68 Ring vib.* COH
1053.99 49.29 Ring vib.
C(6)-H C=O vib.
1077.26 139.93 Ring vib.
* COH
1035.82 9.48 Ring vib.
C(6)-H N(2)-H vib.
C=O
C(3)-H
1058.66 11.57 Ring vib.
* COH
C(3)-H
924.72 1.92 Ring vib. 929.98* 8.08 C(6)-H vib.
Pyrimidinic ring vib.
888.42* 9.52 C(6)-H vib.
Ring vib. 921.32 57.80 C(3)-H vib.Ring vib.
C-O
876.44 0.87 Ring vib.
C(6)-H
C=O
869.48 28.46 Ring vib.
C-O
780.53* 0.12 C(3)-H vib.
Ring vib. 821.98* 18.14 C(3)-H vib.Pyrazolic
ring vib.
737.27* 29.42 C(3)-H vib.
Ring vib. 773.77* 25.34 C(3)-H vib.Ring vib.
703.63* 26.18 N(5)-H vib.
Ring vib. 700.98 3.23 C-O/Ring vib.COH
690.19 2.53 Ring vib. 671.80* 25.32 Ring vib.
N(1)-H
* COH
[image:6.612.121.527.95.629.2]K25 cE1
647.68* 87.37 N(2)-H vib.
N(5)-H vib. Ring vib.
652.06* 5.75 N(1)-H vib.
Ring vib.
OH
619.70* 10.01 N(5)-H vib.
N(2)-H vib. Ring vib.
594.20 0.50 Ring vib.
C-O/COH
584.75 8.11 Ring vib.
C(6)-H N(5)-H vib.
C=O vib.
565.52* 130.95 OH vib.
N(1)-H Ring vib.
545.26* 39.22 N(2)-H vib.
Ring vib. 552.60* 8.35 N(1)-H vib.Ring vib.
OH vib.
525.29* 27.93 N(5)-H vib.
N(2)-H vib. Ring vib.
513.85 0.88 Pyrimidinic
ring vib.
C-O
521.58 3.66 Ring vib.
C(6)-H
C=O
508.45 4.05 Pyrimidinic
ring vib.
C-O
497.59 2.36 Pyrimidinic
ring vib.
C(6)-H
462.99* 37.55 N(1)-H vib.
Ring vib.
307.79 11.16 Ring vib.
C=O vib. 287.50 6.09 Pyrimidinic ring vib.
C-O/OH
261.79* 9.29 Ring vib. 284.57* 2.27 Ring vib.
N(1)-H vib.
189.16* 4.60 Ring vib. 196.91* 2.80 Ring vib.
C(3)-H vib.
146.94* 16.15 C=O vib.
Ring vib. 159.34* 0.00 Ring vib.
[image:7.612.119.501.82.477.2]