Effects of cold storage on cut
Gre
6
illea
‘Sylvia’
inflorescences
Daryl C. Joyce
a,*, Sarah A. Meara
b, Suzan E. Hetherington
b, Peter Jones
c aPosthar6est Laboratory,Cranfield Uni6ersity at Silsoe,Silsoe,Bedfordshire MK45 4DT,UK
bSchool of Land and Food,The Uni
6ersity of Queensland,St Lucia,Qld4067,Australia
cCSIRO IPPP Biometrics Unit,306Carmody Road,St Lucia,Qld4067,Australia Received 5 November 1998; accepted 26 August 1999
Abstract
Gre6illea‘Sylvia’ is a novel cut flower of sub-tropical to tropical origin. Cut inflorescences were dry-stored at 0, 5
or 10°C for 3, 6, 9 or 12 days. Inflorescences stored at 0°C for 3 or 6 days maintained a post-storage vase life of 7 days, which was similar to that of non-stored (control) inflorescences. There was no evidence of chilling injury even after 12 days at 0°C. Stems stored at 5 or 10°C for periods of 9 or 12 days suffered significant loss in vase life. Shortened longevity was associated with increased levels of flower and perianth abscission. Respiration rates of inflorescences stored at 0, 5, 10 or 22°C fell markedly with decreasing temperature. Temperature quotients (Q10)
estimated for 0 – 5, 5 – 10 or 10 – 22°C intervals were 2.1, 3.8 and 3.4, respectively. Inflorescences stored dry at 0°C for 6, 12 or 18 days maintained vase lives about 1 day longer than those stored wet. Vase lives after 6, 12 or 18 days dry storage were 8, 6 and 4 days, respectively. To simulate non-refrigerated export by aircraft, inflorescences were held wet or dry for 2 days at 22°C. Vase life of stored inflorescences was shortened by 1 day compared to non-stored control inflorescences. There was no difference in inflorescence vase life between wet versus dry transport simulation. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Cold storage; Cut flower; Grevillea; Inflorescence; Vase life
www.elsevier.com/locate/postharvbio
1. Introduction
The Gre6illea genus (family Proteaceae) has
numerous species grown for their ornamental flower and foliage value (Olde and Marriott, 1994). Many grevillea, especially hybrids of sub-tropical and sub-tropical species, have large showy
inflorescences. Some tend to flower all year round, and recently, grevillea have been considered as a potential cut flower crop (Joyce et al., 1996). For example, Gre6illea ‘Sylvia’ has bright pink
infl-orescences about 15 cm long (Beal et al., 1995). Postharvest storage characteristics are impor-tant with respect to cut flower marketing. Toler-ance and responsiveness to low storage and transport temperatures is desirable. Cold storage allows regulation of market supply when there is * Corresponding author. Fax: +61-44-1525-863277.
E-mail address:[email protected] (D.C. Joyce)
surplus production, ‘holding-over’ to achieve higher prices, for example on St. Valentine’s day, accumulation of sufficient stock for commercial consignments when supplies are limited, and uti-lization of cost-effective refrigerated sea transport (Nowak and Rudnicki, 1990). When transported as airfreight, cut-flowers are typically packed dry (i.e. stems not stood in water) and are usually subject to fluctuating ambient temperatures.
Storage at between 0 and 1°C is most effective for maintaining the quality of most cut flowers (Reid, 1992). However, some species, especially those of subtropical and tropical origin (e.g. An -thurium) suffer chilling injury at low storage tem-peratures. Symptoms of chilling injury include brown discoloration that reduces visual appeal and, thereby, shortens vase life. No investigation of the response of cut grevillea inflorescences to cold storage has been reported. Being of sub-trop-ical to tropsub-trop-ical origin, Gre6illea ‘Sylvia’ could
suffer from chilling injury. This grevillea is a F2 selection from ‘Pink Surprise’ (Gre6illea banksii× Gre6illea whiteana; Costin and Costin, 1988). The
natural climate of G. banksii is ‘‘sub-tropical to tropical; humid wet summer, sometimes wet win-ter’’ (Olde and Marriott, 1995a). That of G. whiteana is ‘‘summer hot, wet; winter mild to cool, wet or dry’’ (Olde and Marriott, 1995b). Both species are native to coastal Queensland in the latitude range 18 – 28° S.
The hypothesis tested in the present study was that low temperature storage above freezing in-jury temperature (50°C) and/or above some pos-sible chilling injury temperature would extend the post-storage and transport longevity of Gre6illea
‘Sylvia’.
2. Materials and methods
2.1. Plant material
Gre6illea ‘Sylvia’ inflorescences are racemes
comprised of many pairs of 2 – 4 cm long flowers along a single floral rachis (Olde and Marriott 1994). Inflorescences were harvested from 3-year-old unfertilised, irrigated and pruned stock plants grown in a loam soil at a nursery near Brisbane
(27.28° S, 153.01° E), Queensland. They were cut in the morning (ca. 09:00 h) at the harvest matu-rity stage of 50 – 100% looped styles (Vuthapanich et al., 1993). All leaves were trimmed from the stems, which were then laid between sheets of newsprint moistened with deionised water and placed over ice in polystyrene containers. The containers were transported in an air-conditioned car to the laboratory (22°C) within 1 h of harvest.
2.2. Experiment 1:storage temperature and duration
Storage temperatures of 0, 5 or 10°C were used and inflorescences were stored for 3, 6, 9 or 12 days. Vase life was assessed immediately after harvest for non-stored inflorescences. Ten repli-cate single inflorescences were randomly assigned to each of the 13 treatments of non-stored plus three temperatures by four durations.
Inflorescences were recut under water to 40 cm length, weighed, tagged, dipped into a fungicide solution of 1 g Rovral (a.i. iprodione) plus 1 g Benlate (a.i. benomyl) plus 0.1 ml Agral (wetting agent) per l and allowed to dry. Each inflores-cence was individually wrapped in moistened newspaper, and two wrapped stems were placed together in an unperforated plastic flower sleeve with a 20 cm wide top and 40 cm long. Sleeves containing inflorescences allocated to the same treatment were laid into 48×32×10 cm fibre-board cartons with lids. Eight pressure cooling holes in the sides of each carton were covered with perforated 15 mm thick polypropylene film
(W.R. Grace PY30 film; 5 by 1.5 mm diameter holes per cm) to minimize water loss. The cartons were placed into storage rooms at 0°C (range 0.1 – 2.5°C), 5°C (range 4.7 – 7.0°C) or 10°C (range 8.1 – 11.1°C) by 16:30 h on the day of harvest. The cartons were withdrawn from storage after 3, 6, 9 or 12 days, whereupon inflorescences were as-sessed for vase life.
2.3. Vase life assessment
weighed and placed into individual vases contain-ing solution (see below). The inflorescences re-moved from storage were unwrapped and weighed. Relative fresh weights at the end of the storage period were calculated as percent initial fresh weight. The stems were then trimmed under water and weighed again before being placed into vases.
The vases contained around 375 ml of 10 mg available Cl (as dichloroisocyanurate, sodium salt) per l solution to inhibit growth of micro-or-ganisms. The chlorine solution was prepared us-ing deionised water. The mouths of each translucent plastic vase were covered with plastic film, and held in place with a rubber band, to keep out foreign matter and prevent the evapora-tion of the vase soluevapora-tion. One inflorescence stem per vase was inserted through a slit in the plastic cover.
Vase life was assessed in a ventilated controlled temperature room at 2291°C. Relative humidity ranged between 44 and 82%. The light period was 12 h per 24 h and the photon flux density at the flower level was about 10mmol m−2s−1.
Inflorescence weights were recorded separately every morning over seven (experiment 1) or eight (experiments 2 and 3) consecutive days and the relative fresh weights were calculated.
The flowers were assessed daily for visual ap-peal. Vase life was judged to have ended when 50% or more of the flowers on an inflorescence were rated unattractive. Observations of specific indices of deterioration were also recorded, viz. flower opening-reflexing of the looped style, ‘slip-pering’-calyx tube remaining attached to the stigma as it reflexes, wilting of both the style and perianth, flower colour change — bright pink to dull pink, and flower abscission.
2.4. Respiration
Respiration rates were measured on individual inflorescences held at 0, 5, 10 or 22°C. Each inflorescence was sealed in a 1.2 l glass container holding approximately 12 g Purafil (ethylene ab-sorbant; KMnO4-coated pellets). Headspace gas
samples (1 ml) were taken through a rubber sep-tum for CO2 analysis and were injected into a
thermal conductivity detector gas chromatograph fitted with a 1.8 m by 1.5 mm ID stainless steel column packed with Porapak Q (80/100 mesh). The GC operating temperatures were: detector 90°C, injector 80°C, and column 35°C (isother-mal). The carrier gas was He (15 ml min−1). The
standard gas was 0.54790.010% CO2.
Respira-tion rates were calculated on measurements made when the CO2 concentrations in the containers
reached between 0.5 and 2%. These concentra-tions were reached after 382, 339, 169 and 47 h for 0, 5, 10 or 22°C treatments, respectively.
2.5. Experiment 2:wet 6ersus dry storage
Inflorescences were stored under either wet or dry conditions at 0°C for periods of 6, 12 or 18 days. Non-stored inflorescences were taken for vase life assessment immediately after harvest. Nine individual replicate inflorescences were ran-domly assigned to each of the seven treatments of non-stored plus two storage methods by three durations.
Inflorescences were recut to a length of 40 cm, weighed, tagged, dipped in a fungicide solution of 1 g Benlate plus 0.1 ml Agral per l and allowed to dry. For dry storage, individual inflorescences were wrapped in moistened newspaper and then placed individually in an un-perforated plastic flower sleeve 14 cm wide by 40 cm long. The nine inflorescences per storage duration were stood vertically in a test tube rack. For wet storage, individual inflorescences were placed in a flower sleeve, the top of which was folded over and secured. Four 0.5 cm diameter holes were made in each sleeve to allow for the ventilation of respira-tory gases. Each inflorescence was then stood in a test tube containing 35 ml of 10 mg available Cl per l solution, and the flower sleeve was secured around the neck of the test tube using a rubber band.
(range 0.6 – 3.0°C) by 15:45 h on the harvest day. Inflorescences were removed from the cabinet af-ter 6, 12 or 18 days.
2.6. Experiment3:simulated transport
Inflorescences were held for 2 days at 22°C under either wet or dry storage conditions to simulate the conditions experienced by cut flowers when they are exported from Australia to Japan by air. The period and temperature were deter-mined on the basis of conditions recorded for an export cut flower consignment (Joyce, 1994). The temperature by time profile was equivalent to 44 day×°C, hence adoption of the 22°C for a 2 days regime. Non-stored inflorescences were assessed for vase life immediately after harvest.
Inflorescences were prepared for wet and dry storage as described above. The control inflores-cences were the same as those used in experiment 2 because the two experiments were run concurrently.
2.7. Statistical analyses
Each experiment was conducted once. Change in the relative fresh weight of the stems during storage, respiration rates at different tempera-tures, vase life and changes in stem relative fresh weight during vase life were analysed by ANOVA
using Genstat 5 (Lawes Agricultural Trust, Rothamsted Experimental Station). Means were compared by least significant difference (L.S.D.) values calculated ast-value (error degrees of free-dom (e.d.f.),P=0.05) times the S.E. of the differ-ence between means.
3. Results and discussion
3.1. Storage temperature
Gre6illea ‘Sylvia’ inflorescences wrapped in
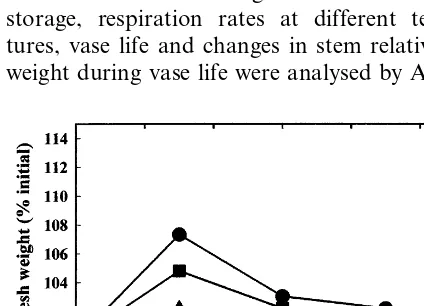
moistened newspaper and stored dry at 0, 5 or 10°C gained weight during the first 3 days of storage (Fig. 1). This gain was at least partially due to condensation and its entrapment by nu-merous small trichomes covering the stems and immature flowers (Olde and Marriott, 1994). Thereafter, all the stems lost weight. There were no significant differences in relative fresh weight at the end of 12 days storage. Packaging in moist-ened newsprint and plastic sleeves clearly pro-vided water and maintained high relative humidity.
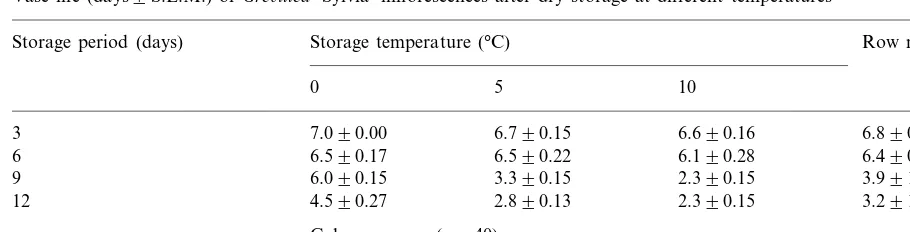
The inflorescence vase life averaged over stor-age durations at 0°C was 6 days (Table 1). Infl-orescences stored at 5°C averaged a vase life of 5 days, and those stored at 10°C averaged a vase life of 4 days. The vase life of inflorescences stored for 3 days at each of the three temperatures did not differ from the vase life of non-stored inflores-cences. Similar deterioration symptoms were ob-served for both non-stored inflorescences and those stored for 3 days. There was a small de-crease of 0.5 days in the post-storage vase life of inflorescences stored for 6 days compared with non-stored inflorescences. Larger vase life losses of 3 and 4 days were recorded after 9 and 12 days of storage, respectively. Vase lives averaged over storage temperatures for durations of 3, 6, 9 and 12 days were 7, 6, 4 and 3 days, respectively. Thus, the magnitude of the decrease between 6 and 9 days of storage was greater than those between the other two intervals. This accelerated deterioration was not associated with loss of wa-ter during storage because the fresh weight was largely maintained (Fig. 1). The relatively greater Fig. 1. Relative fresh weights of Gre6illea ‘Sylvia’
Table 1
Vase life (days9S.E.M.) ofGre6illea‘Sylvia’ inflorescences after dry storage at different temperaturesa
Storage temperature (°C)
Storage period (days) Row means (n=30)
5 10
0
3 7.090.00 6.790.15 6.690.16 6.890.21
6.590.17
6 6.590.22 6.190.28 6.490.23
3.390.15 2.390.15
6.090.15 3.991.9
9
4.590.27
12 2.890.13 2.390.15 3.291.2
Column means (n=40)
4.892.1 4.392.3 6.091.1
aThe vase life of non-stored control inflorescences was 6.990.23 days (n=10). L.S.D. (P=0.05) duration by temperature means=0.52 days.
loss in vase life was characterised by enhanced levels of flower abscission and ‘slippers’. Abscis-sion of flowers and petals in many cut flowers is normally associated with increased ethylene pro-duction (Halevy and Mayak, 1981). Generally, the end of vase life forGre6illea‘Sylvia’ inflorescences
was associated with a combination of moderate levels of flower discolouration, inflorescence wilt-ing and flower openwilt-ing, together with moderate flower abscission. The relative vase life benefit of the 0°C storage treatment for up to 12 days showed thatGre6illea‘Sylvia’ is not chilling
sensi-tive (Table 1), despite being of subtropical or tropical origin.
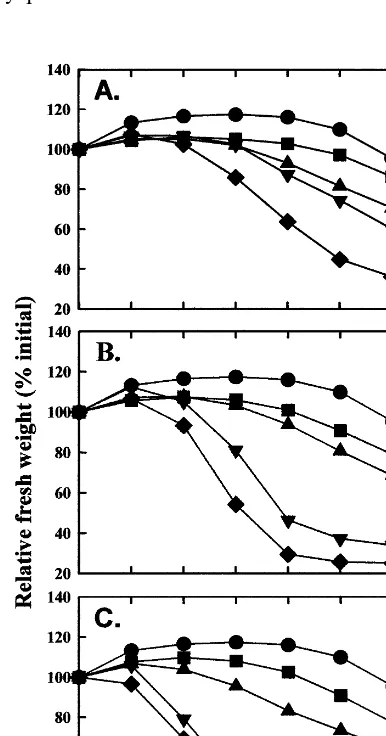
Non-stored inflorescences had comparatively less fresh weight loss during vase life evaluation than inflorescences stored at 0, 5 or 10°C for any of the four periods of time (Fig. 2). In terms of proportions averaged over the vase life evaluation period, inflorescences stored at 10°C had the greatest weight loss of 31%, followed by losses of 26 and 16% for those stored at 5 and 0°C, respec-tively. Inflorescences stored for longer periods had larger losses in fresh weight during vase life evaluation.
Inflorescence respiration rates increased with increasing storage temperature (Table 2). Q10s
over the whole temperature range averaged 3.1 and did not differ markedly between individual temperature increments. Thus, a 3-fold benefit in terms of conservation of respiratory substrates can be anticipated for each 10°C drop in storage temperature. The Q10 for Gre6illea ‘Sylvia’
be-tween 0 and 10°C of 2.8 is similar to that of around 3 reported for carnation (Reid, 1992). However, the Q10 between 10 and 22°C of 3.4 is
only about half that for carnation. Q10s over the
0 – 10 and 10 – 20°C ranges for waxflower of 4.8 and 3.3, respectively, are generally similar to those for Gre6illea ‘Sylvia’ (Joyce, 1988).
3.2. Wet 6ersus dry storage
There was a relatively greater increase in infl-orescence weight during wet storage compared with dry storage for each storage duration tested (Fig. 3). There was no further increase in inflores-cence weight in wet storage after 6 days, but fresh weight was maintained thereafter. During dry storage, inflorescence fresh weight increased dur-ing the first 6 days and then declined notably by 18 days.
The vase life ofGre6illea‘Sylvia’ averaged over
storage duration was 6 days for dry storage (Table 3). This vase life average is 1 day longer than that for wet storage. Non-stored inflores-cences had a vase life of 8 days. Storage of inflorescences under either wet or dry conditions for 6 days did not significantly reduce the vase life compared with non-stored inflorescences. When stored for either 12 or 18 days, vase life declined by 2 and 4 days, respectively. Longer vase life of dry-storedGre6illea‘Sylvia’ inflorescences than of
senes-cence is accelerated in wet-stored flowers (Nowak and Rudnicki, 1990). For example, carnations stored in water respired 25 – 30% faster than those stored dry (Hardenburg et al., 1969), and wet storage promotes sustained slow bud development (Goszczynska and Rudnicki, 1988). Neff (1939) reported ‘‘considerable’’ utilisation of carbohy-drates by carnations stored in water, but only ‘‘slight’’ utilisation when flowers were stored in dry packs.
Table 2
Respiration rates (9S.E.M.) of and temperature quotients for Gre6illea‘Sylvia’ inflorescences during dry storage at different
temperatures
Temperature Storage Respiration rate
quotientQ10b temperature (mmol CO2kg−1h)a
(range °C) (°C)
72950 –
0
104954
5 2.09 (0–5)
3.84 (5–10) 2049137
10
22 8849594 3.39 (10–22)
aL.S.D. (P=0.05)=491
mmol CO2kg−1h. bQ
10=(R2/R1)10/(t2−t1); Wills et al., 1998.
Fig. 2. Relative fresh weights of Gre6illea ‘Sylvia’
inflores-cences during vase life following dry storage at 0 (A), 5 (B) and 10°C (C) for 3 (), 6 (), 9 () and 12 days (") and for the non-stored control ( ). L.S.D. (P=0.05)=7.5% (between curves) and 5.7% (within curves).
Fig. 3. Relative fresh weights of Gre6illea ‘Sylvia’
inflores-cences during wet () and dry () storage at 0°C. L.S.D. (P=0.05)=3.3%.
Table 3
Vase life (days9S.E.M.) of Gre6illea ‘Sylvia’ inflorescences
after wet or dry storage at 0°Ca
Storage pe- Wet storage Dry storage Row means (n=18) riod (days)
6 6.991.45 7.6+0.88 7.390.49 5.091.12 6.091.22
12 5.590.71
18 3.191.45 3.991.17 3.590.57
Column means (n=27) 5.091.9 5.891.9
Inflorescences stored either wet or dry for peri-ods of 12 or 18 days developed limited gray mould on their flowers caused by Botrytis sp. Also, 22% of inflorescences stored dry for 12 days and 11% of inflorescences stored wet for 6 days developed superficial stem discolouration caused byAlternariasp. andStemphyliumsp. (G.I. John-son, personal communication).
Non-stored inflorescences lost less fresh weight during the 8 day vase life evaluation period than either wet- or dry-stored inflorescences (Fig. 4). Overall, inflorescences stored wet had somewhat greater fresh weight loss during vase life than those stored dry. The duration for which inflores-cences were stored prior to vase life determination also had an effect on fresh weight loss during vase life. In terms of percentages averaged over storage
temperatures, inflorescences stored for 6 days had least fresh weight loss of about 16%. The greatest fresh weight loss of approximately 32% was recorded for stems stored for 18 days.
3.3. Simulated transport
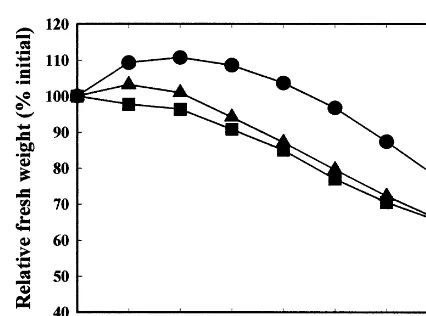
Inflorescences stored wet at 22°C for 2 days showed a large increase of 17.0% in fresh weight compared to a small increase of 2.8% during dry storage at 22°C for 2 days (L.S.D.=5.3%). Over all three experiments the increase in Gre6illea
‘Sylvia’ fresh weight was approximately 12% for wet storage. For comparison, a 20% increase in fresh weight has been recorded for carnation (Goszczynska et al., 1982).
Vase lives for wet- (6.991.5 days; mean9 S.E.M.)) and dry-stored (6.491.1 days) inflores-cences did not differ (L.S.D. (P=0.05)=1.0 day), but were shorter than the corresponding vase life for non-stored inflorescences (7.890.4 days). The vase life decrease of 1.4 days between the non-stored and the dry-stored inflorescences was significant. Wet-stored inflorescences had less flower abscission and wilting during subsequent vase life than either those stored dry or the non-stored inflorescences. Both wet and dry storage were associated with increased flower opening at the end of vase life. During vase life 44% of inflorescences stored wet were observed to de-velop superficial fungal infections by Alternaria alternata, Stemphylium sp. and Trichoderma 6iri -dae (one stem only). No such stem discolouration was observed on non-stored or dry-stored infl-orescences. Dry storage is the more practical method for export, but wet handling in buckets may be easier and less labour intensive for domes-tic marketing. However, superficial stem infec-tions would lessen the appeal to consumers of the blooms.
There were no differences in changes in inflores-cence weight during vase life between wet- or dry-stored inflorescences (Fig. 5). However, stored inflorescences had lower fresh weights during vase life than non-stored inflorescences. Inflorescence weights began to decrease after 2 days in the vase. The ability of inflorescences to maintain fresh weight during vase life has been used as a measure Fig. 4. Relative fresh weights of Gre6illea ‘Sylvia’
Fig. 5. Relative fresh weights of Gre6illea ‘Sylvia’
inflores-cences during vase life after wet () and dry () storage at 22°C for 2 days, and for the non-stored control ( ). L.S.D. (P=0.05)=9.0% (between curves) and 5.2% (within curves).
tion QL95007, Department of Primary Industries, Bris-bane, Queensland.
Costin, R., Costin, S., 1988. TropicalGre6illeahybrids. Aust.
Plants 116, 335 – 341.
Goszczynska, D.M., Rudnicki, R.M., 1988. Storage of cut flowers. Hortic. Rev. 10, 35 – 62.
Goszczynska, D.M., Pietrzkowska, M., Rudnicki, R.M., 1982. Cold storage of carnations cut in bloom. Rosliny Ozdobne Ser. B7, 105 – 117.
Halevy, A.H., Mayak, S., 1981. Senescence and postharvest physiology of cut flowers — Part 2. Hortic. Rev. 3, 59 – 143.
Hardenburg, R.E., Uota, M., Parsons, C.S., 1969. Refrigera-tion and modified atmosphere for improved keeping qual-ity of cut flowers. Prog. Refrig. Sci. Technol. 3, 339 – 347. Joyce, D.C., 1988. Postharvest characteristics of Geraldton
wax flowers. J. Am. Soc. Hortic. Sci. 13, 738 – 742. Joyce, D.C., 1994. Postharvest handling of cut flowers —
some important issues. In: 3rd Natl. Workshop for Aus-tralian Native Flowers, Proceedings. February, 1994. The University of Queensland, Gatton College, Queensland. pp 11-1 – 11-6.
Joyce, D.C., Beal, P., Shorter, A.J., 1996. Vase life character-istics of selectedGre6illea. Aust. J. Exp. Agric. 36, 379 –
382.
Mayak, S., Halevy, A.H., Sagie, S., Bar-Yoseph, A., Bravdo, B., 1974. The water balance of cut rose flowers. Plant Physiol. 31, 15 – 22.
Neff, M.S., 1939. Problems in the storage of cut carnations. Plant Physiol. 14, 271 – 284.
Nowak, J., Rudnicki, R.M., 1990. Storage. In: Postharvest Handling and Storage of Cut Flowers, Florist Greens, and Potted Plants. Timber Press, Portland, OR, pp 67 – 86. Olde, P., Marriott, N., 1994. The Grevillea Book, vol. 1.
Kangaroo Press, Kenthurst, NSW.
Olde, P., Marriott, N., 1995a. The Grevillea Book, vol. 2. Kangaroo Press, Kenthurst, NSW.
Olde, P., Marriott, N., 1995b. The Grevillea Book, vol. 3. Kangaroo Press, Kenthurst, NSW.
Reid, M.S., 1992. Postharvest handling systems: ornamental crops. In: Kader, A.A. (Ed.), Postharvest Technology of Horticultural Crops. University of California, Oakland, CA, pp. 201 – 209 Publication 3311.
Vuthapanich, S., Simons D.H., Turnbull, L.V., 1993. Effects of harvest maturity and postharvest treatments on vase life ofGre6illeacv. Majestic inflorescences. In: 1993
Aus-tralasian Postharvest Conference Proceedings. The Uni-versity of Queensland, Gatton College, Queensland, pp 45 – 51.
Wills, R., McGlasson, B., Graham, D., Joyce, D., 1998. Postharvest: An Introduction to the Physiology and Han-dling of Fruit, Vegetables and Ornamentals. U.N.S.W. Press, Sydney.
of their quality. Typically, cut flowers in the vase initially gain weight and then subsequently lose fresh weight (Mayak et al., 1974; Halevy and Mayak, 1981). In the present study, the higher the storage temperature and the longer the duration of storage, the lower the initial increase in fresh weight and the greater the decrease in fresh weight towards the end of vase life of Gre6illea ‘Sylvia’
inflores-cences (Fig. 2). However, there was no direct quantitative relationship between vase life (y-axis; days) and relative fresh mass at the end of vase life (x-axis; percent initial f.w.) for treatment means pooled across the three experiments (n=23); viz. y=5.9−0.0041x; r2=0.00039, P=0.992.
Acknowledgements
The authors thank Tony Shorter and Jane Lig-awa for technical advice and assistance.
References
Beal, P., Howell, J., Joyce, D., Shorter, A., 1995. Maturity Stages for HarvestingGre6illeafor Cut Flowers.