Plant disease control is entering an exciting period during which transgenic plants showing improved resistance to pathogenic viruses, bacteria, fungi and insects are being developed. This review summarizes the first successful attempts to engineer fungal resistance in crops, and highlights two promising approaches. Biotechnology provides the promise of new integrated disease management strategies that combine modern fungicides and transgenic crops to provide effective disease control for modern agriculture.
Addresses
Zeneca MOGEN, PO Box 628, 2300 AP Leiden, The Netherlands; *e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:147–152
1369-5266/00/$ — see front matter © 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations Avr avirulence
HR hypersensitive response
PR pathogenesis-related
R resistance gene
Introduction
Protection against attack by detrimental micro-organisms and pests is a major challenge for crop production. Fungal diseases have been one of the main causes of crop losses ever since mankind started to cultivate plants [1]. At present, the control of the epidemic spread of fungal diseases mainly involves three strategies: first, husbandry techniques, such as crop rotation and avoiding the spread of infested soil and pathogen-contaminated plant materials; second, breeding of resistant crop cultivars; and third, the application of agro-chemicals. Although conventional plant breeding has made a significant impact by improving the resistance of many crops to important diseases, the time-consuming processes of mak-ing crosses and back-crosses, and the selection of the desired resistant progeny make it difficult to react adequately to the evolution of new virulent fungal races [2,3]. Moreover, these plant breeding techniques will not provide a solution to many major fungal diseases because there are simply no nat-ural sources of resistance available to the breeder.
Farmers, therefore, often use fungicides for crop protec-tion in modern agriculture. The use of these compounds is, however, limited by their high costs and potentially harm-ful impact on the environment [4,5]. In addition, extended application can reduce the efficiency of fungicides because of the evolution of tolerant or resistant pathogens. The growing concern about the environment, together with a strong motivation to decrease production costs, encourages the development of crop cultivars that require less chemi-cals and/or fewer types of chemical. The advent of plant
transformation and advanced molecular techniques for plant breeding provides powerful tools for genetically improving crops so as to enhance their resistance to fungal diseases. The distinct advantage of transgenic technology is that it enables the plant breeder to cross species barriers, allowing genes from non-related plants and other organ-isms to be introduced into crop plants.
In this review, we discuss the current status of engineered resistance to fungal pathogens in crops and highlight two highly promising approaches for generating broad-spec-trum fungus-resistant crops [6]. The first strategy relies on the expression of genes that encode proteins which have either a direct or indirect inhibitory effect on the growth of fungi (i.e. the antifungal protein strategy); the second strat-egy aims to increase fungal resistance in plants by engineering controlled hypersensitive death of host cells on the basis of the specific interaction of the product of the avirulence gene (Avr) and that of the resistance gene (R) (i.e. Avr/Rtwo-component strategy) [7].
Antifungal proteins
Plants have several inducible defense mechanisms that act to limit pathogen infection, including increased lignifica-tion and cell wall cross-linking, the produclignifica-tion of small antibiotic molecules (i.e. phytoalexins), host cell death at the site of infection (i.e. the hypersensitive response; [8] and references therein), and the production of reactive oxygen species [9]. Many plants also develop an increased resistance to subsequent pathogen infection in uninfected tissues. This systemic acquired resistance (SAR) can be effective against viruses, bacteria and fungi, and is accom-panied by the expression of a large set of genes termed pathogenesis-related (PR) genes [10,11].
PR genes probably have an important role in the defense response of plants against fungal infections. The ‘antifun-gal protein’ strategy involves the constitutive expression in transgenic plants of genes encoding proteins that have a fungitoxic or fungistatic capacity and therefore enhance resistance to fungal pathogens. Table 1 presents an overview of the reports of transgenic plants showing increased resistance obtained using the above approach.
The hydrolytic enzymes chitinase and β-1,3-glucanase, which are capable of degrading the major cell-wall con-stituents (i.e. chitin and β-1,3-glucan) of most filamentous fungi, have been extensively studied in plants. Expression of a plant or bacterial chitinase gene in transgenic tobacco was shown to enhance the resistance of plants to Rhizoctonia solani, an endemic, chitinous, soilborne fungus that infects numerous plant species [12,13]. Interestingly, similar trans-genic tobacco plants showed no enhanced tolerance of
Novel genes for disease-resistance breeding
Cercospora nicotianae[14], indicating that results obtained in vitroare difficult to extrapolate to the situation in vivo and that tolerance to a range of pathogens can require more than the simple over-expression of a single gene.
Researchers at Zeneca MOGEN were the first to demon-strate that the constitutive co-expression of tobacco chitinase and β-1,3-glucanase genes in tomato plants con-fers higher levels of resistance to a fungal pathogen (i.e. Fusarium oxysporum) than either gene alone, indicating a synergistic interaction between the two enzymes in vivo [15,16]. The effectiveness of this approach was further demonstrated in tobacco, in which the simultaneous expression of a rice chitinase and an alfalfa glucanase gene yielded a substantially greater protection against the fungal pathogen C. nicotianae than either transgene alone [17]. Similar effects were found after co-expression of genes for class II chitinase, class II glucanase and a type I ribosome-inactivating protein (RIP) from barley in transgenic tobacco [17]. Certain combinations (i.e. chitinase/glu-canase and chitinase/RIP) provided ‘significantly enhanced protection’ against Rhizoctonia solani. J Ryals’ group at Novartis has made a significant effort to evaluate these and other PR proteins for their potential to provoke disease resistance in transgenic tobacco plants (see Table 1). Transgenic tobacco plants constitutively express-ing PR1a, a protein with unknown biochemical function, showed tolerance to infection by two oomycete pathogens,
Peronospora tabacina and Phytophthora parasitica [18]; although, once more, this resistance did not extend to other pathogens tested. An interesting observation from this work was that the apparent disease resistance of a transgenic line was not correlated with the level of expres-sion of the transgene. This is an observation that has been made on several occasions in other reported work.
There are several other reports of enhanced tolerance being conferred by the overexpression of a single gene. Overexpression of tobacco osmotin, a PR-5 protein, in potato significantly delayed the appearence of lesions caused by Phytophthora infestans [19,20]. Interestingly, recent studies with transgenic monocot crops, including rice and wheat, have yielded the first promising results relating to the development of resistance to Rhizoctonia solani[21] and Fusariumsp. [22], respectively, in these eco-nomically most important crops.
In addition to PR proteins, a broad family of small antifun-gal peptides, termed plant defensins, has been discovered. Several of these peptides have been isolated from the seeds of a variety of plants and shown to possess antifungal activ-ity in vitro against a broad spectrum of fungal pathogens. The constitutive expression of Rs-AFP2 (a small cysteine-rich plant defensin from radish) in tobacco conferred a high level of resistance to the foliar pathogen Alternaria longipes on plants that produced high levels of Rs-AFP2 [23]. Table 1
Increased fungal resistance in transgenic plants.
Plant Transgene(s) Pathogen Reference
Tobacco PR-1a, SAR 8.2 Peronospora tabacina, Phytophthora
parasitica, Pythium [18]
Class III chitinase Phytophthora parasitica [18]
Chi-I Rhizoctonia solani [18]
Bean chitinase (CH5B) Rhizoctonia solani [12]
Barley RIP Rhizoctonia solani [48]
Serratia marcescens Chi-A Rhizoctonia solani [48]
Barley Chi + Glu Rhizoctonia solani [13]
Barley Chi + RIP Rhizoctonia solani [13]
Rice Chi + alfalfa Glu Cercospora nicotianae [17]
Radish Rs-AFP Alternaria longipes [23]
Carrot Tobacco Chi-I + Glu-I Alternaria dauci (a)
Tobacco AP24 Alternaria radicina (a)
Cercospora carotae (a)
Erysiphe heracleï (a)
Tomato Tobacco Chi-I + Glu-I Fusarium oxysporum [16]
Brassica napus Bean chitinase Rhizoctonia solani [12]
Tomato/tobacco chitinase Cylindrosporium conc. [29]
Sclerotinia sclerotiorum [29]
Phoma lingam [29]
Potato AP24 Phytophthora infestans [19]
Glux-ox Phytophthora infestans [28]
Verticillium dahliae [28]
Aly AFP Verticillium [22]
Rice Rice Chi Rhizoctonia solani [21]
Wheat Aly AFP Fusarium sp. [22]
Other examples of plant antifungal peptides include thion-ins from monocots and dicots, hevein (a lectin from Urtica dioica) and lipid transfer proteins from various plant species ([24] and references therein). The first reports of the effi-cacy of these peptides in controlling fungal diseases were published recently. Epple and co-workers [25] demonstrat-ed increasdemonstrat-ed protection of Arabidopsis against Fusarium oxysporumby the overexpression of an endogenous thionin (Thi2.1 gene). Interestingly, the induction of the endoge-nous gene for Thi2.1after pathogen attack is independent of PR-gene expression, suggesting that these defenses are activated through independent pathways. Molina and Garcia-Olmedo [26] reported that the expression of a barley non-specific lipid-transfer protein in Arabidopsisand tobac-co tobac-conferred enhanced resistance to the bacterial pathogen Pseudomonas syringae. These proteins have also been shown to demonstrate antifungal effects in vitro.
Finally, there are a number of antifungal proteins that do not fall into any of the classes described above. For exam-ple, the H2O2-producing enzyme oxalate oxidase has been
shown to accumulate in barley that is attacked by powdery mildew, Erysiphe graminis[27]. Interestingly, Wu et al.[28] demonstrated that constitutive expression in potato of another H2O2-generating enzyme, glucose oxidase,
provid-ed disease resistance to a range of plant pathogens, including Erwinia carotovora, Phytophthora infestans and Verticilliumwilt disease.
Most of the promising reports described above are based solely on observations of the increased fungal resistance of
transgenic plants tested in climate-controlled growth cham-bers or greenhouse facilities. Now, the technical challenge is to translate such results into a robust effect in the field. A comprehensive field evaluation of transgenic carrots contain-ing the tobacco class I chitinase and β-1,3-glucanase genes (LS Melchers, MH Stuiver, unpublished data) has demon-strated that these plants have a high level of resistance to major carrot pathogens including Alternaria dauci, Alternaria radicina, Cercospora carotae and Erysiphe heracleï (powdery mildew). Most of the transgenic carrot lines that were resis-tant to one pathogen exhibited significant resistance to all four pathogens. The broad-spectrum fungal resistance in transgenic carrot plants indicates a proof of the concept of the ‘antifungal gene’ strategy. In another field-testing study, transgenic canola (Brassica napus) that constitutively expressed a chimeric chitinase gene showed enhanced resis-tance to Cylindrosporium concentricumand, to a smaller degree, Phoma lingamand Sclerotinia sclerotiorumafter artificial inocu-lation with these pathogens [29].
Future studies will be necessary to identify combinations of defense proteins that might confer effective broad-spec-trum protection.
Hypersensitive-response-based strategies
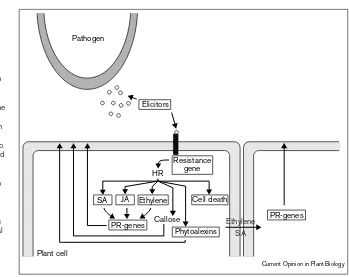
The hypersensitive response (HR) is one of the most power-ful mechanisms by which plants resist pathogen attack (Figure 1). The genetically controlled induction of the HR is triggered in plant–pathogen interactions only if the plant contains a disease-resistance protein (R) that recognizes the corresponding avirulence (Avr) protein from the pathogen. In Figure 1Schematic drawing of defense responses activated in a plant–pathogen interaction. A pathogen secretes elicitor molecules that are perceived via the plant resistance gene products. The resulting HR encompasses many different defense responses, including induction of cell death, callose deposition at the site of pathogen entry, and the production of a number of hormones (e.g. salicyclic acid [SA], jasmonic acid [JA] and ethylene), which trigger production of PR-gene products. Some of the hormones produced may move systemically, leading to PR protein production in the neighbouring cells. The boxed components have all been used in attempts to engineer disease resistance. Elicitors are used in the avirulence gene approach (see text). Resistance genes can be transformed into plants, thereby increasing the plant’s ability to recognise pathogenic invaders. Increased production of active hormones to induce (subsets of) PR genes is the subject of some research, as is the over-expression of specific PR genes (see text). Additionally, the potential for the engineering of phytoalexin production and the induction of localized pathogen-triggered cell death is being explored.
Pathogen
Elicitors
Resistance gene
SA JA Ethylene Cell death
PR-genes PR-genes Callose
Phytoalexins
Plant cell
HR
Current Opinion in Plant Biology
the absence of a functional resistance gene (R) or avirulence gene product, no recognition occurs and the interaction between plant and pathogen results in disease. Resistance genes involved in race-specific interactions often provide full disease resistance, and are well known from conventional breeding programs. In recent years, many disease-resistance genes have been cloned and have been shown to represent several structurally different classes of gene.
The strength of the hypersensitive reaction makes it exquisitely designed for combating a broad spectrum of plant pathogens. The normal use of resistance genes does not, however, allow such a broad spectrum of activity. The exclusive specificity of HR-associated disease resistance for specific pathogen-derived avirulence factors limits their use to only one pathogen, and sometimes even a limited number of races or pathotypes of the pathogen. The engi-neering of broad-spectrum disease resistance relying on the HR is therefore highly desirable.
The interesting so called ‘Avr/R strategy’ proposed by P de Wit [7] involves the transfer of an avirulence gene (i.e. the Cladosporium fulvum avr9gene) into a plant containing the corresponding resistance gene (i.e. the tomato Cf9 gene), and its subsequent expression under the direction of a pro-moter that is rapidly and locally inducible by a wide range of fungal pathogens. Pathogen-induced expression of the avr9 gene will then provoke a resistance reaction mani-fested by a hypersensitive response. A localized HR will be induced that prevents the further spread of any invad-ing pathogen that can be inhibited by an HR, followed by a general defense response. Using this technology we have shown in collaboration with de Wit and co-workers that tomato plants can be generated with increased resistance to a broad-spectrum of diseases: resistance to multiple fungi with different infection modes, and also to viruses, was observed (MH Stuiver et al., unpublished data). Along similar lines, a broad-spectrum responsive HR-induction system in tobacco plants has been generated [30••].
With the continuing elucidation of the signal transduction pathways invloved in plant defense responses, more and more molecular components are becoming available that can be used as tools to induce the hypersensitive response either completely or partially [31]. Karrer et al.[32•] have
used a search for tobacco proteins that, when overex-pressed, are able to induce necrosis or an HR [32•]; these
are interesting starting points. Further understanding of the molecular mechanism responsible for the resistance mediated by the Avr-R genes is critical for establishing durable resistance in genetically engineered crops. In addi-tion, the elucidation of signal transduction components functioning downstream of R genes opens avenues for engineered resistance.
Hypersensitive response signalling
Genetic analysis of HR signalling has revealed a number of key steps in the signal transduction of the HR. One such
step involves a small number of protein molecules that function downstream of a very large number of resistance genes [33]. This convergence is probably the result of shared signal-transduction cascades leading to the execution of the processes during the HR. The Arabidopsis -derived gene products Ndr1 and Eds1 [34] are good candidates for the induction of downstream HR defense responses. Similarly, in the barley–powdery mildew inter-action a large number of R genes feed into one central signalling molecule, Rar1 [35]. The exact function of none of these proteins is known. Eds1 resembles a lipase, sug-gesting that lipid signalling may be activated, whereas Ndr1 can be activated by mutations resembling Ca2+-dependent
protein kinase (CDPK)-phosphorylation (MH Stuiver et al., unpublished data), suggesting that it functions downstream of the Ca2+ influx into the cytoplasm that has been
observed in many HR-inducing systems [36].
Besides protein–protein signalling, the generation of sec-ond messengers involved in the transduction of the HR response, such as the above-mentioned Ca2+ ions and
lipids, may serve as a starting point for engineering resis-tance; so, too, might extracellular nitric oxide [37••] and
reactive oxygen species [31,38,39••], which seem to be
important in mediating at least part of the defense respons-es, and which, when applied together, might signal processes downstream of HR induction. The generation of reactive oxygen species by other means might also induce systemic acquired resistance [40].
Further downstream in the execution of the HR response, there is a probable divergence of signals, in which the induction of cell death is likely to be separated from other aspects of the induction of the HR, such as callose deposition, the accumulation of phenolics and the induction of PR proteins. The hormones salicyclic acid, jasmonic acid and ethylene are important in the induction of PR proteins in the HR and also in normal defense responses triggered in the compatible interaction. These multiple responses are involved in combating pathogen infection [41–45]. It is likely that the coordinated or sep-arate activation of these PR-protein induction pathways [46] leads to a general increased level of resistance. It is envisaged, however, that the level of control might be limited in the strength of the resistance and/or the scope of pathogens inhibited.
Future prospects
useful levels of resistance has not yet been achieved. The increasing number of reports of broad-spectrum resistance to fungi in different transgenic crops, however, indicates that commercial introductions of fungus-resistant crops can be expected within the next 4–8 years.
The growing global demand for increased food production and consumers’ need for high-quality food present consid-erable challenges to scientists in industry, academia and government institutions. Fungicides are expected to con-tinue to form the main strategy permitting growers to achieve high levels of disease control and hence improve crop yields in the near future. Continuous agrochemical research has developed a range of modern fungicides with new modes of action that provide broad-spectrum disease control in economically important crops. It is now clear that the introduction of a single transgene is not sufficient to achieve durable and broad-spectrum disease resistance. A combination of transgenic strategies will therefore be needed to reduce the requirement for agrochemicals to control crop diseases. Future research into disease resis-tance will focus on the successful integration of transgenic strategies into breeding programs to develop the durable resistance of new commercial varieties, in combination with a selective use of low doses of fungicides. In the longer term it is envisaged that farmers will achieve opti-mal disease control in individual crops by balancing the use of both transgenes and fungicides through integrated crop management.
Acknowledgements
We thank Bart van Wezenbeek for critical reading of the manuscript, and Melanie Custers-van Spronsen for her assistance in its preparation.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Oerke EC: Crop Protection and Crop Production. Amsterdam: Elsevier; 1994.
2. Andrade H: Breeding in Ecuador: facing increasing late blight severity. In Proceedings of the Global Initiative on Late Blight Volume 1: 1999 Mar 16-19; Quito, Ecuador. Lima, Peru: GILB; 1999:38-40.
3. Colon LT: Trends in late blight resistance breeding in Western Europe. In Proceedings of the Global Initiative on Late Blight Volume 1: 1999 Mar 16-19; Quito, Ecuador. Lima, Peru: GILB; 1999:40-41.
4. Anon: Agrochemical risks and benefits. Chemistry in Britain 1998,
43:20-24.
5. Hallberg GR: Pesticide pollution of ground water in the humid United States. Agric Ecosyst Environ 1989, 26:299-367.
6. Cornelissen BJC, Melchers LS: Strategies for control of fungal diseases with transgenic plants.Plant Phys1993, 101:709-712.
7. De Wit PJGM: Molecular characterisation of gene-for-gene systems in plant–fungus interactions and the application of avirulence genes in control of plant pathogens.Annu Rev Phytopathol1992,
30:391-418.
8. Bowles DJ: Defense-related proteins in higher plants.
Annu Rev Biochem1990, 59:873-907.
9. Mehdy MC: Active oxygen species in plant defense against pathogens. Plant Phys 1994, 105:467-472.
10. Van Loon LC, Gerritsen YAM, Ritter CE:Identification, purification and characterisation of pathogenesis-related proteins from virus-infected Samsun NN tobacco leaves. Plant Mol Biol 1987, 9:593-609.
11. Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Metraux JP, Ryals JA: Coordinate gene activity in response to agents that induce systemic acquired resistance.Plant Cell1991, 3:1085-1094.
12. Broglie K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvis CJ, Broglie R: Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani.Science1991,
254:1194-1197.
13. Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C: Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco.Plant J1995, 8:97-109.
14. Neuhaus JM, Ahl-Goy P, Hinz U, Flores S, Meins F: High-level expression of a tobacco chitinase gene in Nicotiana sylvestris; susceptibility of transgenic plants to Cercospora nicotianae infection.Plant Mol Biol1991, 16:141-151.
15. Van den Elzen PJM, Jongedijk E, Melchers LS, Cornelissen BJC: Virus and fungal resistance: from laboratory to field.Phil Trans R Soc LondB 1993, 342:271-278.
16. Jongedijk E, Tigelaar H, van Roekel JSC, Bres-Vloemans SA, Dekker I, van den Elzen PJM, Cornelissen BJC, Melchers LS: Synergistic activity of chitinases and ββ-1,3-glucanases enhances fungal resistance in transgenic tomato plants.Euphytica1995, 85:173-180.
17. Zhu Q, Maher EA, Masoud S, Dixon RA, Lamb CJ: Enhanced protection against fungal attack by constitutive co-expression of chitinase and glucanase genes in transgenic tobacco.
Bio/Technology1994, 12:807-812.
18. Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E, Ryals J: Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a.Proc Natl Acad Sci USA
1993,90:7327-7331.
19. Liu D, Raghothama KG, Hasegawa PM, Bressan RA: Osmotin overexpression in potato delays development of disease symptoms.Proc Natl Acad Sci USA1994, 91:1888-1892.
20. Zhu B, Chen THH, Li PH: Analysis of late-blight disease resistance and freezing tolerance in transgenic potato plants expressing sense and antisense genes for an osmotin-like protein.Planta
1996, 198:70-77.
21. Lin W, Anuratha CS, Datta K, Potrykus I, Muthukrishnan S, Datta SK:
Genetic engineering of rice for resistance to sheath blight.
Bio/Technology1995, 3:686-691.
22. Liang J, Wu Y, Rosenberger C, Hakimi S, Castro S, Berg J: AFP genes confer disease resistance to transgenic potato and wheat plants.Abstract no. L-49. In 5th International Workshop on Pathogenesis-related Proteins In Plants; Signalling Pathways and Biological Activities 1998; Aussois, France 1998.
23. Terras FRG, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, van Leuven F, Vanderleyden J et al.:
Small cysteine-rich antifungal proteins from radish (Rhaphanus sativus L.): their role in host defence.Plant Cell1995, 7:573-588.
24. Broekaert WF, Cammue BPA, De Bolle MFC, Thevissen K,
de Samblanx GW, Osborn RW: Antimicrobial peptides from plants.
Crit Rev Plant Sci1997, 16:297-323.
25. Epple P, Apel K, Bohlman H: Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum.Plant Cell1997, 9:509-520.
26. Molina A, Garcia-Olmedo F: Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein LTP2.Plant J1997, 12:669-675.
27. Zhang Z, Collinge DB, Thordal-Christensen H: Germin-like oxalate oxidase, a H2O2producing enzyme, accumulates in barley
attacked by the powdery mildew fungus.Plant J 1995, 8:139-145.
28. Wu G, Shortt BJ, Lawrence EB, Levine EB, Fitzsimmons C, Shah DM:
Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants.
Plant Cell1995, 7:1357-1368.
Brassica napus constitutively expressing a chimeric chitinase gene.Nat Biotechnol1996, 14:643-646.
30. Keller H, Pamboukdjian N, Ponchet M, Poupet A, Delon R, Verrier JL, •• Roby D, Ricci P: Pathogen-induced elicitin production in
transgenic tobacco generates a hypersensitive response and nonspecific disease resistance.Plant Cell1999, 11:223-235. This paper describes interesting experiments with transgenic tobacco that harbour a pathogen-inducible promotor–elicitor fusion. Fungal resistance to various (hemi)biotroph and also necrotrophic fungi is observed in these transgenic plants.
31. Desikan R, Reynolds A, Hancock JT, Neill SJ: Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures.Biochem J1998, 330:115-120.
32. Karrer EE, Beachy RN, Holt CA: Cloning of tobacco genes that • elicit the hypersensitive response.Plant Mol Biol1998, 36
:681-690.
The authors present an elegant strategy for the isolation of plant genes involved in various aspects of the HR. They reveal some surprises in the cod-ing sequences identified. Down-regulation of ubiquitin also induces HR and provides a positive control for the strategy.
33. Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JD: Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses.Plant Cell1999, 11:273-287.
34. Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE:
Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis.Proc Natl Acad Sci USA1998, 95:10306-10311.
35. Lahaye T, Shirasu K, Schulze-Lefert P: Chromosome landing at the barley Rar1 locus.Mol Gen Genet1998, 260:92-101.
36. Scheel D: Resistance response physiology and signal transduction.Curr Opin Plant Biol1998, 4:305-310.
37. Delledonne M, Xia Y, Dixon RA, Lamb C: Nitric oxide functions as a •• signal in plant disease resistance.Nature1998, 394:585-588. A classic paper describing the central role of nitric oxide in plant defence and the HR using experiments on soybean suspension cells and intact
Arabidopsis leaves.
38. Keller T, Demude HG, Werner D, Doerner P, Dixon RA, Lamb C:
A plant homolog of the neutrophil NADPH oxidase gp91phox
subunit gene encodes a plasma membrane protein with Ca2+
binding motifs.Plant Cell1998, 10:255-266.
39. Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C: •• Reactive oxygen intermediates mediate a systemic signal
network in the establishment of plant immunity.Cell1998,
92:773-784.
An authoritative paper describing the role of reactive oxygen species in the local induction of defence and providing evidence that reactive oxygen plays a role in the more distant signalling leading to systemic acquired resistance. 40. Molina A, Volrath S, Guyer D, Maleck K, Ryals J, Ward E: Inhibition of protoporphyrinogen oxidase expression in Arabidopsis causes a lesion-mimic phenotype that induces systemic acquired resistance.Plant J1999, 17:667-678.
41. Dong X: SA, JA, ethylene and disease resistance in plants.
Curr Opin Plant Biol1998, 1:316-323.
42. Solano R, Stepanova A, Chao Q, Ecker JR: Nuclear events in ethylene signaling: a transcriptional cascade mediated by ethylene-insensitive3- and ethylene-response-factor 1.Genes Dev
1998, 23:3703-3714.
43. Beale MH, Ward JL: Jasmonates: key players in the plant defence.
Nat Prod Rep 1998, 15:533-548.
44. Solano R, Ecker JR: Ethylene gas: perception, signaling and response.Curr Opin Plant Biol 1998, 1:393-398.
45. Penninckx IA, Thomma BP, Buchala A, Métraux JP, Broekaert WF:
Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene.
Plant Cell1998, 10:2103-2113.
46. Fraissinet-Tachet L, Baltz R, Chong J, Kaufmann S, Fritig B, Saindrenan P: Two tobacco genes induced by infection, elicitor and salicylic acid encode glucosyltransferases acting on phenylpropanoids and benzoic acid derivatives, including salicylic acid.FEBS Lett1998, 437:319-323.
47. Dangl J, Jones JD: Plant–microbe interactions. Affairs of the plant: colonization, intolerance, exploitation and co-operation in plant–microbe interactions.Curr Opin Plant Biol 1998, 1:285-287.
48. Logemann J, Jach G, Tommerup H, Mundy J, Schell J: Expression of a barley ribosome inactivating protein leads to increased fungal protection in transgenic tobacco plants.Bio/Technology1992,