Effectiveness of indigenous and non-indigenous isolates
of arbuscular mycorrhizal fungi in soils from degraded
ecosystems and man-made habitats
Batkhuugyin Enkhtuya
∗, Jana Rydlová, Miroslav Vosátka
Institute of Botany, Academy of Sciences of the Czech Republic, 252 43 Pru◦honice, Czech Republic Received 19 April 1999; received in revised form 2 March 2000; accepted 6 March 2000
Abstract
Culturing in soils from degraded ecosystems significantly influenced the effectiveness of indigenous arbuscular mycorrhizal fungi (AMF) isolated from disturbed and undisturbed soils. The AMF isolates from degraded or artificially created habitats (acid rain polluted site, power station fly ash deposits, spoil banks, pyrite deposit), were not, in most cases, more effective than those from undisturbed soils, when grown in symbiosis with maize in the disturbed soils. Significant effects of soil or substrate on plant growth were found, while the influence of the AMF inoculant was much less pronounced. The development of AMF isolates was reduced in soils with more adverse chemical properties irrespective of the isolate origin. The length of extraradical mycelium of AMF and NADH-diaphorase activity of the mycelium were good indicators of negative effects of stress factors in the soil. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Glomus; Heavy metals; Mycorrhiza; Soil contamination; Soil disturbance
1. Introduction
Serious ecological problems have arisen due to exploitation of natural resources in Central Europe, particularly in the northwestern part of the Czech Republic, which is one of the most polluted areas in Europe. It is a part of the area called ‘Black Triangle’ — the region where several anthropogenic stress fac-tors combine to cause degradation of natural ecosys-tems and also the establishment of man-made habitats with very unfavorable conditions for plant growth. The most serious problem is the effect of air pollu-tion on mountain forest, which is caused by burning
∗Corresponding author. Tel.:+42-02-6775-0022;
fax:+42-02-6775-0022.
E-mail address: [email protected] (B. Enkhtuya)
highly sulfurous brown coal by power stations. As a consequence of emissions, drastic changes occur in the soil chemistry leading to lowered soil pH. Acid-ification processes induce aluminum and manganese toxicity and a shortage of calcium and magnesium which cause nutrient imbalances and depletion of populations of soil microorganisms (Esher et al., 1992). Mining of the coal leads to the establishment of spoil banks. These are composed of materials mined from depths of about 200 m, mostly Miocene clays with unfavorable physical properties such as liability to erosion, low drainage ability, and dry or hypoxic conditions. Sedimentation ponds for power station fly ash are a type of artificial habitat with mostly alkaline substrates and adverse physical properties such as low water-holding capacity. Another type of sedimen-tation pond is that for waste from pyrite processing.
Substrates in these waste disposal sites show high concentrations of toxic heavy metals such as Mn, Fe and Al, high salinity and pH fluctuations.
Successful survival and growth of plants in soils degraded by industrial activity is greatly dependent not only upon the abiotic properties of the soil but also on the activity of microbial populations (Visser, 1985). The presence of arbuscular mycorrhizal fungi (AMF) may reduce negative effects of stresses caused by lack of nutrients or organic matter, by adverse soil structure, extreme pH or by pathogens (Sylvia and Williams, 1992). AMF can also enhance the resis-tance of plants to drought stress and high salinity due to the increased absorption zone of mycorrhizal roots (Hardie, 1985). An important feature of AMF might be a protective role of mycorrhiza against stress in-duced by high concentrations of heavy metals (Galli et al., 1994). Schuepp et al. (1987) have postulated that AMF can serve as a filtration barrier against trans-fer of heavy metals to the plant shoots. Better P nu-trition and increase in plant biomass have also been proposed as possible reasons for a higher tolerance to heavy metals (Haselwandter et al., 1994). However, different populations or geographical isolates of AMF were found to show high variability in their tolerance to heavy metals and associated stress (Leyval et al., 1991; Weissenhorn et al., 1993; Bartolome-Esteban and Schenck, 1994).
Elimination of AMF populations leads to prob-lems with plant establishment and survival (Pfleger et al., 1994). Even if AMF are ubiquitous in terres-trial ecosystems, mechanical or chemical disturbance of the soil can substantially reduce AMF population vigor and functioning (Sylvia and Williams, 1992). Numbers of spores and root colonization are often reduced by soil disturbance (Waaland and Allen, 1987), but AMF isolates adapted to local soil con-ditions are still able to stimulate plant growth at that site compared with non-indigenous isolates. It seems probable that such AMF ecotypes result from long-term adaptation to soils with extreme properties (Sylvia and Williams, 1992). Isolation of indigenous and presumably stress-adapted AMF is a potential biotechnological tool for inoculation of plants in dis-turbed ecosystems (Dodd and Thompson, 1994). The isolation and study of these ‘stress-tolerant’ isolates might contribute to knowledge of the ecophysiology of AMF under stress conditions.
The aim of the present study was to study the effec-tiveness of indigenous AMF isolates from disturbed soils and non-indigenous isolates from undisturbed soils in symbiosis with maize (a model universal host plant for AMF) growing in disturbed soils.
2. Materials and methods
2.1. Site characteristics
2.1.1. Sedimentation ponds
The Chvaletice (CHV) and Opatovice (OPA) sedi-mentation ponds are located in the eastern part of the Labe river basin (50◦02′28′′N, 15◦26′39′′E, altitude
204 m, and 50◦04′00′′N, 15◦50′00′′E, altitude 208 m).
The Tusimice (TUS) sedimentation pond is located in the North Bohemia (50◦04′00′′N, 15◦50′00′′E,
alti-tude 208 m). Waste from a smelter factory processing pyrite raw materials has been stored in the Chvalet-ice sedimentation pond. The OPA and TUS ponds contain fly ash from a power station burning brown coal. The OPA and CHV ponds were abandoned in the 1980s, whereas TUS is still being used for fly ash storage. Vegetation spontaneously developed on the CHV pond, dominated by Calamagrostis epigejos with small hardwoods such as birch, poplar and wil-low. The OPA and TUS ponds have been vegetated by a mixture of grasses (Festuca rubra, Poa pratensis), but C. epigejos has become gradually dominant over the sown grass species. Soil from the CHV pond is characterized by a high content of sulfides, and there-fore a low pH. Weathering of the soil causes strong acidification followed by an increase in salinity. The soil also shows high concentrations of heavy metals, mainly Mn, Fe and Al (Rauch, 1996). Fly ash mixtures from the OPA sedimentation pond are acid, whereas those from TUS are alkaline and both are rich in the soluble elements, Ca, Mg, K and Na, which influences the toxicity of other elements and compounds. Both soils are very vulnerable to erosion and drought.
2.1.2. Spoil banks
were selected: (1) the Albrechtice (ALB) spoil bank (50◦33′31′′N, 13◦31′58′′E, altitude 250 m), 31-year
old, with spontaneous plant succession. Initial plant colonization occurred 1 year after mining and the majority of plants are annuals. After about 15 years, the site was completely covered by herbaceous veg-etation, although, the establishment of hardwoods is rather limited with scarce occurrence of birch and willow (Prach, 1987); (2) the Brezno (BRE) spoil bank (50◦28′03′′N, 13◦32′56′′E, altitude 250 m), with
a 2-year old plantations of Acer pseudoplatanus and (3) the Velebudice (VEL) spoil bank (50◦28′33′′N
13◦38′23′′E, altitude 270 m), with an 8-year old
plantations of A. pseudoplatanus and Fraxinus
excelsior.
2.1.3. Acid rain polluted site
The Lesna (LES) site is located in a clear-cut area in the Krusne hory Mountains (50◦34′02′′N,
13◦26′12′′E, altitude 890 m) where the original spruce
forest died off due to acid rain pollution. The site is a 30-year old plantation of Sorbus aucuparia with ground cover of Calamagrostis villosa. As the pH of the soils exposed to acid rain decreased, the acidify-ing process induced Al toxicity and led to a decrease in nutrient availability.
2.2. Soil analysis
The pH was determined on 50 g air-dried soil sub-samples extracted by distilled water (pH actual) or by 0.1 M KCl (pH exchangeable) and stirred for 10 min, using a Radiometer TT2 pH meter (Kub´ıková, 1972).
Other 5 g soil sub-samples were ground to a pow-der of maximum particle size 0.1 mm, weighed into the Al containers and analysed for C and N by the CHN-Rapid Heraeus Elemental Analyser. After com-bustion at 950◦C and reduction of NO
x the contents
of C and N were determined by thermo-conductibility detection (Monar, 1972).
Soil sub-samples (50 g) were air dried and ground to a maximum particle size of 2 mm and extracted with 0.5 M NaHCO3 (pH 8.5) for extractable P
determination. The content of P–PO3 was
deter-mined by a photometric method with ammonium molybdenate-sulfuric acid reagent (Olsen, 1954), using the UV–VIS Spectrometer Unicam UV4-200.
For exchangeable ions (Ca, Mg, K, Na, Mn, Zn) determination, 50 g soil sub-samples were air dried and ground to the maximum particle size of 2 mm and extracted with 1 M ammonium acetate (pH 7) for Fe with pH 4.8. In the resulting solution, Ca, K and Na content was determined by flame atomic emission spectroscopy, Mg and heavy metals content was de-termined using the AAS Spectrometer Unicam 9200X (Moore and Chapman, 1986).
2.3. Experimental design
The indigenous AMF were isolated during 2 years of successive trapping in pots with various host plants cultivated in a greenhouse. Pure cultures of AMF were established from multiple spore sub-cultures and identified on the basis of spore morphology (Walker, 1992) and isozyme analysis of spores (Dodd et al., 1996). Sixty-four treatments were included in a two-factorial design. The first factor was inoculation: seven AM fungi (indigenous isolates: G. mosseae — spoil bank ALB; G. fistulosum — CHV sedimentation pond; G. etunicatum — TUS sedimentation pond;
G. intraradices — OPA sedimentation pond; the
non-indigenous isolates from the Bank of European Glomales (BEG): G. mosseae, BEG25; G. fistulosum, BEG23; G. geosporum, BEG11) and non-inoculated control. The second factor was growing substrate: seven soils collected from the sites and sand as an in-ert substrate (Table 1). There were five replicates for each treatment. Maize (Zea mays L.) as the universal host plant for AMF was used to compare effective-ness of mycorrhizal symbiosis. Plant growth response to inoculation and development of mycorrhizal colo-nization was measured after a 14-week period of cul-turing in a greenhouse with no additional fertilization and no supplementary light.
Shoot dry mass was assessed after drying in an oven at 80◦C for 48 h. The root system was cut into 1 cm
modi-Table 1
Characteristics of substrates and soils used in the experimenta
Substrate SAN ALB VEL BRE OPA TUS LES CHV
pH H2O 7.0 6.7 6.6 7.3 7.5 6.2 3.6 7.0
KCl 6.2 6.0 6.2 7.0 7.5 5.8 3.1 6.9
C, N (%) C 0.3 2.9 2.9 1.2 2.2 3.0 13.8 1.7
N 0.0 0.2 0.2 0.04 0.04 0.1 0.7 0.1
CN – 12.5 19.1 28.1 57.8 47.7 21.3 31.7
Macroelements (mg kg−1) P 30 6.0 7.7 0.7 9.0 5.9 2.9 16.0
Ca 416 2506 2774 713 2401 785 194 10356
Mg 37 1597 1484 318 44 101 29 383
K 55 536 361 136 416 218 119 155
Na 5 106 76 52 72 170 0 3
Heavy metals (mg kg−1) Fe 0.7 <0.1 0.4 0.4 <0.1 0.6 25.8 1.1
Mn 2.6 6.0 1.3 1.7 0.8 1.1 1.9 186
Zn 0.1 0.3 0.1 0.1 0.1 0.1 0.7 0.2
Cu 0.1 0.3 <0.1 <0.1 <0.1 <0.1 0.7 0.2
Cd <0.03 0.2 0.2 0.6 0.3 0.1 <0.03 <0.03
Ni <0.1 0.3 0.2 0.1 0.2 0.4 0.1 <0.1
Pb <0.03 <0.03 <0.03 <0.03 0.1 0.2 9.4 <0.03
aSAN — sand; ALB — Albrechtice spoil bank; VEL — Velebudice spoil bank; OPA — Opatovice fly ash disposal site; BRE — Brezno
spoil bank; TUS — Tusimice fly ash disposal site; LES — Lesna acid rain polluted site; CHV — Chvaletice pyrite waste disposal site.
fied gridline intersect method (Giovannetti and Mosse, 1980) using an ocular grid at 100×magnification.
The length and NADH diaphorase activity of the ex-traradical mycelium (ERM) were estimated. A 15 ml core was removed from the middle part of each pot, homogenized by hand in a dish and a 5 g sub-sample was mixed in a blender. The suspension (1 ml) was pipetted onto a membrane filter (24 mm in diameter and 0.45mm pore size) and vacuum filtered. The
mem-brane filter was then placed on a microscope slide and stained with 0.05% Trypan Blue solution in lac-toglycerine. The remaining content of the blender was sieved through two sieves (0.25 and 0.036 mm). The ERM clusters from the finer sieve were collected us-ing sharp tweezers and put into an Eppendorf micro-tube with 300ml of the NADH diaphorase staining
solution (Sylvia, 1988). Staining solution for NADH diaphorase (Sylvia, 1988) was prepared by dissolving 1 mg ml−1of iodonitrotetrazolium in 0.5 ml of 100%
ethanol and vortexing for 5 min in an Eppendorf tube. Then 3 mg ml−1 NADH were added to 0.2 M Tris
buffer (pH 7.4) and the final solution was then stirred for 1 h on a magnetic stirrer. The microtubes were in-cubated at 28◦C for 14 h in the dark. The total length
of mycelium was evaluated under an Olympus BX60 microscope using a grid inside the eyepiece at 100×
magnification (Brundrett et al., 1994). The results were expressed as centimeters of mycelium in 1 g of dry soil. The percent proportion of mycelium length which contained red precipitate (NADH diaphorase activity) was measured after mounting mycelium clusters from Eppendorf tubes in glycerol on the microscope slides at magnification of 400×.
2.4. Statistical analysis of data
Statistical analysis was carried out with SOLO 4 (BMDP Statistical Software). Data showing nor-mal distribution were analyzed by two-way ANOVA followed by the Duncan Multiple Range test. Data with non-normal distribution were logarithmi-cally transformed and analyzed by non-parametric Kruskal–Wallis and Connover tests. Relationships between all measured parameters were tested using correlation analysis.
3. Results
3.1. Plant growth
effect of AMF was not significant. Maize shoot dry mass was not reduced in disturbed soils in any AMF treatment as compared to plants growing in sand (Table 2). Non-inoculated host plants had higher shoot dry mass in soils from the VEL spoil bank and the CHV sedimentation pond than in sand, BRE spoil bank and the LES site. Host plants growing in soil from the ALB spoil bank had higher shoot dry mass than plants growing in most other soils in all mycorrhizal treatments. In contrast, in the acidified substrate from LES shoot dry mass was very low (irrespective of inoculation with AMF). Maize root lengths were not reduced in disturbed soils in any inoculation treatment in comparison with root lengths of plants growing in sand (Table 2). Plants growing in soils from the ALB and VEL spoil banks had higher root length than plants growing in most other soils in all mycorrhizal treatments.
Shoot dry mass of maize was positively influenced by AMF only in soil from ALB (with the exceptions of G. geosporum BEG11 and G. mosseae indigenous isolates). In substrate from CHV, inoculation of host plants with G. intraradices, G. fistulosum BEG23,
Table 2
Shoot dry mass and root length of maize grown in sand and seven soils from the disturbed ecosystems and man-made habitats listed in Table 1, uninoculated or inoculated with indigenous and non-indigenous isolates of arbuscular mycorrhizal fungia
AMF/ Control G. intraradices G. fistulosum G. etunicatum G. geosporum G. etunicatum G. mosseae G. mosseae
substrate isolate BEG23 isolate BEG11 isolate BEG25 isolate
Shoot dry mass (g)
SAN 1.2 bcd (k) 1.3 bcd (k) 1.6 bcd (k) 1.7 bcd (k) 1.3 cd (k) 1.3 cd (k) 1.6 bc (k) 1.2 bcd (k) ALB 2.4 ab (m) 4.5 a (kl) 4.6 a (kl) 6.6 a (k) 3.5 a (lm) 4.6 a (kl) 4.7 a (kl) 3.2 a (lm) VEL 2.5 a (k) 2.2 ab (k) 2.3 ab (k) 2.8 ab (k) 2.7 ab (k) 2.4 ab (k) 1.7 b (k) 2.5 ab (k) BRE 1.0 cd (k) 0.9 cd (k) 1.5 bcd (k) 1.4 cd (k) 1.5 cd (k) 1.2 cd (k) 1.5 bc (k) 1.1 cd (k) OPA 1.8 abc (k) 1.5 bc (k) 1.6 bc (k) 1.8 bc (k) 1.9 abc (k) 1.5 bc (k) 1.4 bc (k) 1.3 abc (k) TUS 1.6 abc (k) 1.6 bc (k) 1.9 abc (k) 2.2 abc (k) 1.8 abc (k) 1.9 abc (k) 2.1 ab (k) 1.4 abc (k) LES 0.4 d (k) 0.5 d (k) 0.6 d (k) 0.6 d (k) 0.5 d (k) 0.5 d (k) 0.7 c (k) 0.4 d (k) CHV 2.7 a (k) 1.3 bcd (l) 1.3 cd (l) 1.8 bcd (kl) 1.6 bc (kl) 1.3 cd (l) 2.2 ab (l) 1.2 bcd (l)
Root length (cm g−1)
SAN 7.9 abc (k) 7.3 bc (k) 6.7 bc (k) 5.5 cd (k) 5.6 bcd (k) 5.0 cd (k) 6.2 bc (k) 8.1 bc (k) ALB 12.0 a (m) 22.7 a (k) 11.0 a (m) 22.6 a (kl) 12.1 ab (lm) 11.5 a (m) 23.2 a (k) 15.8 a (klm) VEL 10.6 ab (k) 10.9 ab (k) 8.4 ab (k) 11.5 a (k) 13.4 a (k) 12.1 a (k) 10.7 ab (k) 13.4 ab (k) BRE 9.8 ab (k) 8.1 bc (k) 6.6 bc (k) 8.5 abc (k) 6.0 abc (k) 5.5 bcd (k) 7.0 bc (k) 4.8 c (k) OPA 11.6 ab (k) 6.7 bc (k) 10.2 ab (k) 10.0 ab (k) 11.3 ab (k) 9.4 abc (k) 10.5 ab (k) 12.7 ab (k) TUS 6.5 bc (lm) 10.6 ab (k) 9.2ab (klm) 5.6 cd (m) 5.9abc (lm) 11.2 ab (kl) 5.9 bc (m) 8.3 abc (klm) LES 3.3 c (l) 2.9 c (l) 6.0 bc (kl) 3.3 d (l) 2.9 d (l) 8.6 abcd (k) 9.8 ab (k) 4.3 c (kl) CHV 7.7 abc (k) 4.4 c (lm) 3.5 c (m) 6.1bcd (kl) 3.9 cd (m) 4.1 d (lm) 4.4 c (lm) 6.1 c (kl)
aMeans followed by the same letter (a–d) within columns (comparisons between soils) and within rows (k–n) (comparisons between
AMF) are not significantly different according to Duncan’s Multiple Range test (p<0.05, n=5).
G. geosporum and G. mosseae had negative effects on
shoot dry mass and root length.
3.2. Development of arbuscular mycorrhizal symbiosis
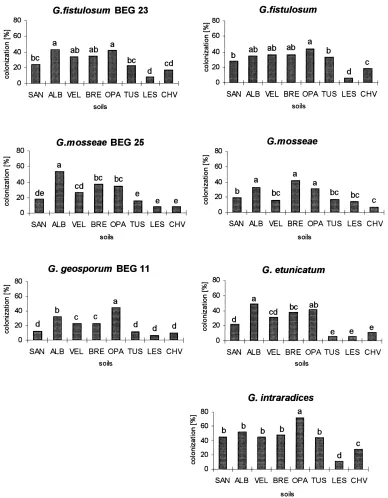
Mycorrhizal colonization was significantly influ-enced by both substrate and fungus (p<0.001).
Sub-strate and AMF isolate interactions were significant for mycorrhizal colonization (p<0.01), and for length
of ERM (p<0.001). The development of mycorrhizal
Table 3
Comparison of mycorrhizal colonization of indigenous isolate G. intraradices with other AMF isolates associated with maize grown in sand and seven soils from disturbed ecosystems and man-made habitatsa
AMF/Substrate SAN ALB VEL OPA BRE TUS LES CHV
G. fistulosum BEG23 * n.s. n.s. * * * n.s. n.s. G. fistulosum isolate * * n.s. * * * n.s. n.s. G. geosporum BEG11 * * * * * * n.s. * G. etunicatum isolate * n.s. * * * * n.s. * G. mosseae BEG25 * n.s. * * * * n.s. * G. mosseae isolate * * * * n.s. * n.s. *
aSubstrate abbreviations are as in Table 1. Asterisks
indi-cate significantly higher colonization in G. intraradices; n.s.: non-significant according to Duncan’s Multiple Range test (p<0.05).
significantly higher percentages of mycorrhizal colo-nization compared to treatments inoculated with all other isolates.
ERM length and NADH-diaphorase activity were significantly influenced only by substrate (Table 4). Length of ERM was highest in soil from the ALB spoil bank for most of the AMF isolates. In substrates with more unfavorable chemical characteristics (from
Table 4
NADH-diaphorase activity and total length of extraradical mycelium associated with roots of maize grown in sand and seven soils from disturbed ecosystems and man-made habitats listed in Table 1, inoculated with indigenous and non-indigenous isolates of arbuscular mycorrhizal fungia
AMF/ G. intraradices G. fistulosum G. fistulosum G. geosporum G. etunicatum G. mosseae G. mosseae
substrate isolate BEG23 isolate BEG11 isolate BEG25 isolate
NADH-diaphorase activity (%)
SAN 54 bc (k) 44 bcd (k) 65 ab (k) 50 bcd (k) 51 bcd (k) 46 bc (k) 52 bcd (k) ALB 63 ab (l) 80 a (k) 72 a (kl) 57 abc (l) 71 a (kl) 74 a (k) 70 a (kl) VEL 55 bc (k) 53 bc (k) 51 bcd (k) 61 ab (k) 63 abc (k) 51 bc (k) 48 cd (k) BRE 78 a (k) 62 ab (l) 68 ab (kl) 64 ab (l) 67 abc (kl) 70 a (kl) 65 abc (l) OPA 58 abc (lm) 56 ab (m) 67 ab (kl) 66 a (kl) 71 a (k) 60 ab (lm) 72 a (k) TUS 58 abc (kl) 53 bc (lmn) 55 bcd (klm) 13 cd (mn) 0 d (n) 57 ab (klm) 68 ab (k)
LES 0 d (k) 0 d (k) 0 d (k) 12 d (k) 0 d (k) 11 d (k) 14 d (k)
CHV 41 cd (k) 37 cd (k) 43 d (k) 41 cd (k) 45 cd (k) 38 bc (k) 50 cd (k)
ERM total length (cm g−1)
SAN 20 b (lmn) 29 b (lm) 103 a (k) 54 ab (l) 34 ab (l) 4 b (a) 29 bc (lm) ALB 94 a (k) 81 a (k) 101 a (k) 64 a (lm) 49 a (m) 81 a (k) 94 a (k) VEL 17 b (k) 12 bc (k) 14 b (k) 27 cd (k) 8 c (k) 14 b (k) 21 bc (k) BRE 21 b (k) 14 bc (k) 6 b (k) 20 cd (k) 22 bc (k) 22 b (k) 20 bc (k) OPA 17 b (k) 26 bc (k) 13 b (k) 30 bc (k) 20 bc (k) 19 b (k) 32 b (k)
TUS 14 b (k) 7 bc (k) 14 b (k) 3 de (k) 2 c (k) 7 b (k) 3 c (k)
LES 0 b (k) 2 c (k) 0 b (k) 1 e (k) 0 c (k) 0 b (k) 0 c (k)
CHV 3 b (k) 7 bc (k) 1 b (k) 11 cde (k) 2 c (k) 2 b (k) 0.4 c (k)
aSubstrate abbreviations and methods of comparing treatment means are as for Table 2.
LES and CHV sites), there was a tendency for reduced growth of ERM as compared to other soils. Some iso-lates, which were very successful in some substrates, had very low ERM length in others (e.g. G. fistulosum in sand and in the soil from the ALB spoil bank ver-sus the same isolate in the OPA sedimentation pond and BRE spoil bank). The AMF growing in soil from ALB had higher NADH-diaphorase activity in com-parison with most other substrates, the lowest one was found in acidified soil from the LES site. Some AMF isolates showed no NADH-diaphorase activity in the most adverse substrates (G. intraradices, G. fistulosum BEG23, G. fistulosum, G. etunicatum in soil from the LES acidified forest site and the indigenous isolate
G. etunicatum in substrate from TUS).
Table 5
Comparison of development of native isolates from disturbed and man-made habitats (G. intraradices, G. mosseae, G. etunicatum and G. fistulosum) and from undisturbed soils (G. mosseae BEG25, G. geosporum BEG11 and G. fistulosum BEG23) associated with maize grown in sand and seven soils from disturbed ecosystems and man-made habitatsa
Substrate Mycorrhizal ERM NADH-diaphorase colonization length activity of ERM
SAN * * n.s.
ALB n.s. n.s. n.s.
VEL n.s. n.s. n.s.
BRE * n.s. n.s.
OPA n.s. n.s. *
TUS n.s. n.s. n.s.
LES n.s. n.s. n.s.
CHV n.s. n.s. n.s.
aSubstrate abbreviation are as in Table 1. Asterisks indicate
higher values of measured parameters for AMF isolates indige-nous in disturbed soils; n.s.: non-significant according to Duncan’s Multiple Range test (p<0.05).
mycorrhizal parameters such as mycorrhizal colo-nization and NADH-diaphorase were correlated with each other.
4. Discussion
AMF can stimulate plant growth and survival, thus reducing stress caused by unfavorable edaphic condi-tions, while, as stated by Pfleger et al. (1994), their reduction can lead to plant growth depression. This
Table 6
Correlations between parameters characterizing plant growth and AMF development in sand and seven soils from disturbed ecosystems and man-made habitatsa
Substrate SDM×MC ERM×MC ERM×ACT MC×ACT RL×ERM RL×MC RL×SDM
SAN n.s. n.s. 0.343* 0.338* n.s. n.s. n.s.
ALB 0.464** n.s. n.s. 0.399* n.s. n.s. 0.394**
VEL n.s. n.s. n.s. n.s. n.s. n.s. n.s.
BRE n.s. n.s. n.s. 0.369* n.s. n.s. n.s.
OPA n.s. n.s. n.s. 0.387* n.s. 0.316* n.s.
TUS n.s. 0.571** 0.470** 0.685*** n.s. n.s. n.s.
LES n.s. n.s. n.s. n.s. n.s. n.s. 0.358*
CHV n.s. n.s 0.415* n.s. 0.423* n.s. 0.403*
aSubstrate abbreviations are as for Table 1. SDM — shoot dry mass, MC — mycorrhizal colonization, ERM — length of extraradical
mycelium, ACT — NADH-diaphorase activity of extraradical mycelium, RL — root length. Correlation coefficients (*p<0.05, **p<0.01, ***p<0.001; n.s.: non-significant). Combinations of parameters with no significant correlation in any soil (SDM×ERM, SDM×ACT and RL×ACT) are not presented.
has probably been the case for acidified soil from the LES site, where the plants were very stunted and very poorly colonized by AMF. The acidified soil showed the highest phyto-toxicity and the main reason for such a depression of AMF was probably a high content of heavy metals, particularly the toxic Al but also Fe and Pb released by soil acidification. In addition, de-creased phosphorus availability probably contributed to poor plant growth. Mycorrhizal colonization, length and activity of ERM of all AMF isolates were very low in the LES soil, whereas the substrates from the ALB and VEL spoil banks and from the OPA fly ash deposit which were relatively less toxic allowed much better development of mycorrhizal symbiosis.
less attention has been paid to the interaction of AMF tolerance and plant tolerance to stress factors. Inouhe et al. (1994) found that in monocotyledons, particu-larly grasses, the uptake of heavy metals is generally lower as compared to dicotyledons. A high tolerance of maize to edaphic stresses can be one of the expla-nations for ineffective mycorrhizal symbiosis in soils from degraded ecosystems. By contrast, Weissenhorn et al. (1995) found higher biomass and lower concen-trations of Cd, Cu, Zn and Mn in mycorrhizal maize compared to non-mycorrhizal.
Most successful in terms of mycorrhizal devel-opment in different soils was the indigenous isolate of G. intraradices from the OPA fly ash deposit. Such effectiveness and tolerance could be explained (apart from the possibility of high effectiveness of this particular isolate itself) by the fact that this was the only isolate kept throughout the isolation pro-cess and sub-culturing in its original soil sterilized by g-irradiation. The other indigenous isolates were
sub-cultured in inert media, e.g. sand, vermiculite or atapulgite clay Terragreen, and therefore they could lose their adaptation and tolerance to the original stress factor. This would indicate that the ability of AMF isolates to maintain their adaptations and stress-tolerance could change during the sub-culturing process depending on the cultivation substrate used. This could have been the reason why in most toxic soils the indigenous AMF isolates developed signifi-cantly better than non-indigenous isolates. However, there are indications that even some non-indigenous isolates such as G. mosseae BEG25 can exhibit very high plasticity and adapt to various edaphic stresses. To maintain stress tolerance gained through the pro-cess of long-term exposure to chronic stress, the AMF isolates for further research should be cultured in original soil or in media where stress is maintained by addition of sterile original soil or soil extract or chemical solution inducing stress.
The results of comparative studies of tolerance or adaptation of indigenous versus non-indigenous AMF isolates are still controversial. Koomen et al. (1987) concluded that indigenous isolates are not necessar-ily highly effective in terms of mycorrhizal growth response in the soils of their origin. Rydlová (1998) found that AMF from spoil banks had low effective-ness in association with native plants colonizing dis-turbed soils, even if mycorrhizal colonization of roots
was relatively high. By contrast, the non-indigenous isolate G. fistulosum BEG23 increased plant growth significantly. In another experiment, Rydlová (un-published data) observed much higher mycorrhizal growth response in lettuce inoculated with G. mosseae BEG25 compared to several indigenous isolates from contaminated soils and G. mosseae exhibited very distinct tolerance to high salinity and heavy metal contamination of substrates from the pyrite waste site. High infectivity of indigenous AMF but low ef-fectiveness in mycorrhizal growth response compared to some non-indigenous isolates are in agreement with findings of, e.g. D´ıaz and Honrubia (1995), who found similar differences between AMF isolated from heavy metal contaminated soils and non-indigenous
G. fasciculatum.
In contrast, several authors reported high sensitiv-ity of non-indigenous isolates not-adapted to stress of contaminated soils (Gildon and Tinker, 1981; Weis-senhorn et al., 1993, 1994) and higher heavy metal tol-erance and effectiveness of AMF from contaminated soils (Gildon and Tinker, 1983; Leyval et al., 1991). Shetty et al. (1995) found that AMF from soil contam-inated by zinc were more effective in regard to positive growth response of host plant when grown at higher concentration of zinc, while at lower concentration non-indigenous AMF isolates from non-polluted soils were more efficient. Weissenhorn et al. (1993, 1994) found particularly high sensitivity in stage of spore germination rather than ERM development. However, Vosátka et al. (1997) did not find any effects of the same soils used in this experiment on the germination of indigenous and non-indigenous AMF isolates since no isolates were found to be sensitive at the spore germination stage. Nevertheless, we have found the growth of ERM and its NADH-diaphorase activity to be highly sensitive indicators of the stress effects in this experiment.
and adaptation throughout culturing and inoculum production under conditions where stress factors were removed.
Acknowledgements
The authors want to thank M.Sc. Radka Malcová and M.Sc. Jan Jansa for their help with the experi-ment and to Dr. Tomáš Frant´ık for help with statistical analysis. We acknowledge the cooperation of Dr. John Dodd IIB MIRCEN, Canterbury, Kent, UK in isola-tion of fungal material used in this study. The study was financed by the Grant Agency of the Academy of Sciences (grant A6005705) and the Grant Agency of the Czech Republic (grant 526/99/0895).
References
Bartolome-Esteban, H., Schenck, N.C., 1994. Spore germination and hyphal growth of arbuscular mycorrhizal fungi in relation to soil aluminium saturation. Mycologia 86, 217–226. Brundrett, M., Melville, L., Peterson, R. L., 1994. Practical
Methods in Mycorrhizal Research. Mycologue Publications, Waterloo, Canada, 80 pp.
Chao, C.C., Wang, Y.P., 1990. Effects of heavy metals on the infection of vesicular–arbuscular mycorrhizae and the growth of maize. J. Agric. Assoc. China 152, 34–45.
D´ıaz, G., Honrubia, M., 1995. Effect of native and introduced arbuscular mycorrhizal fungi on growth and nutrient uptake of Lygeum spartum and Anthyllis cytisoides. Biol. Plantarum 37 (1), 121–129.
Dodd, J.C., Thompson, B.D., 1994. The screening and selection of inoculant arbuscular mycorrhizal and ectomycorrhizal fungi. Plant Soil 159, 149–158.
Dodd, J.C., Rosendahl, S., Giovannetti, M., Broome, A., Lanfranco, L., Walker, C., 1996. Inter- and intraspecific variation within the morphologically-similar arbuscular mycorrhizal fungi Glomus mosseae and Glomus coronatum. New Phytol. 133, 113–122. Esher, R.J., Marx, D.H., Ursic, S.J., Baker, R.L., Brown, L.R.,
Coleman, D.C., 1992. Simulated acid rain effects on fine roots, ectomycorrhizae, micro-organisms, and invertebrates in pine forests of the Southern United States. Water Air Soil Pollut. 61, 269–278.
Galli, U., Schuepp, H., Brunold, Ch., 1994. Heavy metal binding by mycorrhizal fungi. Physiol. Plantarum 92, 364–368. Gildon, A., Tinker, P.B., 1981. A heavy metal-tolerant strain of a
mycorrhizal fungus. Trans. Br. Myc. Soc. 77 (3), 648–649. Gildon, A., Tinker, P.B., 1983. Interactions of vesicular–arbuscular
mycorrhizal infection and heavy metals in plants. I. The effects of heavy metal on the development of vesicular–arbuscular mycorrhizas. New Phytol. 95, 247–261.
Giovannetti, M., Mosse, B., 1980. An evaluation of techniques to measure vesicular–arbuscular infection in roots. New Phytol. 2, 489–500.
Hardie, K., 1985. The effect of removal of extraradical hyphae on water by vesicular–arbuscular mycorrhizal plants. New Phytol. 101, 677–684.
Haselwandter, K., Leyval, C., Sanders, F.S., 1994. Impact of arbuscular mycorrhizal fungi on plant uptake of heavy metals and radionuclides from soil. In: Gianinazzi, S., Schuepp, H. (Eds.), Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. Birkhauser Verlag, Basel, Switzerland, pp. 179–189.
Inouhe, M., Ninomyia, S., Tohoyama, H., Joho, M., Murayama, T., 1994. Different characteristics of roots in the cadmium-tolerance and Cd-binding complex formation between monocotyledonous and dicotyledonous plants. J. Plant Res. 107, 201–207. Killham, K., Firestone, M.K., 1983. Vesicular–arbuscular
mycorrhizal mediation of grass response to acidic and heavy metal deposition. Plant Soil 72, 39–48.
Koomen, I., Grace, C., Hayman, D.S., 1987. Effectiveness of single and multiple mycorrhizal inocula on growth of clover and strawberry plants at two soil pHs. Soil Biol. Biochem. 19, 539–544.
Kub´ıková, J., 1972. Geobotanické praktikum-SPN, Praha, p.105 (in Czech).
Leyval, C., Berthelin, J., Schontz, D., Weissenhorn, I., Morel, J.L., 1991. Influence of endomycorrhizas on maize uptake of Pb, Cu and Cd applied as mineral salts and sewage sludge. In: Farmer, J.G. (Ed.), Heavy Metals in the Environment. CEP Consultants Ltd., Edinburg, Great Britain, pp. 204–207.
Monar, I., 1972. Analyseautomat zur simultanen Mikrobestimmung von C, H und N. Mikrochim. Acta 6, 784–806.
Moore, P.D., Chapman, S.B., 1986. Methods in Plant Ecology. Blackwell Scientific Publications, Oxford.
Olsen, R.S., 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Dept. Agric. Stat., No. 939.
Pfleger, F.L., Stewart, E.L., Noyd, R.K., 1994. Role of VAM fungi in mine land revegetation. In: Pfleger, F.L., Linderman, R.G. (Eds.), Mycorrhizae and Plant Health. The American Phytopathological Society, MN, pp. 47–82.
Philips, J.M., Hayman, D.S., 1970. Improved procedure for clearing roots and staining parasitic and VAM fungi for rapid assessment of infection. Trans. Br. Myc. Soc. 55, 158–161. Prach, K., 1987. Succession of vegetation on dumps from strip
coal mining. Folia Geobot. Phytotax. 22, 339–354.
Rauch, O., 1996. Comparison of selected elements in substrates of sedimentation ponds and contamination dynamics of soil solution. In: Kovár, P., Hroudová, Z. (Eds.), Biotic Interactions at Succession on Toxic Substrates. Final report of the grant 260/93/2256, Ms. (Dep. in Grant Agency of the Czech Republic, Prague), pp. 7–9 (in Czech).
Schuepp, H., Dehn, B., Sticher, H., 1987. Interaktionen zwischen VA-Mykorrhizen und Schwermetallbelastungen. Angew. Bot. 61, 85–96.
Shetty, K.G., Hetrick, B.A., Schwab, A.P., 1995. Effects of mycorrhizae and fertilizer amendments on zinc tolerance of plants. Environ. Pollut. 88, 307–314.
Sylvia, D.M., Williams, S.E, 1992. Vesicular–arbuscular mycorr-hizae and environmental stresses. In: Bethlenfalvay, G.J., Linderman, R.G. (Eds.), Mycorrhizae in Sustainable Agri-culture. ASA No 54, Madison, WI, USA, pp. 101–124. Sylvia, D.M., 1988. Activity of external hyphae of vesicular–
arbuscular mycorrhizal fungi. Soil Biol. Biochem. 20, 39–43. Visser, S., 1985. Management of microbial processes in surface
mined land reclamation in western Canada. In: Tade, R.L., Klein, D.A. (Eds.), Soil Reclamation Processes: Microbiological Analyses and Applications. Marcel Dekker, New York, Basel, pp. 203–241.
Vosátka, M., Dodd, J.C., Rydlová, J., Batchuugjin, E., Paroulek, M., 1997. The isolation and study of AMF from polluted soils.
In: EC COST Workshop on The Impact of AM Fungi on Ecosystem Restoration, Reykjavik, Iceland.
Waaland, M.E., Allen, E.B., 1987. Relationships between VA mycorrhizal fungi and plant cover following surface mining in Wyoming. J. Range Manage. 40, 271–276.
Walker, C., 1992. Systematics and taxonomy of the arbuscular endomycorrhizal fungi (Glomales) — a possible way forward. Agronomie 12, 887–897.
Weissenhorn, I., Glashoff, A., Leyval, C., Berthelin, J., 1994. Differential tolerance to Cd and Zn of arbuscular mycorrhizal (AM) fungal spores isolated from heavy metal-polluted and unpolluted soils. Plant Soil 167, 189–196.
Weissenhorn, I., Leyval, C., Berthelin, J., 1993. Cd-tolerant arbuscular-mycorrhizal (AM) fungi from heavy-metal polluted soil. Plant Soil 157, 247–256.