99.76%
Originality0.24%
Similarity7
SourcesExcluded as citation or reference Web sources: 37 sources found
1. RESEARCH METHODOLOGY
Determination of Antimicrobial Activity by Inhibitory Zone Analysis After 24 Hour
Incubation
Application of Zinc Oxide Based Antimicrobial Film on Deboned Chicken Meat
Inoculation of Escherichia coli culture
1.2 Preparation of Zinc-Oxide (ZnO) Based Antimicrobial Film
DryZnOnanoparticlesfromSigma-Aldrichwithprimarysizes<100nmwereprepared. A
preset amounts of dry ZnO nanoparticles, including 0.3 ; 0.5 ; 0.7 g were mixed with 45 mL
distilled water in a glass beaker. The glass beaker was placed in an ultrasonicator (Delta
Ultrasonic Cleaner D150 H) for 60 minutes. After that, 5 mLofpolyethylene glycol(PEG
400) as dispersant was used to improve the stability of the suspension (Xihong Liet al.,
2009). Theglassbeakerwasplacedagaininanultrasonicatorfor180minutes. Asolutionof
alginic acidsodium salt wasthen prepared, by mixing1 g of alginicacidsodium salt powder
with50mL distilledwaterinaglassbeakerandstirredusingamagneticstirrerinahotplate
at240rpmuntilallthechemicalsweredissolved.The ZnOnanofluidwasthenmixedwith
the alginic acid sodium salt solution, and placed in an ultrasonicator for 60 minutes. The
mixedsolutionofZnOnanofluidandalginicacidsodiumsaltwastaken40mLandpoured
intoa15cmdiameterglasspetridisc. Thepetridiscswereplacedonahotplateandheatedat
100oC for 1 hour and placed in an oven at 65 C until the solution dried and turned into a sheeto
of film. Driedfilmwassoakedinto2%of CaCl solutionfor 2 minutestopreventcurlingof 2
thefilmsduringdrying(Rhim,2004)andthendriedagainatroomtemperature. Filmmade
bymixing 2g ofalginic acid sodiumsalt alonewith100 mL distilled water wasused asa
control.
1.3 Determination of Mechanical Properties of Zinc Oxide Based Antimicrobial Film
Tensile Strength(TS)and elongationat break(E)were evaluated using aTexture Analyzer
(Lotun ScienceCo. Ltd)according totheASTMstandard methods(ASTM D882and ASTM
D6287). Pre-conditionedfilmscutinto 2.5cm (W)x7.2 cm(L)stripsand mountedbetween
the grips of the machine. Sample was pulled until break at a cross head speed of 1 mm/second
and trigger force of 5 kg. TS and E were calculated using the following relationship:
Theresultsof TSwereexpressedbyMPaandtheresultsof Ewereexpressedbypercentage
1.4 Determination of Antimicrobial Properties of Zinc Oxide Based Antimicrobial Film
1.4.1 Preparation of Mueller Hinton II Agar (MHA) for Agar Diffusion Method
Nutrient agarplates were preparedby adding 38 gMHApowder with 1 L distilledwater in a
glass beakerand shakeduntilcompletelydissolved.After that, MHAsolutionweresterilized
(121oC, 15 min), and then poured into petridishes asseptically.
1.4.2 Culturing Escherichia coli and Salmonella sp.
Stock cell cultures were activatedby taking one colony of each bacteriainto a 9 mL buffered
peptone water and vortexed to homogenized thebacterial culture. The cell cultures were then
inoculated onto a nutrient agar plate and incubated for 24 hours in an agitating incubator.
1.4.3 Analysis of Inhibitory Zone
The agar diffusion method was used for determination of antibacterial effects of ZnO
nanoparticles based films on bacterial strains. The films with different nano ZnO loaded were
cut into 15 mm diameter disks and then placed on agar plates which had been seeded with 0.1
mL ofactivatedcellculture.Initialnumberofbacteriawasintherangeof104–107CFU/ml, and the diameters of inhibitory zone on agar plates after 24 hour incubation were analyzed.
1.5 Application of Zinc Oxide Based Antimicrobial Film to Deboned Chicken Meat
1.5.1 Preparation of Tryptic Soy Agar (TSA) for Bacteria Growth
TheTSA powder(38 g)were initiallymixed with1Ldistilled waterinaglass beakerand
shakeduntilcompletelydissolved. Afterthat,TSA solutionwere sterilized(121 C, 15min) o
then poured into petridishes asseptically.
1.5.2 Culturing Escherichia coli and Salmonella spp.
Stock cell cultures were activatedby taking one colony of each bacteriainto a 9 mL buffered
peptone water and vortexed to homogenized thebacterial culture. The cell cultures were then
inoculated onto a nutrient agar plate and incubated for 24 hours in an agitating incubator.
1.5.3 Application of Antimicrobial Film to Deboned Chicken Meat
Deboned chicken meat was purchased from local supermarket and stored at refrigeration
temperatureprior toantimicrobialtests. The debonedchickenmeatwas cutintosmall pieces
that,thesterilizedmeatswereinoculatedwith0,1mlbacterialculturewithinitialnumberof
the bacteria was 10 CFU/ml andwrapped with films (ZnO incorporated and/orcontrol), then 2
stored at temperature 7 C for 72 hours.o
1.5.4 Microbial Growth Analysis
After72hoursincubation,thewrappingfilmsfromonmeatsampleswereremovedandthe
meatsamplesweremixedandwashedwith20mL peptonewaterfor about1minute. After
that, 1 mL peptone water used for washing the meat samples were taken using 1000 µL
micropipet (Socorex Acura 825) following placed in 9 mL peptone water to reach a 10 -1
dilution. Thedilutionwascontinueduntil10 dilutionsolutionwasreached. Analiquot0,1 -3
mL of each dilution was taken, and placed onto a TSA plates, and spreaded on the agar
surfacewithasterilehockeystick.Thedillutionsamplesweretakenduplicate.Thesamples
were incubated in the incubator at 37 C for 24 hours. Finally,o Escherichia coli and Salmonellaspp.colonies were counted manually and expressed as colony-forming units per
mililiter. For computation, total colony per plate was divided by dillution factor and it is
expressed as CFU/mL.
1.6 Statistical Analysis
Results ofphysical properties and microbial growthanalysis were analyzed using one-way
analysis of variance (ANOVA). The significant differences between treatment means were
determined using Duncan Test New Multiple Range Test (DNMRT) at 95% confidence level.
2. RESULTS AND DISCUSSION
2.1 Zinc Oxide Based Antimicrobial Film
Figure 1. showsthe results ofcontrolantimicrobialfilm and ZnO based antimicrobialfilm
with 3 different concentrations, including0.3 ; 0.5; and 0.7 gsof ZnO. As expected,alginate
filmswithoutZnO (control)weretransparentandpliable,conversely, the antimicrobialfilms
Tensilestrength(TS)andelongationatbreak(E)aretheimportantmechanicalpropertiesin
almost everypackaging applications. Tensile strength is measured for film strength during
stretching andelongation atbreak isthestretch abilityprior tobreakage (Krochta& Johnson
1997in Pranoto,Salokhe,&Rakshit,2005). DifferentTSandEvalueswereobservedwith
respect to ZnO contents, although all the films have the same duration and same
concentration of CaCl soaking treatment. CaCl is a salts with multivalent cations which 2 2
increasethegelstrengthduetothedevelopmentofcross-linkingbetweencarboxylgroupof
alginate and Ca . Therefore, the difference of TS and E values between the film were caused2+
bythedifferentcompositionofalginicacidandZnOnanoparticles. Controlfilmshowedthe
greatest tensile strength (29,48 ± 2,61 MPa) among the test films, as reported previously
(Rhim, 2004). The greatest TS in control film was regarded as 2 g of alginic acid used,
comparedto1 gofalginicacidintheantimicrobialfilms.As thealginicacidscontentswere
reduced, the strength of films were also decreased. This is due to the fact that alginic acid is a
biopolymer and has colloidal properties, including stabilizing, thickening, film forming,
because of the presence of ZnO nanoparticles. The presence of ZnO nanoparticles in the films
probably interferes with ionic interactions between Ca ions and alginate, which were
supposed to help in forming a network, thus, cause a loss of TS values (Pranoto et al., 2005).
Generally,as TSvalue increases, the elongationatbreak (E) valuedecreasesasshown inthe
Table 1. The E values between control, 0.3 g and 0.5 g ZnO containing films were not
significantlydifferent,whilefilmwith0.7gZnOshowed thehighestpercentageofEvalue
andsignificantly greaterthan others.Fromthose results,TSandEinverselyproportionalto
each other, whereas the TS value increases, the E value decreases, and the interaction are
shown in Figure 5-6. The present results agree with previous study by Lee, Shim, & Lee
(2004). As explained previously, tensile strength (TS) is measured for film strength during
stretching and elongation at break (E) is the stretch ability prior to breakage (Krochta &
Johnson1997 in Pranoto, Salokhe, & Rakshit, 2005). Therefore, ifthe TS value increases,
thus the film strength during stretching is increasing. As a result of the increases of film
strength, the stretch ability of the film prior to breakage is decreasing. Thus, it can be
explained why TS value and E value inversely proportional.
Table 1. Effects of ZnO Concentration on Physical Properties of Antimicrobial Films Concentration of ZnO (g) Tensile Strength (Mpa) Elongation at Break (%)
Control 29.48 ± 2.61a 1.46 ± 0.004a
0.3 5.90 ± 2.02c 2.19 ± 0.004a
0.5 10.09 ± 2.32b 1.52 ± 0.004a
0.7 2.64 ± 0.23c 3.22 ± 0.008b
a,b,c
Figure 3. Results of Tensile Strength
Figure 4.Results of Elongation at Break
2.3 Inhibitory Zone
2.4 Results of Escherichia coli
The effects of different ZnO concentrations on growth inhibition ofEscherichia coli were
accomplished andthecontrolfilm didnotshow effective antibacterial properties. As seen on
the figures, the control film did not have an effective antibacterial property against
Escherichia colias illustrated in Figure 7-10. The results of antimicrobial films containing
nano ZnO were not as expected, where clear zones could not be determined. The results
could be due to the insufficient diffuse of antimicrobial agents through agar gel. Furthermore,
films were expected to increase too, thus increasing the diameter of the inhibitory zone
(Hosseini,Razavi,&Mousavi,2009). TheZnObasedantimicrobialfilmsdidnotshowany
inhibitory zone despite the antimicrobial activity of ZnO, this might be due to the ZnO agents
didnotdiffusedthroughtheadjacentagarmediaduring theagardiffusiontestmethod,thus
the organisms did not go through anydirect contact with the active sites of ZnO and the
antimicrobial agents could not inhibit the microorganism surrounding the film strips
(Hosseini et al., 2009).
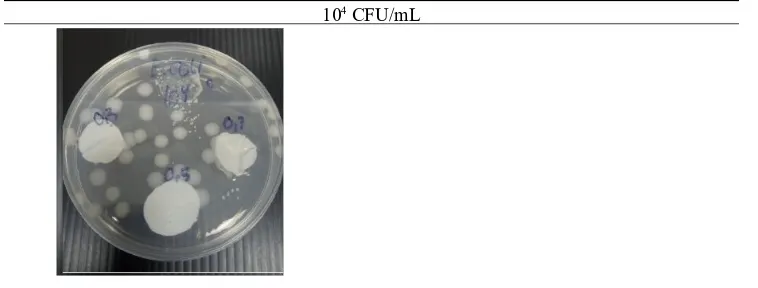
104 CFU/mL
Figure 5. Zone of Inhibition of Escherichia coli with Initial Number of 10 CFU/mL4
105 CFU/mL
Figure 6. Zone of Inhibition of Escherichia coli with Initial Number of 10 CFU/mL5
Figure 7. Zone of Inhibition of Escherichia coli with Initial Number of 10 CFU/mL6
107 CFU/mL
Figure 8. Zone of Inhibition of Escherichia coli with Initial Number of 10 CFU/mL7
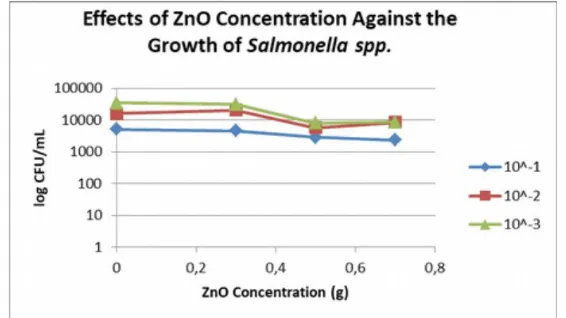
2.5 Results of Salmonella spp.
The antimicrobial effects of different ZnO concentrations againstSalmonella spp.were
shown on Figure 11-14. As seen on the figures, thecontrol film had no effective antibacterial
property againstSalmonella spp.The results of antimicrobial films with addition of ZnO
were also not as expected where the ZnO based antimicrobial films did not show any
inhibitory zonedespite theantimicrobial activityofZnO, this maybe due tothe samereason
in the results of antimicrobial properties of ZnO based antimicrobial films onEscherichia
coli.
Figure 9. Zone of Inhibition of Salmonella spp. with Initial Number of 104
CFU/mL
105 CFU/mL
Figure 10. Zone of Inhibition of Salmonella spp. with Initial Number of 10 CFU/mL5
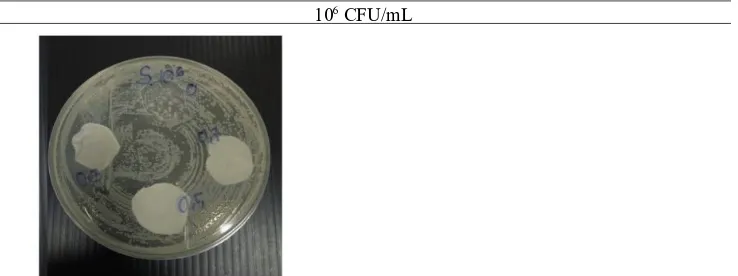
106 CFU/mL
Figure 11. Zone of Inhibition of Salmonella spp. with Initial Number of 10 CFU/mL6
Figure 12. Zone of Inhibition of Salmonella spp. with Initial Number of 10 CFU/mL7
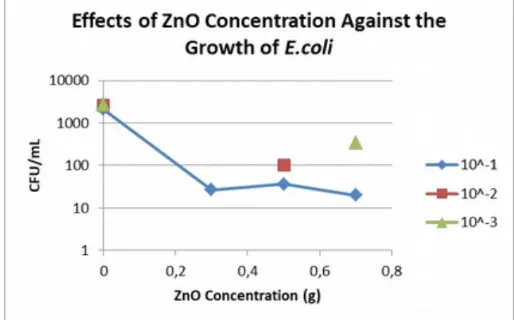
average of log CFU/mL of Escherichia coli in contact with the control film were significantly
greater thantheothers, and coloniesdevelopedfrom ZnO based antimicrobialfilms wrapped
products decreased, whereas the initial number ofEscherichia coli added to the surface of the
deboned chickenmeat were log 2 CFU/mL.Theresults alsoshowsthat the average oflog
antimicrobial film with 0.5 g of ZnO were slightly higher than the 0.3 g of ZnO, although the
results were not significantly different.
Metalsandmetallicoxideareknowntobetoxicinrelativelyhighconcentrations,butsince
thezincelementisanessentialcofactorinavarietyofcellularprocesses,thusZnOhasnot
shown toxicity atrelatively low concentrations. Those resultswere similar toresults reported
by PadmavathyandVijayaraghavan(2008)inEspitia etal. (2012),indicatingthatthezinc-
oxide nanoparticlesin low concentrations were not effective againstEscherichia coli, and the
presenceof soluble zinc-oxide ionsmayactasanutrientsfor thismicroorganism. Moreover,
the solubility of metal oxides such as ZnO, is a function of their concentration and time
(Wanget al., 2009inEspitiaet al., 2012). Therefore,lowconcentrationsofsolubilizedZn 2+
can cause a relatively high tolerance by the microorganism.
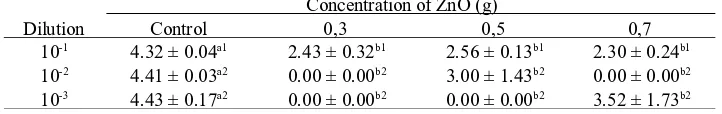
Table 2. Effect of ZnO Concentration Against the growth of Escherichia coli (log CFU/ml)
*Within the same column or row, values not followed by the same superscript are significantly different (P <
0.05). Control is a film without addition of zinc oxide.
Figure 13. Effect of Zinc Oxide Against the Growth of Escherichia coli From Different Dillution
bacteriostaticactionoftheZnObasedantimicrobialfilmsisincreasingastheconcentration
of ZnOincreases (Liu et al.,2009). Theresults alsoshowedthatthe averageoflog CFU/mL
ofSalmonella spp. resulted from antimicrobial film with 0.7 g of ZnO were the lowest
compared tofilms with0.3 and0.5 gof ZnO.However,nofurther decreases of logCFU/mL
Table 3. Effect of ZnO Concentration Againts the Growth of Salmonella spp. (log CFU/ml)
Dilution
Concentration of ZnO (g)
Control 0,3 0,5 0,7
10-2 5.20 ± 0.10a2 5.31 ± 0.02a2 4.75 ± 0.02b2 4.93 ± 0.14b2
10-3 5.54 ± 0.04a3 5.49 ± 0.12a3 4.90 ± 0.11b3 4.94 ± 0.26b3
*Within the same column or row, values not followed by the same superscript are significantly different (P <
0.05). Control is a film without addition of zinc oxide.
Figure 14. Effects of Zinc Oxide Against the Growth of Salmonella spp. from Different Dillution
The antimicrobial effects of ZnO to inactivate microorganims were affected primarily by
surface area and the concentration, therefore the larger the surface area and higher the
concentrationof theZnO, thegreater antimicrobialeffects. Smallerordispered particlesalso
have a muchbetter bacteriostatic activity. Therefore, the ZnO nanoparticles suspensionhas
beentreatedwithultrasonicator(Zhanget al., 2007in Liu et al.,2009). Themechanismof
theantimicrobialeffectsofZnOcouldbeexplainedintwoways,thefirstwayiscausedby
the different charges between the bacteria cell surface and ZnO nanoparticles, resulted in
electrostatic forces. According to Stoimenovet al. (2002), the global charges of bacterial
cells are negative at biological pH, due to the excess of the carboxylic groups which are
dissociatedand resultedinnegativecharges.Meanwhile,ZnOnanoparticleshaveapositive
interaction between the bacterial surface and ZnO nanoparticles also cause the internalization
of ZnO nanoparticles in bacterial cells due to the disruption and collapse of the cell wall,
resultingintheinhibitionofcellgrowth.Thesecondwayiscaused bythereactiveoxygen
species (ROS), especially hydrogen peroxide (H2O2) which is a strong oxidizing agent
contributes to the antimicrobial activity of ZnO nanoparticles, thus causing a stress to
microorganism’s sensitivity (Raghupathi, Koodali, & Manna, 2011).
BothEscherichia coli andSalmonella spp. are a Gram negative bacteria and could grow
better at an environment with a warm constant temperature and have a high concentrations of
free amino acidsand sugars(Winfiel & Groisman, 2003). Gramnegative bacteriahave much
morecomplexcellwalls comparedto thegrampositivebacteria. ThecellwalloftheGram
negative bacteria consists of about 2 nm thick peptidoglycan layer and only accounts for 10%
ofthe cellwall. However, theyhave outer membrane consists of50% lipopolysaccharides,
35% phospholipids, and 15% lipoproteins, and it is about 6-18 nm thick and accounts for
90% of the cellwall. Thus, the peptidoglycan and outer membrane provide protection and
influence the sensitivity ofthe antimicrobialagentshence, reduce the absorptionof ROS into
the cell (Espitia et al., 2012). Consequently, it would need higher concentration of ZnO
nanoparticles if the expectedresults were complete inhibition of the bacterial cells growth, as
the generation of H2O2would increase too, because asseen on the Table2. and Table3. there was still agrowth of bacterialcells, even onthe results of antimicrobial film with addition of
0.7 g of ZnO nanoparticles.
As seen inthe Table2 and3, the growth ofSalmonella spp. was better thantheEscherichia
coli, indicating the ZnO based antimicrobial films have more effective antimicrobial activity
againsttheEscherichia colithantheSalmonella spp.This isregarded bythedifferences in
the metabolic processes ofZn ions, which depends on the internalcharacteristics of each 2+
microorganism. Therefore,differencesintoxicitythresholdsofZnOnanoparticles to various
environmentalfluctuations, likewise,Salmonella was more resistant to bactericidal activity
bybioticfactors,suchasmicrobialpredatorsorcompetingorganisms,thusitcanberelated
3. CONCLUSION AND SUGGESTION
3.1 Conclusion
As theZnO nanoparticle contents increased, the tensile strength(TS) values decreased and
elongationat break(E) values increased, because the presence ofZnO nanoparticles inthe
films probablyinterfereswithionic interactionspresentedbyCa ions,whichhelp informing
a network. The antimicrobial effects of ZnO to inactivate microbial growth were affected
primarily with surface area and concentration, whereas the larger surface area and higher
concentrationoftheZnO,thebetter antimicrobialeffects,thusadditionof0.7gramofZnO
nanoparticlesintheantimicrobialfilmdemonstrated thebestinhibitory effecton thegrowth
of microorganisms among of test films. Moreover, ZnO nanoparticles have more
antimicrobial activity againstEschericia coli ratherthanSalmonella spp., becausemetabolic
processes of Zn ions are affected by intrinsic characteristics of each microorganism, whereas2+
Salmonella is more resistant to bactericidal activity by biotic factors.
3.2 Suggestion
For futureresearch, higher concentrationofZnO nanoparticles are takenintoaccount, thus
thecompleteinhibitionon microbialgrwoth couldbeobtained.Moreover,scanningelectron
microscopy(SEM) used to examine morphological changes of microorganisms before and
4. REFERENCES
Espitia,P.J.P.,Soares,N.deF.F.,Coimbra,J.S.dosR.,de Andrade, N.J.,Cruz,R.S.,& Medeiros,E. A. A. (2012).Zinc Oxide Nanoparticles:Synthesis, Antimicrobial Activity and Food Packaging Applications. Food and Bioprocess Technology, 5 (5), 1447 –1464. https://doi.org/10.1007/s11947-012-0797-6
Hosseini, M. H., Razavi, S. H., & Mousavi, M. A. (2009). Antimicrobial, physical and
mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamonessentialoils. Journal of Food Processing and Preservation, 33(6), 727–743. https://doi.org/10.1111/j.1745-4549.2008.00307.x
Lee, K. Y., Shim, J., & Lee, H. G. (2004). Mechanical properties of gellan and gelatin composite films. Carbohydrate Polymers, 56(2), 251–254. https://doi.org/10.1016/j.carbpol.2003.04.001
Liu, Y., He,L.,Mustapha, A., Li, H.,Hu, Z.Q., &Lin,M.(2009).Antibacterial activitiesof zinc oxide nanoparticles against Escherichia coli O157:H7. Journal of Applied Microbiology 107, (4), 1193–1201. https://doi.org/10.1111/j.1365-2672.2009.04303.x
Li, Xihong., Xing, Y., Jiang, Y., Ding, Y., & Li, W. (2009). Antimicrobial activities of ZnO powder-coated PVC film to inactivate food pathogens. International Journal of Food Science and Technology, 44(11), 2161–2168. https://doi.org/10.1111/j.1365-2621.2009.02055.x
Pranoto, Y., Salokhe, V. M.,& Rakshit,S. K.(2005). Physicalandantibacterialpropertiesof
alginate-based edible film incorporated with garlic oil. Food Research International , 38(3), 267–272. https://doi.org/10.1016/j.foodres.2004.04.009
Raghupathi,K.R.,Koodali,R.T.,&Manna, A.C. (2011). Size-dependentbacterialgrowth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir:
nanoparticles as bactericidal agents. Langmuir, 18 (17), 6679–6686. https://pubs.acs.org/doi/abs/10.1021/la0202374
Winfiel,M.D., &Groisman,E.A.(2003).Role ofNonhostEnviroments intheLifestyles of