www.elsevier.com / locate / bres
Research report
Co-localization of NMDA receptors and AMPA receptors in neurons
of the vestibular nuclei of rats
a ,1 b a ,
*
L.W. Chen
, K.K.L. Yung , Y.S. Chan
a
Department of Physiology, Faculty of Medicine, The University of Hong Kong, 5 Sassoon Road, Hong Kong, China
b
Department of Biology, Hong Kong Baptist University, Waterloo Road, Hong Kong, China Accepted 22 August 2000
Abstract
We are interested in studying the co-localization of NMDA glutamate receptor subunits (NR1, NR2A / B) and AMPA glutamate receptor subunits (GluR1, GluR2, GluR2 / 3 and GluR4) in individual neurons of the rat vestibular nuclei. Immunoreactivity for NR1, NR2A / B, GluR1, GluR2, GluR2 / 3 and GluR4 was found in the somata and dendrites of neurons in the four major subdivisions (superior, medial, lateral, and spinal vestibular nuclei) and in two minor groups (groups x and y) of the vestibular nuclei. Double immuno-fluorescence showed that all the NR1-containing neurons exhibited NR2A / B immunoreactivity, indicating that native NMDA receptors are composed of NR1 and NR2A / B in a hetero-oligomeric configuration. Co-expression of NMDA receptor subunits and AMPA receptor subunits was demonstrated by double labeling of NR1 / GluR1, NR1 / GluR2 / 3, NR1 / GluR4 and NR2A / B / GluR2 in individual vestibular nuclear neurons. All NR1-containing neurons expressed GluR2 / 3 immunoreactivity, and all NR2A / B-containing neurons expressed GluR2 immunoreactivity. However, only about 52% of NR1-immunoreactive neurons exhibited GluR1 immunoreactivity and 46% of NR1-containing neurons showed GluR4 immunoreactivity. The present data reveal that NMDA receptors are co-localized with variants of AMPA receptors in a large proportion of vestibular nuclear neurons. These results suggest that cross-modulation between NMDA receptors and AMPA receptors may occur in individual neurons of the vestibular nuclei during glutamate-mediated excitatory neurotransmission and may in turn contribute to synaptic plasticity within the vestibular nuclei. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Excitatory amino acid receptors: ligand-gated channels
Keywords: NMDA receptor; AMPA receptor; Co-localization; Vestibular nucleus; Immunocytochemistry
1. Introduction the central nervous system [26]. On the basis of sequence homologies and agonist-affinities, ionotropic glutamate Glutamate, a major excitatory neurotransmitter in the receptors are classified into N-methyl-D-aspartate
central nervous system, has been proposed as the principal (NMDA), a -amino-3-hydroxyl-5-methyl-4-isoxazole-pro-neurotransmitter at the excitatory synapses in the vestibular pionic acid (AMPA), and kainate (KA) receptors. Structur-nucleus [33,34,41,45]. This neurotransmitter is released ally, these receptors are composed of subunits: glutamate from the vestibular afferent nerve terminals in mammals receptor 1–4 (GluR1–4) subunits in the case of AMPA [15,23,45], and drives the excitatory synaptic neurotrans- receptors, GluR5–7 and KA1–2 subunits in the case of KA mission mainly via glutamate-gated ion channels [26]. receptors, and NMDAR1 (NR1) and NR2A-D subunits in Various ligand-gated ion channels (i.e. ionotropic gluta- the case of NMDA receptors [16,20,25,26].
mate receptors) mediate the glutamate neurotransmission in Physiological and pharmacological data indicate that NMDA and AMPA receptors play important roles in the mediation of glutamate neurotransmission and synaptic *Corresponding author. Fax:1852-28559730.
plasticity in the vestibular nuclei. Vestibular nuclear
neu-E-mail address: [email protected] (Y.S. Chan).
1 rons are activated by the primary vestibular afferent
Dr. Liang-Wei Chen is on leave from Institute of Neuroscience, The
Fourth Military Medical University, Xi’an, P.R. China. synaptic inputs via NMDA or AMPA receptors in vivo and 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
in vitro [19,21,23,40,43]. Electrophysiological recording overnight at 48C. The brain stems were then serially cut shows that the monosynaptic excitatory postsynaptic po- into frontal sections (40 mm thickness) with a freezing tential of second-order vestibular nuclear neurons in frog is microtome and then processed for the following immuno-mediated in part by NMDA receptors and in part by AMPA cytochemical reactions.
receptors [40]. In addition, electrophysiological studies Double immunofluorescence was employed to demon-([2,7,15,23]; see review: [13]) have demonstrated that strate the co-localization of different receptor subunits in glutamatergic input from the ipsilateral vestibular nerve is individual vestibular nuclear neurons in sections of eight mediated predominantly by AMPA receptors. A recent rats. Alternate sections of the brain stem that contained the study [31] has suggested that AMPA receptors are also vestibular nuclei were processed to double immunofluores-involved in non-primary vestibular excitatory transmission. cence. A mixture of one mouse antibody and one rabbit The AMPA receptors are therefore involved in both the antibody in 0.01 M phosphate-buffered saline (PBS) primary and non-primary vestibular transmission. Further- containing 3% normal goat serum and 0.1% Triton X-100 more, NMDA receptors, with the characteristics of high was used. The following primary antibodies that were
21
Ca permeability, play more roles in the long-term obtained from commercial source (Chemicon International, modulation (or long-term potentiation, LTP) of synaptic Temcula, CA) were employed in the present study: mouse-neurotransmission [3,6,11,32,42]. Recent evidence indi- anti-NR1 monoclonal antibody (1:1000), rabbit-anti-cates that functional AMPA receptors are important for the NR2A / B polyclonal antibody (1:500), rabbit-anti-GluR1 maintenance of neuronal dendritic spines where most polyclonal antibody (1:500), mouse-anti-GluR2 monoclo-central excitatory synapses reside [1]. It is of interest that nal antibody (1:1000), rabbit anti-GluR2 / 3 polyclonal progressive co-expression of functional NMDA receptors antibody (1:500), and rabbit GluR4 polyclonal anti-and AMPA receptors was observed in hippocampal neu- body (1:500). The following double immunofluorescence rons during postnatal development and LTP [24,28] We was then performed: NR1 and NR2A / B (written as NR1 / hypothesize that the co-localization of NMDA receptors NR2A / B), NR1 / GluR1, NR1 / GluR2 / 3, NR1 / GluR4 and and AMPA receptors in individual vestibular nuclear NR2A / B / GluR2. The sections were incubated in the neurons provides a substrate for cross-talk between the mixtures of primary antibodies for 24–48 h at 48C. receptors during excitatory synaptic events of the vestibu- Subsequently, the sections were washed in PBS (33PBS) lar nuclear neurons. The AMPA receptors (GluR1, GluR2 / and incubated for 2–4 h at room temperature in a mixture 3, and GluR4 subunits) were previously reported in the of secondary antibodies, i.e., either donkey anti-mouse IgG chinchilla vestibular nuclei [31]. Several NMDA receptor conjugated with tetramethyl rhodamine isothiocyanate proteins and their mRNAs were also found in vestibular (TRITC, 1:100–200; Chemicon) and donkey anti-rabbit nuclear neurons [29,33,44]. However, it is not yet known IgG conjugated with dichlorotriazinyl aminofluroscein whether or not NMDA and AMPA receptors are co- (DTAF; 1:100–200; Chemicon), or donkey anti-mouse expressed in individual vestibular nuclear neurons. The IgG conjugated with aminomethylcoumarin (AMCA, co-localization of NMDA and AMPA receptor subunits, 1:100–200; Chemicon) and donkey anti-rabbit IgG conju-viz. NR1, NR2A / B, GluR1, GluR2, GluR2 / 3 and GluR4, gated with TRITC. After several washes in PBS, the was therefore investigated in individual vestibular nuclear sections were mounted in flurosave medium (Calbiochem). neurons of the rat with the use of double immunofluores- The sections were examined both with fluorescence micro-cence. The present study provides the basis for elucidating scope (Axioplan, Zeiss) and a laser scan confocal micro-the interaction between NMDA receptors and AMPA scope (LSM 510, Zeiss) for DTAF-, AMCA- and TRITC-receptors in excitatory synaptic neurotransmission and labeled neurons. In addition, other sections of four rats synaptic plasticity within the vestibular nucleus. were processed with immunoperoxidase procedures using 3,39-diaminobenzidine (DAB) as the chromogen (ABC kit, Vector Labs.) to visualize the cellular localization of NR1,
2. Materials and methods NR2A / B, GluR1, GluR2, GluR2 / 3 and GluR4 in the rat vestibular nuclei. The sections were reacted in a single Eight adult male Sprague–Dawley rats weighing 230– immunoperoxidase reaction for comparison of the pattern 260 g were used in the present study. All procedures in of distribution and number of immunopositive neurons in each preparation conformed to the Principles of Labora- the regions of the vestibular nuclei.
tory Animal Care and were approved by the University of For the control experiments of immunofluorescence and
Hong Kong Committee on the Use of Live Animals in immunocytochemistry, the primary antibody was substi-Research. Briefly, the animals were anesthetized with tuted with normal mouse serum (for NR1 and GluR2) or sodium pentobarbital (60–80 mg / kg, i.p.), and then per- normal rabbit serum (for NR2A / B, GluR1, GluR2 / 3 and fused transcardially with 100 ml of saline, followed by 500 GluR4) respectively. The sections were then processed ml of 0.1 M phosphate buffer (PB; pH 7.4) containing 4% with the same double immunofluorescence or immuno-paraformaldehyde. The brain stems were removed immedi- peroxidase reaction sequence as described above.
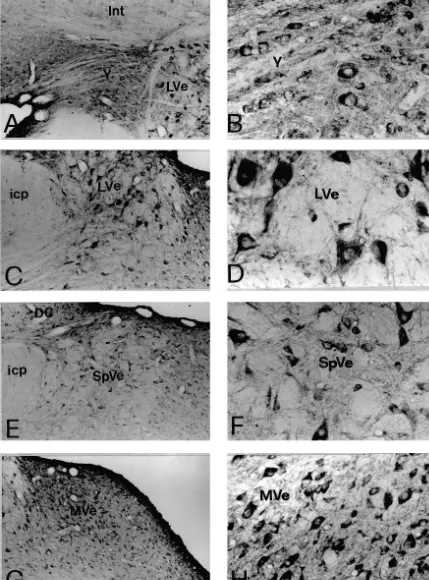
immuno-positive cells was measured under the light microscope NR1-IR neurons in the SpVe were triangle, oval, or multi-with the use of a morphometric micrometer. For semi- polar in shape, and their diameters ranged from 10mm to quantification of immunopositive cells in representative 30mm (Fig. 1F). The NR1-IR neurons in groups x and y sections, double-labeled neurons in eight randomly select- subnuclei were mainly oval in shape and their diameters ed fields (403 objective) of each of the following nuclei, ranged from 10mm to 20mm (Fig. 1B).
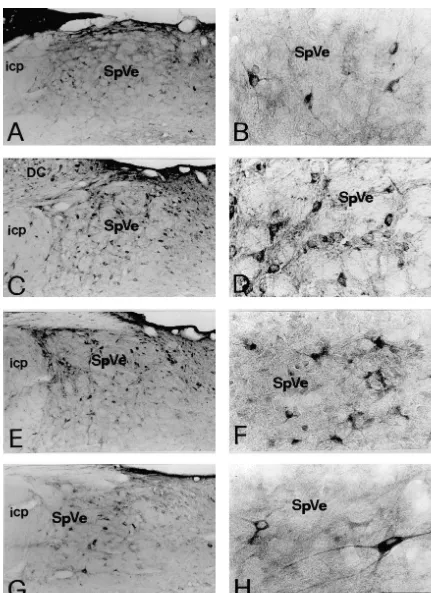
namely the superior vestibular nucleus (SuVe), medial Immunoreactivity for NR2A / B (Fig. 2A–D), GluR2 vestibular vestibular nucleus (MVe), lateral vestibular (Figs. 3C–D, 4C–D) and GluR2 / 3 (Figs. 3E–F, 4E–F) nucleus (LVe), spinal vestibular nucleus (SpVe), group x subunits was also found to be abundant in the vestbular and group y were counted. nuclei. The immunoreactive neurons were similar to the NR1-IR neurons in terms of cell number and distribution pattern in the SuVe, MVe, LVe, SpVe and group x, y
3. Results subnuclei. Immunoreactivity for GluR1 (Figs. 3A–B, 4A– B) or GluR4 (Figs. 3G–H, 4G–H), however, was less 3.1. Control experiments for immunocytochemistry abundant than that for NR1. The GluR1-IR or GluR4-IR neurons were also fewer in number than NR1-IR neurons In those sections that were incubated in normal mouse in different regions of the vestibular nuclei.
or rabbit serum in place of the primary antibodies, no In addition, astrocyte-like immunoreacitve cells are immunoreactivity was detected (data not shown). For prominent in NMDA2A / B antibody-incubated sections. double labeling procedures, only single immunoreactivity Weakly stained astrocytes were also seen in GluR1 and was detected when one of the two primary antibodies was GluR2 / 3-stained sections. GluR4-like immunoreactive as-substituted by normal serum during the reaction sequence. trocytes were hardly detected in our experiment (data not When two primary antibodies were substituted, no im- show). These results were consistent with previous report munoreactivity was detected (data not shown). [30].
3.2. Immunoreactivity for NMDA and AMPA receptor 3.3. Co-localization of NMDA and AMPA receptor
subunits in different regions of the vestibular nuclei subunits
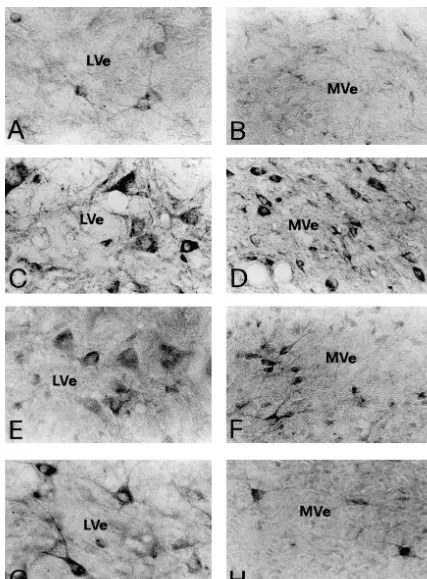
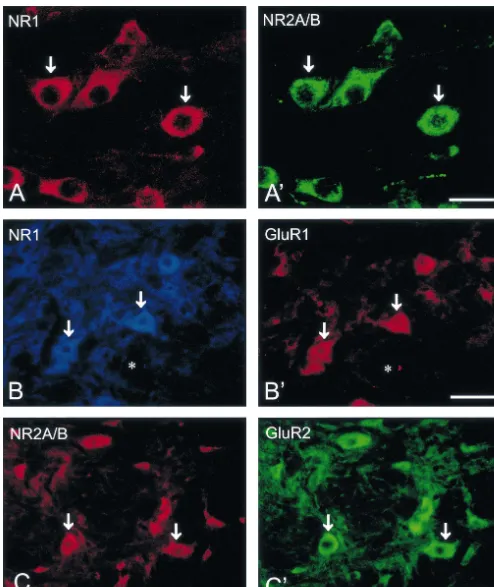
Immunoreactivity for NR1, NR2A / B, GluR1, GluR2, Co-expression of immunoreactivity for NMDA receptor GluR2 / 3 and GluR4 was identified by the presence of subunits, NR1 and NR2A / B, was observed in individual DAB immunoreaction products in the sections of the rat vestibular nuclear neurons (Fig. 5). Double labeled neu-brain stem. The nomenclature and boundaries defined in rons were found throughout all subdivisions of the vestibu-the rat brain atlas of Paxinos and Watson [27] were utilized lar nucleus including the SuVe, MVe, LVe, SpVe and group in this study. In the vestibular nuclei, immunoreactivity for x, y subnuclei. Virtually all the NR1-IR neurons were NR1 (Fig. 1), NR2A / B (Fig. 2), GluR1, GluR2, GluR2 / 3 found to exhibit NR2A / B immunoreactivity (Table 1). or GluR4 (Figs. 3 and 4) was predominantly observed in Fig. 5A, A9 illustrates individual SpVe neurons displaying neuronal somata and in their proximal dendrites. The co-localization of NR1 and NR2A / B immunoreactivity in immunopositive neurons were primarily distributed in the the somata and dendritic processes. Similarly, co-expres-superior vestibular nucleus (SuVe), medial vestibular nu- sion of immunoreactivity for NR2A / B and GluR2, NR1 cleus (MVe), lateral vestibular nucleus (LVe), spinal ves- and GluR1, NR1 and GluR2 / 3, or NR1 and GluR4, was tibular nucleus (SpVe) and group x, y subnucleus (Figs. also found in individual vestibular nuclear neurons. Double 1–4). NR1 immunoreactivity was highly expressed in the labeled neurons were found in the SuVe, MVe, LVe, SpVe LVe (Fig. 1C–D), SpVe (Figs. 1E–F) and MVe (Figs. and group x, y subnuclei. In addition, all the vestibular 1G–H). In SuVe, however, only low NR1-immunoreactivi- neurons that expressed GluR1 (Fig. 5) or GluR4 (Fig. 5) ty was observed (data not shown). Moderate expression of immunoreactivity were found to express NR1 immuno-NR1 immunoreactivity was found in the groups x and y reactivity. However, the NR1 / GluR1 and NR1 / GluR4 subnuclei (see Fig. 1A–B for y subnucleus). Within the double-labeled neurons were fewer in number. This was MVe, a majority of NR1-immunoreactive (IR) neurons was particularly evident in the SuVe, MVe and group x, y spherical, oval or fusiform in shape (Fig. 1 H). The size of subnuclei. Representative NR1 / GluR1 and NR2A / B / these neurons ranged from small to medium with a GluR2 double labeled neurons in the SpVe are shown in diameter between 10 mm to 20 mm. The LVe was Fig. 5B, B9, C, C9.
characterized with the presence of giant NR1-IR neurons,
which were multi-polar with diameters of 30mm to 50mm 3.4. Semi-quantification (Fig. 1D). A number of small and medium-sized NR1-IR
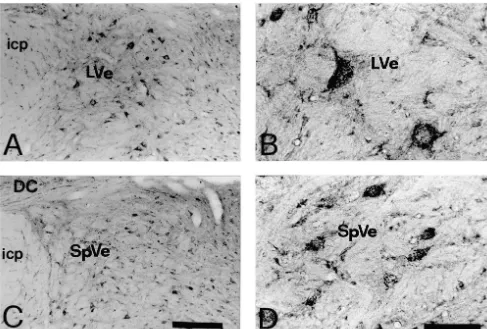
Fig. 2. Photomicrographs showing the immunoreactivity for NR2A / B in the vestibular nuclei. The NR2A / B-immunoreactive neurons are shown in lateral vestibular nucleus (LVe) (A1B) and spinal vestibular nucleus (SpVe) (C1D). Abbreviations as in Fig. 1. Scale bars: 300mm (in C for A); 80mm (in D for B).
to display NR2A / B and GluR2 / 3 immunoreactivity. Simi- subunits in individual vestibular nuclear neurons, and (c) larly, all of the NR2A / B-IR neurons displayed GluR2 NMDA receptors and AMPA receptors are co-localized in immunoreactivity. Only a proportion of NR1-IR neurons, a large proportion of vestibular nuclear neurons.
however, exhibited GluR1 or GluR4 immunoreactivity.
About 52% of the NR1-IR neurons displayed GluR1 4.1. Glutamate and neurotransmission between the immunoreactivity, while 46% of NR1-IR neurons dis- vestibular afferents and vestibular nuclear neurons played GluR4 immunoreactivity.
Glutamate-containing axonal terminals have been shown to make synaptic contacts with the somata and the
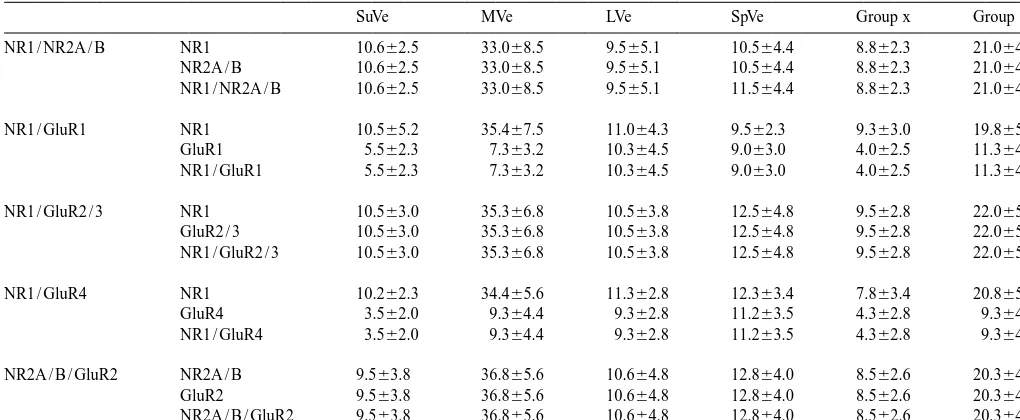
Table 1
a
Neurons doubly labeled with NMDA and AMPA receptor subunits in the vestibular nuclei (n55)
SuVe MVe LVe SpVe Group x Group y
NR1 / NR2A / B NR1 10.662.5 33.068.5 9.565.1 10.564.4 8.862.3 21.064.5
For each pair of receptor subunits, the number (average6SD) represent the immunoreactive neurons in the square area of 2503250mm from randomly selected fields of the distinct vestibular regions in 40mm sections (403objective).
4.2. Distribution and colocalization of NMDA and 5,11,17,32,38]. In addition, AMPA receptor channels are
AMPA receptors in the vestibular afferents and functionally dominated by GluR2 subunits that show low
21
vestibular nuclear neurons permeability to Ca [18] whereas NMDA receptors have
21
the characteristics of high Ca permeability [16]. The The present results show that all individual neurons in mechanism by which these two receptors cross-modulate the vestibular nuclei co-express NR1 and NR2A / B subunit in regulating cellular functions remains to be explored. It is immunoreactivity, indicating that native NMDA receptors of interest to note that during hippocampal development, in vestibular nuclear neurons are most likely assembled silent synapse which initially had only NMDA receptors from these subunits in a hetero-oligomeric configuration. became functional only after the acquisition of AMPA On the other hand, the distributions of GluR1 and GluR4 receptors [24,28]. Most central excitatory synapses occur subunits are uneven among central vestibular neurons. The on dendritic spines of neurons. In hippocampal neurons, LVe and SpVe showed an abundant expression of these the stability and maintenance of these spines depend both subunits. There was less expression in the SuVe, MVe, and on AMPA receptors and spontaneous glutamate release [1]. groups x, y subnuclei. In addition, another interesting
finding is the co-expression of NMDA receptors and the 4.4. Functional implications in the vestibular system AMPA receptor subunit GluR2 in individual vestibular
nuclear neurons. The present data do provide the possible cellular
expres-sion of NMDA and AMPA subunits in the central vestibu-4.3. Comparison with other models lar neurons. The present data however, do not directly provide functional data of the ionotropic glutamate re-Co-expression of NMDA and AMPA receptor subunits ceptors in these neurons. Previous physiological studies in individual neurons was also reported in other systems, report that NMDA receptors contribute to the regulation of such as in the hippocampus [38] and in the substantia nigra the resting discharge [14,37] and long-term modulation of [46]. However, the physiological significance of the co- their synaptic transmission [6] in vestibular neurons. expression of multiple glutamate receptor subunits in NMDA receptors also play a crucial role in the synaptic individual neurons remains to be elucidated. One possi- plasticity associated with vestibular compensation bility is that the AMPA receptors most likely contribute to [8,10,12,39]. However, the role of the AMPA receptors in
21
the relief of the voltage-dependent Mg block of NMDA regulating the functions of central vestibular neurons has receptors [4,38]. On the other hand, the NMDA receptor, received less attention though they are known to involve in
21
receptor-normal and hemilabrythectomized guinea pig, Exp. Brain Res. 81 mediated components [40], suggesting that functional
(1990) 125–133. interaction between NMDA and AMPA receptors occurs in
[15] K.T. Doi, T. Tsumoto, Matsunaga, Actions of excitatory amino acid vestibular nuclear neurons. The intracellular signal cascade antagonists on synaptic inputs to the rat medial vestibular nucleus, and physiological consequence that follow from the cross- an electrophysiological study in vitro, Exp. Brain Res. 82 (1990) modulation between NMDA receptor and AMPA receptor 254–262.
[16] M. Hollmann, S. Heinemann, Cloned glutamate receptors, Annu. in vestibular nuclear neurons deserve further investigation.
Rev. Neurosci. 17 (1994) 31–108.
[17] R.R. Johnson, X. Jiang, A. Burkhalter, Regional and laminar differences in synaptic localization of NMDA receptor subunit NR1
Acknowledgements splice variants in rat visual cortex and hippocampus, J. Comp. Neurol. 368 (1996) 335–355.
[18] P. Jonas, C. Racca, B. Sakmann, P.H. Seebury, H. Monyer, This work was supported by the Hong Kong Research 21
Difference in Ca permeability of AMPA-type glutamate receptor Grants Council (HKU 7246 / 98 M) to YSC and KKLY. channels in neocortical neurons caused by differential gluR-B The authors are grateful to Mr. Simon S.M. Chan for Subunit expression, Neuron 12 (1994) 1281–1289.
excellent technical assistance. [19] G.A. Kinney, B.W. Peterson, N.T. Slater, The synaptic activation of
N-methyl-D-aspartate receptors in the rat medial vestibular nucleus, J. Neurophysiol. 72 (1994) 1588–1595.
[20] T. Kutsuwada, N. Kashiwabuchi, H. Mori, K. Sakimura, E. Kushiya,
References K. Araki, H. Meguro, H. Masaki, T. Kumanishi, M. Arakawa, M.
Mishina, Molecular diversity of the NMDA receptor channel, Nature 358 (1992) 36–41.
[1] P. Andersen, A.F. Soleng, A thorny question: how does activity
[21] T. Knopfel, Evidence for N-methyl-D-aspartic acid
receptor-me-maintain dendritic spines, Nature Neurosci. 2 (1999) 5–7.
diated modulation of the commissural input to central vestibular [2] A. Babalian, N. Vibert, G. Assie, M. Serafin, M. Muhlethaler, P.P.
neurons of the frog, Brain Res. 426 (1987) 212–224. Vidal, Central vestibular networks in the guinea-pig: functional
characterization in the isolated whole brain in vitro, Neuroscience 81 [22] C.H. Lai, Y.S. Chan, Properties of otolith-related vestibular nuclear (1997) 405–426. neurons in respect to bidirectional off-vertical axis rotation of the [3] H. Bading, M.M. Segal, N.J. Sucher, H. Dudek, S.A. Lipton, M.E. rat, Brain Res. 693 (1995) 39–50.
Greenberg, N-methyl-D-aspartate receptors are critical for mediating [23] M.R. Lewis, K.D. Phelan, P. Shimnick-Gallagher, J.P. Gallapger, the effects of glutamate on intracellular calcium concentration and Primary afferent excitatory transmission recorded intracellularly in immediate gene expression in cultures hippocampal neurons, Neuro- vitro from rat medial vestibular neurons, Synapse 3 (1989) 149–
science 64 (1995) 653–664. 153.
[4] J.M. Bekkers, C.F. Stevens, NMDA and non-NMDA receptors are [24] D. Liao, X. Zhang, R. O’Brien, M.D. Ehlers, R.L. Huganir, colocalized at individual synapses in cultured rat hippocampus, Regulation of morphological postsynaptic silent synapses in de-Nature 341 (1989) 228–233. veloping hippocampal neurons, Nature Neurosci. 2 (1999) 37–43. [5] A. Caicedo, M. Eybalin, Glutamate receptor phenotypes in the [25] H. Monyer, R. Sprengel, R. Schoepfer, A. Herb, M. Higuchi, H.
auditory brainstem and mid-brain of the developing rat, Eur. J. Lomeli, N. Burnashev, B. Sakmann, P.H. Seeburg, Heteromeric Neurosci. 11 (1999) 51–74. NMDA receptors: molecular and functional distinction of subtypes, [6] G. Capocchi, G. Della-Torre, S. Grassi, V.E. Pertorossi, M. Science 256 (1992) 1217–1221.
Zampolini, NMDA receptor-mediate long term modulation of elec- [26] S. Nakanishi, Molecular diversity of glutamate receptors and trically evoked field potentials in the rat medial vestibular nuclei, implications for brain function, Science 258 (1992) 597–603. Exp. Brain Res. 90 (1992) 546–550. [27] G. Paxinos, C. Watson, in: The Rat Brain in Stereotaxic Coordinates, [7] D.O. Carpenter, N. Hori, Neurotransmitter and peptide receptors on 4th edn, Academic Press, San Diego, 1998.
medial vestibular nucleus neurons, Ann. NY Acad. Sci. 656 (1992) [28] R.S. Petralia, J.A. Esteban, Y.-X. Wang, J.G. Partridge, H.-M. Zhao,
668–686. R.J. Wenthold, R. Malinow, Selective acquisition of AMPA
re-[8] C.L. Darlington, H. Flohr, P.F. Smith, Molecular mechanism of ceptors over postnatal development suggests a molecular basis for brainstem plasticity. The vestibular compensation model, Mol. silent synapses, Nature Neurosci. 2 (1999) 31–36.
Neurobiol. 5 (1991) 355–368. [29] R.S. Petralia, Y.X. Wang, R.J. Wenthold, The NMDA receptor [9] Y.S. Chan, The coding of head orientations in neurons of bilateral subunits NR2A and NR2B show histological and ultrastructural vestibular nuclei of cats after unilateral labyrinthectomy: response to localization patterns similar to those of NR1, J. Neurosci. 14 (1994) off-vertical axis rotation, Exp. Brain Res. 114 (1997) 293–303. 6102–6120.
[10] C.L. Darlington, P.F. Smith, The effects of N-methyl-D-aspartate [30] R.S. Petralia, N. Yokotani, R.J. Wenthold, Light and electron antagonists on the development of vestibular compensation in the microscope distribution of the NMDA receptor subunits NMDAR1 guinea pig, Eur. J. Pharmacol. 174 (1989) 273–278. in the rat central nervous system using a selective anti-peptide [11] K. Deisseroth, H. Bito, R.W. Tsien, Signaling from synapse to antibody, J. Neurosci. 14 (1994) 667–696.
nucleus: postsynaptic CREB phosphorylation during multiple forms [31] P. Popper, J.P. Rodrigo, J.C. Alavarez, I. Lopez, V. Horrubia, of hippocampal synaptic plasticity, Neuron 16 (1996) 89–101. Expression of the AMPA selective receptor subunits in the vestibular [12] C. de Waele, M. Abitbol, M. Chat, C. Menini, J. Malet, P.P. Vidal, nuclei of the chinchilia, Mol. Brain Res. 44 (1997) 21–30.
Distribution of glutamatergic receptors and GAD mRNA-containing [32] E.M. Quinlan, B.D. Philpot, R.L. Huganir, M.F. Bear, Rapid, neurons in the vestibular nuclei of normal and hemilabyrinthectom- experience-dependent expression of synaptic NMDA receptors in ized rats, Eur. J. Neurosci. 6 (1994) 565–576. visual cortex in vivo, Nature Neurosci. 2 (1999) 352–357. [13] C. de Waele, M. Muhlethaler, P.P. Vidal, Neurochemistry of the [33] J. Raymond, A. Nieoullon, D. Dememes, A. Sans, Evidence for
central vestibular pathways, Brain Res. Brain Res. Rev. 20 (1995) glutamate as a neurotransmitter in the cat vestibular nerve:
24–46. radioautographic and biochemical studies, Exp. Brain Res. 56
[14] C. de Waele, N. Vibert, M. Baudrimont, P.P. Vidal, NMDA receptors (1984) 523–531.
and glutamate immunoreactivity in frog and rat vestibular afferents, vestibular afferent fibers in the frog: differential synaptic activation J. Comp. Neurol. 349 (1994) 603–614. of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors, [35] I. Reichenberger, H. Straka, O.P. Ottersen, P. Streit, N.M. Gerrits, N. Neuroscience 70 (1996) 697–707.
Dieringer, Distribution of GABA, glycine, and glutamate immuno- [41] H. Straka, I. Reichengerger, N. Dieringer, Size-related properties of reactivities in the vestibular nuclear complex of the frog, J. Comp. vestibular afferent fibers in the frog: uptake of and immunoreactivity Neurol. 377 (1997) 149–164. for glycine and aspartate / glutamate, Neuroscience 70 (1996) 685– [36] N. Sakai, H. Ujihara, K. Ishihara, M. Sasa, C. Tanaka, Electro- 696.
physiological and pharmacological characteristics of ionotropic [42] Y. Takahashi, M.P. Takahashi, T. Tsumoto, K. Doi, T. Matsunaga, glutamate receptors in medial vestibular nucleus neurons: a whole Synaptic input-induced increase in intraneuronal Ca21in the medial cell patch clamp study in acutely dissociated neurons, Jpn. J. vestibular nucleus of young rats, Neurosci. Res. 21 (1994) 59–69. Pharmacol. 72 (1996) 335–346. [43] Y. Takahashi, T. Tsumoto, T. Kubo, N-methyl-D-aspartate receptors [37] M. Serafin, A. Khateb, C. de Waele, P.P. Vidal, P.P. Muhlethaler, contribute to afferent synaptic transmission in the medial vestibular
Medial vestibular nuclei in the guinea-pig: NMDA-induced oscilla- nucleus of young rats, Brain Res. 659 (1994) 287–291.
tions, Exp. Brain Res. 88 (1992) 187–192. [44] M. Watanabe, M. Mishina, Y. Inoue, Distinct distribution of five [38] S.J. Siegel, W.G. Janssen, J.W. Tullai, S.W. Roggers, T. Moran, S.F. NMDA receptor channel subunit mRNAs in the brainstem, J. Comp.
Heinemann, J.H. Morrison, Distribution of the excitatory amino acid Neurol. 343 (1994) 520–531.
receptor subunits GluR2(4) in monkey hippocampus and colocaliza- [45] T. Yamanaka, M. Sasa, T. Matsunaga, Glutamate as a primary tion with subunits GluR5-7 and NMDAR1, J. Neurosci. 15 (1995) afferent neurotransmitter in the medial vestibular nucleus as detected
2707–2719. by in vivo microdialysis, Brain Res. 762 (1997) 243–246.
[39] P.F. Smith, C.L. Darlington, J.L. Habbard, Evidence that NMDA [46] K.K.L. Yung, Localization of ionotropic and metabotropic glutamate receptors contribute tosynaptic function in the guinea-pig medial receptors in distinct neuronal elements of the rat substantia nigra, vestibular nucleus, Brain Res. 513 (1990) 149–151. Neurochem. Int. 33 (1998) 313–326.