Brain Research 881 (2000) 69–72
www.elsevier.com / locate / bres
Short communication
Effects of nigrostriatal dopamine denervation on ionotropic glutamate
receptors in rat caudate-putamen
a,b ,
*
a,b a,bFrank I. Tarazi
, Kehong Zhang
, Ross J. Baldessarini
a
Mailman Research Center, McLean Division of Massachusetts General Hospital, 115 Mill Street, Belmont, MA 02478, USA
b
The Consolidated Department of Psychiatry and Neuroscience Program, Harvard Medical School, Boston, MA 02115, USA
Accepted 8 August 2000
Abstract
Changes in ionotropic glutamate NMDA, AMPA and KA receptor binding in rat caudate-putamen were examined by quantitative in vitro receptor autoradiography 5 weeks after lesioning nigrostriatal dopaminergic projections. In this animal model of Parkinson’s disease, density of binding in caudate-putamen increased at KA, but not NMDA or AMPA receptors. The findings indicate that nigrostriatal dopamine denervation can selectively enhance KA receptor levels in rat basal ganglia, suggest that KA receptors contribute to the pathophysiology of Parkinson’s disease, and may suggest innovative treatments. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters and receptors
Topic: Excitatory amino acid receptors: physiology, pharmacology and modulation
Keywords: Autoradiography; Caudate-putamen; Glutamate receptors; 6-Hydroxydopamine; Parkinson’s disease
Ionotropic glutamate (Glu) receptors are of two major types may, respectively, stimulate and inhibit Glu release types: N-methyl-D-aspartic acid (NMDA) receptors and in CPu. In addition, we found that NMDA and KA
two non-NMDA receptor types defined by their preferen- autoreceptors occur on the terminals of the hippocampal tial interactions with a-amino-3-hydroxy-5-methyl-4-isox- Glu projections innervating rat CPu, along with NMDA azolepropionic acid (AMPA) or kainic acid (KA) [11]. receptors on corticostriatal terminals, and they, too, may These m receptors have been implicated in the patho- control the functional availability of Glu in CPu [15]. physiology of psychotic disorders, particularly schizophre- Several laboratories, including ours, have examined nia, may contribute to the actions of antipsychotic drugs levels of ionotropic Glu receptors in CPu 1 week after [14], and may also be involved in the pathophysiology of removing mesencephalic DA projections in rat forebrain by Parkinson’s disease [20]. injecting the neurotoxin 6-hydroxydopamine (6-OHDA) Autoradiographic analyses have demonstrated abundant into substantia nigra (SN). Such lesioning has usually led levels of all three ionotropic Glu receptor subtypes on to significant early decreases in CPu levels of NMDA, neurons in rat caudate putamen (CPu) [1,18]. This region AMPA and KA receptor binding, suggesting that all three receives dense glutamatergic afferent projections from ionotropic Glu receptors are expressed at presynaptic cerebral cortex, and dopaminergic input from the sub- dopaminergic terminals [16,23,24]. Neurophysiological stantia nigra zona compacta [2]. We recently provided and neurochemical studies also indicate that presynaptic evidence that NMDA autoreceptors are co-localized with NMDA, AMPA and KA heteroceptors are expressed on dopamine (DA) D heteroceptors on terminals of corticos-4 terminals of nigrostriatal DA afferents and contribute to triatal projections [16,17]. These complementary receptor increases of DA release in CPu [4,6].
We now report on later, presumably adaptive, effects of nigrostriatal DA denervation on the regulation of the three
*Corresponding author. Tel.: 11-617-855-3176; fax: 1
1-617-855-ionotropic Glu receptors in rat CPu, based on changes in
3479.
E-mail address: [email protected] (F.I. Tarazi). receptor density at 5 weeks after unilateral injection of
70 F.I. Tarazi et al. / Brain Research 881 (2000) 69 –72
6-OHDA into SN. Given evidence of close and typically 75 mM spermine; nonspecific binding was determined by 20 mM ketamine. After incubation, slides were washed reciprocal functional interactions between DA and Glu
(2320 min) in ice cold buffer and air-dried. neuronal systems [3], we hypothesized that DA depletion
For non-NMDA (mainly AMPA) receptors, sections may alter the terminal excitability of glutamatergic
projec-were preincubated for 60 min in 50 mM Tris–HCl buffer tions innervating CPu and subsequently alter expression of
(pH 7.3) at 48C, and then incubated in fresh buffer Glu receptors.
3
containing 20 nM [ H]CNQX for 60 min at 48C. Non-Materials used included radioligands from New England
specific binding was determined with 20 mM unlabeled Nuclear (Boston, MA) and drug substances from
RBI-CNQX. After incubation, slides were washed (3315 s) in Sigma (Natick, MA; St. Louis, MO). Young adult, male
ice cold buffer and dried. SD rats (Charles River Labs., Wilmington, MA), initially
For KA receptors, sections were preincubated for 60 200–225 g, were maintained in a USDA-inspected animal
min in 50 mM Tris–HCl buffer (pH 7.0) at 48C, and then research facility of the Mailman Research Center of
3
incubated in buffer containing 20 nM [ H]KA for 60 min McLean Hospital, with approval by the Institutional
Ani-at 48C, with 20 mM unlabeled KA as a blank. After mal Care and Use Committee (IACUC). Animals were
incubation, slides were washed (3310 s) in ice-cold buffer anesthetized with sodium pentobarbital (60 mg / kg, i.p.).
and air dried. Rats were pretreated with pargyline hydrochloride (30
Radiolabeled slide-mounted sections and calibrated mg / kg, i.p.) 30 min prior to stereotaxic microinfusion of
3
[ H]standards were exposed to Hyperfilm (Eastman-6-OHDA (20mg in 2ml of physiological saline containing
3
Kodak, Rochester, NY) for 10 ([ H]b-CIT), 14 1 mM ascorbic acid as an antioxidant) over 2 min, with
3 3 3
([ H]CNQX), 21 ([ H]KA), or 30 d ([ H]MK-801) at 48C, another 4 min allowed for diffusion. A small-animal
then developed and fixed. Medial and lateral CPu were stereotaxic apparatus (David Kopf, Tujunga CA) was used
outlined and their optical density (OD) was quantified with to place the neurotoxin unilaterally in SN at coordinates
an MCID-M4 image analyzer (Imaging Research; St. (A–P525.8, D–V527.5, L52.5 mm). DA nerve terminal
Catharines, Ontario). Analysis of variance (ANOVA) with degeneration was verified autoradiographically as
unilater-3
post-hoc Dunnett t-test was used for planned statistical al loss of striatal DA transporters labeled with [ H]b-CIT
comparisons between control vs. lesioned sides; results [10]. Frozen coronal brain sections (10 mm) representing
were considered significant at P,0.05 (two-tailed; NS5
medial and lateral CPu were cut in a cryostat at 2208C,
not significant). Autoradiographic data are presented as mounted on gelatin-coated microscopic slides, and stored
mean fmol / mg tissue6S.E.M. binding for N57 subjects / at2808C until used for autoradiographic analysis.
condition. For DA transporter autoradiography, brain sections
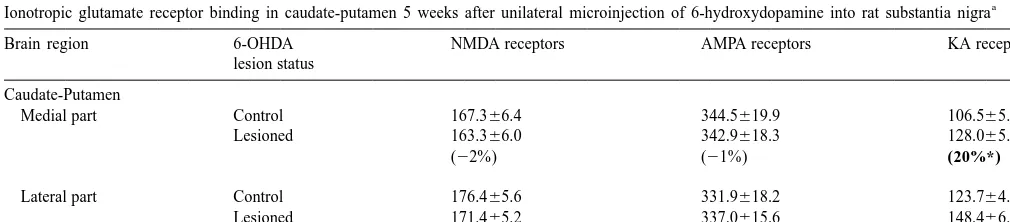
Compared to unlesioned sides, unilateral injection of SN were preincubated (60 min, room temp.) in 50 mM Tris–
with 6-OHDA produced.85% loss of ipsilateral striatal HCl buffer (pH 7.7) containing 120 mM NaCl and 4 mM
3
DA transporters labeled with [ H]b-CIT (not shown). After MgCl , then incubated in fresh buffer (60 min) containing2
3
5 weeks, such extensive degeneration of nigrostriatal DA 2 nM [ H]b-CIT. Nonspecific binding was defined with 1
neurons did not produce significant changes in binding of
mM GBR-12909. Slides were washed (235 min) in
ice-3
[ H]MK-801 to NMDA receptors (F [1,12 df ]50.61, P5
cold fresh buffer and air-dried. Ionotropic Glu receptors
3
0.33), or of [ H]CNQX to AMPA / KA receptors (F [1,12 were quantified by previously detailed methods [15,16,18].
df ]50.14, P50.65) in CPu (Table 1). In contrast, the same For NMDA receptor autoradiography, tissue sections
3
were preincubated (60 min, room temp.) in 50 mM Tris– procedure significantly increased [ H]KA binding to KA HCl buffer (pH 7.4) and then incubated for 150 min at receptors in both medial and lateral CPu by 20% (both
3
room temp. in fresh buffer containing 10 nM [ H]MK-801 F [1,12 df ]57.50, P50.02; Table 1).
3
and 100mM L-Glu, 100mM L-glycine, 1 mM EDTA, and Absence of significant changes in [ H]MK-801 binding
Table 1
a
Ionotropic glutamate receptor binding in caudate-putamen 5 weeks after unilateral microinjection of 6-hydroxydopamine into rat substantia nigra
Brain region 6-OHDA NMDA receptors AMPA receptors KA receptors
lesion status
Caudate-Putamen
Medial part Control 167.366.4 344.5619.9 106.565.5
Lesioned 163.366.0 342.9618.3 128.065.6
(22%) (21%) (20%*)
Lateral part Control 176.465.6 331.9618.2 123.764.7
Lesioned 171.465.2 337.0615.6 148.466.7
(23%) (2%) (20%*)
a
F.I. Tarazi et al. / Brain Research 881 (2000) 69 –72 71
to striatal NMDA receptors contrasts to reported increases depleted rats and potentiate the beneficial effects ofL
-3,4-3
in NMDA-selective [ H]Glu binding 3 months after DA dihydroxyphenylalanine in rats and monkeys with ex-depletion [23], as well as early decreases at 1 week perimental parkinsonism [7,9]. The present findings indi-post-lesioning [16,23,24]. The MK-801 binding site within cate further that increases in KA receptors may also the NMDA receptor-linked cation channel may respond to contribute to the expression of parkinsonian symptoms, DA denervation differently from the NMDA receptor- and suggest that KA receptor antagonists might provide a binding site itself. Differential regulation of MK-801- novel therapeutic strategy for the treatment of Parkinson’s labeled and NMDA receptor binding sites for Glu in disease.
response to 6-OHDA-induced lesioning is consistent with In conclusion, ionotropic Glu receptors in CPu showed findings in other conditions. These include a decrease in differential changes at 5 weeks after nigrostriatal DA
3
striatal [ H]MK-801 binding vs. an increase in striatal denervation by 6-OHDA in the rat. Whereas tissue labeling
3
NMDA-selective [ H]Glu binding after surgical lesioning of KA receptors was selectively and significantly increased of the cerebral cortex [16,22], as well as decreased cortical by 20%, binding at NMDA or AMPA receptors did not MK-801 binding vs. increased cortical NMDA binding differ from that in control tissue. Further evaluation of the after repeated administration of haloperidol [18,19,21]. timing, location, and degree of differential adaptive re-The present findings also contrast to reported decreases sponses of specific Glu receptors to DA depletion may help in binding to AMPA receptors in CPu labeled with to clarify their physiological and pharmacological roles,
3
[ H]AMPA at 4 weeks [24], or no change in AMPA including their interactions with DA receptors and contri-receptor levels in the same brain region at 3 and 6 months bution to the actions of antiparkinsonian drugs.
[13,23] after 6-OHDA lesioning of the medial forebrain
3
bundle. Our use of [ H]CNQX as a radioligand to assess AMPA receptors, as well as the site of lesioning and timing
Acknowledgements
all may have contributed to this reported variability in the effects of brain lesions on binding of radioligands targeting
Supported by NIH grants MH-34006 and MH-47370, a ionotropic Glu receptors. CNQX has some interaction with
grant from the Bruce J. Anderson Foundation, and by the KA as well as AMPA receptors [12], but appears to have
McLean Private Donors Neuropharmacology Research labeled mainly AMPA sites in the present study in view of
Fund. a lack of effect of 6-OHDA lesioning on labeling with
3 3
[ H]CNQX despite increased binding of [ H]KA (Table 1).
3
The significant increase found in [ H]KA binding (Table References 1) indicates that compensatory mechanisms which increase
postsynaptic striatal Glu receptors after DA depletion were [1] R.L. Albin, R.L. Makowiec, Z.R. Hollingsworth, L.S. Dure IV, J.B. more effective for KA receptors than for the other two Glu Penney, A.B. Young, Excitatory amino acid binding sites in the receptor types. Alternatively, the observed increase in KA basal ganglia of the rat: a quantitative autoradiographic study,
Neuroscience 46 (1992) 35–48.
receptor labeling may reflect combined increases of
post-[2] R.J. Baldessarini, F.I. Tarazi, Brain dopamine receptors: A primer
synaptic KA receptors in CPu and presynaptic KA
re-on their current status, basic and clinical, Harvard Rev. Psychiatry 3
ceptors located on terminals of hippocampostriatal
projec-(1996) 301–325.
tions innervating CPu [15]. Increased expression of KA [3] M. Carlsson, A. Carlsson, Interactions between glutamatergic and receptors may represent a functional adaptation, perhaps in monoaminergic systems within the basal ganglia-implications for schizophrenia and Parkinson’s disease, Trends Neurosci. 13 (1990)
response to loss of tonic inhibition of Glu release by DA,
272–276.
tending to normalize glutamatergic neurotransmission
[4] C.L. Christoffersen, L.T. Meltzer, Evidence for N-methyl-D
-aspar-within CPu. In contrast, in a study on the effects of
tate and AMPA subtypes of the glutamate receptor on substantia
surgical lesions of the median forebrain bundle, there were nigra dopamine neurons: possible preferential role for N-methyl-D -no differences in expression of KA receptors in rat CPu at aspartate receptors, Neuroscience 67 (1995) 373–381.
3 months [23]. This unexplained difference may reflect [6] M.O. Krebs, J.M. Desce, M.L. Kemel, C. Gauchy, G. Godeheu, A. Chermay, J. Glowinski, Glutamatergic control of dopamine release
dissimilarities in the lesioning procedures or timing of
in the rat striatum: evidence for presynaptic N-methyl-D-aspartate
observations following lesioning.
receptors on dopaminergic nerve terminals, J. Neurochem. 56
Disturbances in glutamatergic neurotransmission are (1991) 81–85.
implicated in Parkinson’s disease. Labeling of NMDA [7] T. Klockgether, L. Turski, NMDA antagonists potentiate
antipar-receptors was increased in postmortem CPu tissue from kinsonian actions of L-dopa in monoamine-depleted rats, Ann. Neurol. 28 (1990) 539–546.
patients diagnosed with Parkinson’s disease [20]. In
addi-[8] T. Klockgether, L. Turski, Toward an understanding of the role of
tion, intrastriatal injection of non-toxic doses of NMDA
glutamate in experimental parkinsonism: agonist-sensitive sites in
can produce parkinsonism-like behavioral effects in rats the basal ganglia, Ann. Neurol. 34 (1993) 585–593.
[8], whereas treatment with NMDA and AMPA receptor [9] T. Klockgether, L. Turski, T. Honore, Z.M. Zhang, D.M. Gash, R.
72 F.I. Tarazi et al. / Brain Research 881 (2000) 69 –72
antiparkinsonian effects in monoamine-depleted rats and MPTP- [18] F.I. Tarazi, W.J. Florijn, I. Creese, Regulation of ionotropic gluta-treated monkeys, Ann. Neurol. 30 (1991) 717–723. mate receptors following subchronic and chronic treatment with [10] N.S. Kula, R.J. Baldessarini, F.I. Tarazi, R. Fisser, S. Wang, J. typical and atypical antipsychotics, Psychopharmacology 128 (1996)
3
Trometer, J.L. Neumeyer, [ H]b-CIT: A radioligand for dopamine 371–379.
transporters in rat brain tissue, Eur. J. Pharmacol. 385 (1999) [19] J. Ulas, L. Nguyen, C.W. Cotman, Chronic haloperidol treatment
291–294. enhances binding to NMDA receptors in rat cortex, NeuroReport 4
[11] D.T. Monaghan, R.J. Bridges, C.W. Cotwan, The excitatory amino (1993) 1049–1051.
acid receptors: their classes, pharmacology, and distinct properties in [20] J. Ulas, F.B. Weihmuller, L.C. Brunner, J.N. Joyce, J.F. Marshall, the function of the central nervous system, Ann. Rev. Pharmacol. C.W. Cotman, Selective increases of NMDA-sensitive glutamate Toxicol. 29 (1989) 365–402. binding in the striatum of Parkinson’s disease, Alzheimer’s disease, [12] A.V. Paternain, A. Vicente, E.Q. Nielsen, J. Lerma, Comparative and mixed Parkinson’s disease /Alzheimer’s disease patients: an
antagonism of kainate-activated kainate and AMPA receptors in autoradiographic study, J. Neurosci. 14 (1994) 6317–6324. hippocampal neurons, Eur. J. Neurosci. 8 (1996) 2129–2136. [21] L. Nguyen, C.W. Cotman, Chronic haloperidol treatment enhances [13] R.H.P. Porter, J.G. Greene, D.S. Higgines Jr., J.T. Greenamyre, binding to NMDA receptors in rat cortex, NeuroReport 4 (1993)
Polysynaptic regulation of glutamate receptors and mitochondrial 1049–1051.
enzyme activities in the basal ganglia of rats with unilateral [22] U. Wullner, D.G. Standaert, C.M. Testa, G.B. Landwehrmeyer, M.V. dopamine depletion, J. Neurosci. 14 (1994) 7192–7199. Catania, J.B. Penney, A.B. Young, Glutamate receptor expression in [14] C.A. Tamminga, Schizophrenia and glutamatergic transmission, rat striatum: Effects of deafferentation, Brain Res. 647 (1994)
Crit. Rev. Neurobiology 12 (1998) 21–36. 209–219.
[15] F.I. Tarazi, A. Campbell, R.J. Baldessarini, Effects of hippocampal [23] U. Wullner, C.M. Testa, M.V. Catania, A.B. Young, J.B. Penney, kainic acid lesion on striatolimbic ionotropic glutamate NMDA, Glutamate receptors in striatum and substantia nigra: Effects of AMPA and KA receptors, Neurosci. Lett. 250 (1998) 13–16. medial forebrain bundle lesions, Brain Res. 645 (1994) 98–102. [16] F.I. Tarazi, A. Campbell, S.K. Yeghiayan, R.J. Baldessarini, Locali- [24] K. Zavitsanou, A. Mitsacos, P. Giompres, E.D. Kouvelas, Changes
3 3
zation of glutamate receptor subtypes in caudate-putamen and in [ H]AMPA and [ H]KA binding in rat caudate-putamen and nucleus accumbens septi: Comparison of NMDA, AMPA and KA nucleus accumbens after 6-hydroxydopamine lesions of the medial receptors, Synapse 30 (1998) 227–235. forebrain bundle: An autoradiographic study, Brain Res. 731 (1996) [17] F.I. Tarazi, A. Campbell, S.K. Yeghiayan, R.J. Baldessarini, Locali- 132–140.
zation of dopamine receptor subtypes in caudate-putamen and nucleus accumbens septi of rat brain: Comparison of D -, D -, and1 2