compounds and digestible proteins renders seafood easily perishable, often spoiling in a short period of time even under refrigeration. F ish and shellfish carry a variety of micro-organisms from both aquatic and terrestrial sources. In addition to spoilage micro-organisms, seafood may contain various potential pathogens which are public health hazards. It is often difficult to maintain the quality of seafood and seafood products because of the considerable distance between consumers and harvesting areas, which pro-vides opportunities for microbial growth and recontamination. T his paper covers the quan-titative and qualitative aspects of micro-organisms found in fish and the factors affect-ing seafood quality. Emphasis will be placed on spoilage bacteria, which causes degrada-tion and organisms that presents risk to the public health.
Fish spoilage
M ost fish are caught in the wild in nets, or with lines with baited hooks, and hence it is difficult to control the initial quality of the raw material with any degree of repeatability. F ish farming has to some extent reduced the varia-tion of fresh fish quality. T he stress and mechanical damage caused during capture, the structure and composition of fish, rate of post-mortem change, minimal pH drop, and storage temperature prior to landing all act to induce rapid spoilage in fish. Like red meat, fish spoils through the combined effects of chemical reactions, via the continuing activi-ties of endogenous enzymes and through bacterial growth. Since fish are poikilother-mal, their metabolism and the commensal bacterial flora of their skin, gills and intestines are adapted to lower temperatures than those of mammals. H ence, chilling has less effect on slowing spoilage when compared with chilled red meat and poultry, especially for fish caught in temperate and polar regions, where-as chilled fish caught in tropical waters tend to spoil more slowly, since the initial micro flora is not adapted to growing in chilled condi-tions. T he initial stages of spoilage in fish characterised by the loss of characteristic odour and taste are mainly due to autolytic degradation, while the final stages of fish quality deterioration characterised by soften-ing or toughensoften-ing of flesh texture and Nutrition & Food Science
Number 6 · November/December 1998 · pp. 325–329 © M CB University Press · ISSN 0034-6659
a nd spoila ge in fi sh
(pa rt II) –
m icrobiologica l
induce d de t e riora t ion
Owen P. Fraser and
S am S umar
The authors
Ow en P. Fraseris a Researcher at the Food Research Centre of South Bank University, London, UK.
Sam Sumaris the Reader in Food Science at the South Bank University, London, UK.
Abstract
production of volatile unpleasant – smelling odours and flavours are mainly due to micro-bial activity. G enerally, the rates at which autolytic and microbial spoilage occur are dependent on the degree of microbial conta-mination and flora, storage temperature and packaging.
M icrobial fl ora in f resh fi sh
T he wide range of fish species, the vastly different environments from which they are harvested and the variety of microbiological sampling techniques used has resulted in widely ranging reports of numbers of organ-isms and flora on fish. T he bacterial flora of cold water fish are dominated by the psy-chrotropic gram-negative genera (Shewan, 1977). O rganisms involved in the genera, A cinetobacter, Flavobacterium, M oraxella, S hewanella and Pseudomonas; members of the V ibrionaceae (V ibrio and Photo bacterium and A eromonadaceae (A eromonas spp.) are also common aquatic bacteria, while gram-posi-tive organisms such as Bacillus, M icrococcus, Clostridium, Lactobasillus and coryneforms can also be found in varying proportions (H uss, 1995). Shewan (1977) concluded that the gram-positive Bacillus, and M icro coccus domi-nate on fish from tropical waters; however, other workers have found that the micro flora on tropical species is very similar to that on temperate species but with a higher load of gram-positive and enteric bacteria (H uss, 1995). It is apparent from the above that potentially there are a very diverse range and number of organisms on fish. T he significance of these organisms in terms of spoilage of fish varies considerably on their growth. M any of the organisms present on spoilt fish are thought to play no active role in spoilage (H uss, 1995). Alur et al.(1989) showed that despite prolific growth of M icrococcus colpo-genes (107-108cells/ml) in mackerel
homogenate there were no signs of spoilage, thus indicating that total bacterial count does not serve as a reliable index of freshness for processed seafood (Alur et al., 1971). T hus, it is a common mistake to think the micro-organisms present in the highest numbers are the cause of spoilage and deterioration in fish. A clear distinction should always be made between the terms spoilage flora and spoilage bacteria. Spoilage flora refers to the bacteria present on the fish when considered spoiled, whereas spoilage bacteria are the bacteria
responsible for the production of off-odours and off-flavours in the spoilt fish. Examples of compounds produced by microbial metabo-lism during spoilage are given in Table I. Some of these spoilage compounds are also produced by the action of autolytic enzymes naturally present in the fish.
Bact erial det eriorat ion of fi sh
T he fish’s regulatory mechanisms, which prevent invasion of the tissues by bacteria, cease to function after death. Bacteria then invade the fish body through the skin, enter the body cavity and belly via intestines, and penetrate the gill tissue and kidney by way of the vascular system. T he low molecular weight compounds and soluble proteins yielded from the fish body during autolysis after rigor mortis provide rich nutrients for bacterial growth.
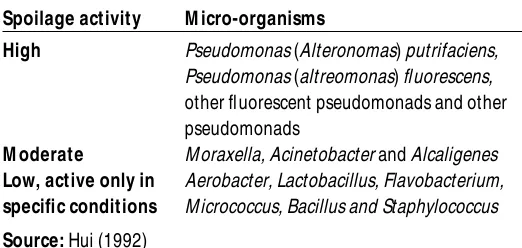
Various proteases and other hydrolytic enzymes secreted by psychrophilic and psy-chrotrophic bacteria can act on the fish muscle even at low temperatures (Venugopal, 1990). T he factors that influence the micro-bial contamination and growth include fish species and size, method of catch, on-board handling, fishing vessel sanitation, processing, and storage condition (C hen and C hai, 1982; Ward and Baj, 1988). F ish are subjected to rapid microbial contamination and growth if handling and storage are inadequate. It is estimated that about 10 per cent of the total world catch is lost due to bacterial spoilage (James, 1986). Various micro-organisms involved in spoilage are listed in Table II in descending order of spoilage activity. Some organisms cause spoilage in different degrees depending on the total microbial flora, fish
Table I Bacterial spoilage compounds
Specific spoilage bacteria Spoilage compounds
Shew anella putrifaciens TM A, H2S, CH3SH, (CH3)2S, HX
Photobacterium phosphoreum TM A, HX
Pseudomonas spp. Ketones, aldehydes, esters,
non-H2S sulphides
Vibrionacaea TM A, H2S
Aerobic spoilers NH3, acetic, butyric and
propionic acid
Note:TM A = trimethylamine, H2S = hydrogen sulphide, CH3SH = methylmercarptan, (CH3)2S = dimethylsulphide, HX = hypoxanthine, NH3= ammonia
quality, handling and packaging methods, and storage temperature.
Refrigerated fresh haddock fillet contain about 105g–1of initial bacteria, predominated by M oraxella, A cinetobacter and Corynebacteri-um. After storage at 1°C for 14 days the bacte-rial numbers reaches 2.1 ×108g–1and the fish enters the spoilage stage. Pseudomonas
(A lteromonas) putrifaciens and fluorescent pseudomonads were the organisms responsi-ble for the spoilage of refrigerated haddock (C hai, 1968). T hese spoilage bacteria account only for about 1 per cent of the initial micro-bial load but increase to at least 30 per cent of the spoilage flora. T his indicates that whenev-er Pseudomonas putrifaciens and fluorescent pseudomonads reaches 30 per cent of the total bacterial count, fish spoilage will result regardless of total bacterial level.
As Pseudomonas putrifaciens, fluorescent pseudomonads and other potential spoilage bacteria increase rapidly in the initial stages of spoilage, they produce vast amounts of prote-olytic and hydrprote-olytic enzymes (Shewan, 1961). Proteins are degraded by proteases to peptides and amino acids and then further broken down to indole, amines, acids, sul-phide compounds, and ammonia (Liston, 1980). Lipases break down lipids to form fatty acids, glycerol and other compounds.
Trim ethylam ine production
F ish have a unique osmoregulatory mecha-nism to avoid dehydration in marine environ-ments and waterlogging of tissue in fresh water. O ne important osmoregulant is trimethylamine oxide (T M AO ) found in concentrations of up to 1 per cent in teleost (e.g. cod, salmon), and up to 1.5 per cent in elasmobranches (e.g. sharks, skates), together with 2 per cent urea. T M AO occurs in the feed of fish, is pH neutral and non-toxic. H owever, gram-negative bacteria such as S hewanella putrifaciens can obtain energy from
T M AO by reducing it to T M A (trimethy-lamine). Endogenous enzymes present in fish can also reduce T M AO to D M A (dimethy-lamine) and FA (formaldehyde). While T M AO is non-odorous, T M A is a component in the odour of stale fish. T he levels of T M A in fish has for sometime been used as an indicator of microbial deterioration of fish. F ish with less than 1.5mg T M A-N 100 g–1is considered of good quality while 10-15mg T M A-N 100g–1is considered the the limit of acceptability (H uss, 1988). H owever, these limits cannot be applied universally as an indicator of freshness of fish. Ababouch et al. (1996) have shown that while less than 1mg T M A-N 100 g–1is considered first grade, 5-10mg T M A-N 100 g–1is the limit of acceptability in sardine. T he reduction of T M AO to T M A is pH dependent and will therefore vary depending on the overall condi-tion of the fish. H owever, the presence of T M A in fish is universally used as an indicator of microbial deterioration.
P roduction of other volatiles
M any other volatile compounds are produced as a result of bacterial catabolism of fish con-stituents and are sometimes used as indicators of microbial degradation of fish. Volatile sulphide compounds and H2S gas is produced from sulphur-containing amino acids (G ram et al., 1990). Ammonia is produced as a result of bacterial degradation of other non-protein nitrogen compounds such as urea which is in significantly large amounts in elasmobranch-es. Bacterial catabolism of amino acids in fish muscle also result in the formation of ammo-nia and other volatile bases. Limits of accept-ability for total volatile base nitrogen (T VB-N ) in some fish were 30-35mg 100 g–1(H uss, 1988); however, Ababouch et al.(1996) found that 25-35mg T VB-N 100g-1was the limit of acceptability in sardine, while El M arrakchi et al. (1990) found the limit of acceptability in another fish sample to be 25-30mg T VB-N 100 g–1. T hese findings suggest that the development of T VB-N in fish may vary widely and is dependent on a number of intrinsic and extrinsic factors.
Volatile acids such as acetic, propionic, butryic and other short chain fatty acids are also produced as a result of microbial degra-dation of fish. T hese compounds contribute to the odour of spoilt fish. Ethanol, propanol, isopropanol and phenethyl alcohol are Table II Activity of micro-organisms involved in fish spoilage
Spoilage activity M icro-organisms
High Pseudomonas (Alteronomas) putrifaciens, Pseudomonas (altreomonas) fluorescens,
other fluorescent pseudomonads and other pseudomonads
M oderate M oraxella, Acinetobacter and Alcaligenes
Low, active only in Aerobacter, Lactobacillus, Flavobacterium,
specific conditions M icrococcus, Bacillus and Staphylococcus
produced by a number of fish spoilers (C hen et al., 1974).
H istam ine production
Scrombroid fish ( tuna, albacore ) contain characteristically high levels of free histidine in their muscle tissue (Tayloret al., 1989), which can be converted to histamine by the action of bacterial decarboxylase. Bacteria known to be capable of decarboxylating histi-dine include V ibrio, Proteus morganii, and K lebsiela pneumoniae, all having minimum growth temperatures between 8-15°C . T he formation of histamine in fish is purely from microbial origin and therefore could be used as an indicator of bacterial activity in fish. T he presence of histamine in fish is insignificant in its contribution to the sensory acceptability of fish; however, its presence could appreciably compromise its safety when consumed. T he guideline established by the U S Food & D rug Administration (F DA, 1996), for histamine in fish is 5 mg/100g (50ppm). F ish with levels above this limit are prohibited from being sold for human consumption. Since histamine is only produced at temperatures above 8°C , adequate temperature control is necessary to reduce or prevent its formation. D uring storage for extended periods at ambient tem-perature the level of histamine may begin to reduce by bacteria having histaminase activity (Taylor, 1986); however, the fish would have been completely spoilt by the time the hista-mine levels have reduced to a legally accept-able level. O ther biogenic amines formed as a result of bacterial activity on free amino acids in fish muscle are cadaverine and tyramine.
Pat hogenic fl ora of f resh fi sh
M any pathogenic bacteria make up part of the natural micro flora of fresh fish. Some aquatic pathogens found in fish include V ibrio para-heamolyticus, V ibrio vulnificus, Listeria monocy-togenes, A eromonas hydrophilla, Yersinia entero-coltica, Clostridium perfringens and Clostridium botulinum. T he activity of these pathogens in fish is dependent on the storage, handling and processing of the raw material. Since fish is stored at low temperatures (< 2°C ),
psychrotropic strains of Clostridium botulinum and other psychrotropic pathogens such as A eronomas, Listeria and Yersinia, are of greater importance because they can render food unsafe for human consumption. When con-sidering the safety of fish, non-proteolytic,
psychrotropic strains of Clostridium botulinum is of particular importance. T hese strains can grow and produce toxins without producing any overt signs of spoilage, which may also be absent as a result of an inhibition of the normal spoilage flora, e.g. in bulk stored, modified atmosphere packaged (M AP) fish or conditions in which an anaerobic environ-ment is created. Strains of type E and non-proteolytic types B and F are a major concern in M AP as they are able to grow at tempera-tures as low as 3.3°C , albeit slowly, and, as they do not putrefy proteins they may not show obvious signs of spoilage (C hurch, 1998).
D uring storage, handling and processing fish may become contaminated with other pathogens such as S almonella typhimurium and Escherichia coli. T hese and other pathogens may cause degradation leading to intoxication and spoilage of fish and fish products stored at elevated temperatures. T his is very common when fish is stored in the home refrigerator where the temperature is not not monitored or controlled (Parry, 1993).
Conclusion
there-fore necessary to ensure safe, good quality seafood.
Ref erences
Ababouch, L.H., Souibri, L., Rhaliby, K., Ouadhi, O., Battal, M . and Busta, F.F. (1996), “ Quality changes in sardines (Sardina pilchardus) stored in ice and at ambient temperature” ,Food M icrobiology, Vol. 13, pp. 123-32.
Alur, M .D., Venugopal, V. and Nerkar, D.P. (1989), “ Spoilage potential of some contaminant Bacteria isolated from Indian mackerel” (Rastrelliger kanagurta), J. Food Sci., Vol. 54 No. 5.
Alur, M .D., Lew is, N.F., M adhavan, D.L. and Kumta, U.S. (1971), “ Spoilage potential of predominant organ-isms and radiation survivors in fisheries products” , Ind J. Exp. Bio., Vol. 9 No. 48.
Chai, T. (1968), “ Detection and incidence of specific species of spoilage bacteria in fish. II. Relative incidence of Pseudomonas putrifaciens and fluores-cent pseudomonads on haddock fillets” ,Applied M icrobiology, Vol. 16, pp. 1738-41.
Chen, H.C. and Chai, T. (1982), “ M icro flora of drainage from ice in fishing vessel fish holds” , Applied Environmental M icrobiology, Vol. 43, pp. 1360-5. Chen, T.C., Nawar, W.W. and Levin, R.E. (1974), “
Identifi-cation of major high-boiling volatile compounds produced during refrigerated storage of haddock fillets” ,Applied M icrobiology, Vol. 28, pp. 679-80. Church, N. (1998), “ M AP fish and crustacean-sensory
enhancement” , Food Science and Technology Today, Vol. 12 No. 2, pp. 73-83.
El M arrakchi, A., Bennour, M ., Bouchriti, N., Hamama, A. and Tagafait, H. (1990), “ Sensory, chemical and microbiological assessments of M oroccan sardines” , J. Food Protect, Vol. 53, pp. 600-5.
FDA (1996), “ Decomposition and histamine in raw, frozen tuna and mahi-mahi, canned tuna; and related species” , Compliance Policy Guides 7108.240, Sec. 540.525.
Gram, L., Wedell-Neergaard, C. and Huss, H.H. (1990), “ The bacteriology of fresh and spoiling Nile perch” , Int. J. Food M icrobiology, Vol. 10, pp. 303-16. Hui, Y.H. (1992),Encyclopedia of Food Science and
Tech-nology, Vol. 2 (E-H), Wiley, p. 870. Huss, H.H. (1988), “ Fresh fish quality and quality
changes” , FAO Fisheries Series No. 29, FAO, Rome. Huss, H.H. (1995), Quality and Quality Changes in Fresh
Fish, FAO Fisheries Technical Paper No. 348., FAO. Rome.
James, D.G. (1986), “ The prospects of fish for the under-nourished food and nutrition” , FAO, Vol. 12, pp. 20-7.
Liston, J. (1980), “ M icrobiology in fishery science” , in Connel, J.J. (Ed.), Advances in Fish Science and Technology, Fishing News Books Ltd, Surrey. Parry, R.T. (1993), in Parry, R.T. (Ed.), Principles and
Appli-cations of M odified Atmosphere Packaging of Food, Blackie Academic and Professional, London. Shewan, J.M . (1961), “ The microbiology of sea-water
fish” , in Borgstrom, G. (Ed.),Fish as Food, Vol. 1, Academic Press, Inc., Orlando, Florida.
Shewan, J.M . (1977), “ The bacteriology of fresh and spoiling fish and biochemical changes induced by bacterial action” , in Proceedings of the Conference on Handling, Processing and M arketing of Tropical Fish, Tropical Products Institute, London, pp. 51-66. Taylor, S.L. (1986), “ Histamine food poisoning: toxicology and clinical aspects” ,CRC Crit. Rev. Toxicol., Vol. 17, pp. 91-128.
Taylor, S.L., Stratton, J.E. and Nordlee, J.A. (1989), “ Hista-mine poisoning (Scrombroid fish poisoning): an allergy-like intoxication” , Clin.Toxicol, Vol. 27, pp. 225-40.
Venugopal, V. (1990), “ Extra cellular proteases of contami-nant bacteria in fish spoilage: a review,” J. Food Prot., Vol. 53, pp. 341-50.