Efficacy and safety of atorvastatin in hyperlipidemic, type 2
diabetic patients. A 34-week, multicenter, open-label study
Carlos A. Aguilar-Salinas
a,*, Francisco J. Go´mez-Pe´rez
a, Carlos Posadas-Romero
b,
Cuauhte´moc Va´zquez-Cha´vez
c, Eduardo Meaney
d, Alfonso Gulı´as-Herrero
a,
Luz E. Guille´n
a, Alma Alvarado Vega
b, Enrique Mendoza Pe´rez
b,
Luis Eduardo Romero-Nava
b, Rita Ange´lica Go´mez-Dı´az
c, Sau´l Salinas-Orozco
c,
Rafael Moguel
d, Germa´n Novoa
eaInstituto Nacional de la Nutricio´n‘Sal6ador Zubira´n’de la SSA(Secretarı´a de Salud),Mexico City,Mexico bInstituto Nacional de Cardiologı´a‘Ignacio Cha´6ez’de la SSA,Mexico City,Mexico
cHospital de Cardiologia,Centro Me´dico Nacional Siglo XXI del IMSS(Instituto Mexicano del Seguro Social),Mexico City,Mexico dHospital1°de Octubre del ISSSTE(Instituto de Seguridad y Ser6icios Sociales para los Trabajadores del Estado),Mexico
eParke-Da6is,Mexico
Received 22 March 1999; received in revised form 16 November 1999; accepted 13 December 1999
Abstract
Hyperlipidemia is common in type 2 diabetic patients and is an independent risk factor for cardiovascular disease. The aim of this trial was to evaluate the efficacy and safety of once-daily atorvastatin 10−80 mg for the treatment of hyperlipidemia in type 2 diabetics with plasma low-density lipoprotein cholesterol (LDL-C) levels exceeding 3.4 mmol/l (130 mg/dl). One hundred and two patients met the study criteria and received 10 mg/day atorvastatin. Patients who reached the target LDL-C level of 52.6 mmol/l (100 mg/dl) maintained the same dosage regimen until they had completed 16 weeks of treatment. Patients not reaching the target LDL-C underwent dose titration to atorvastatin 20, 40 and 80 mg/day at Weeks 4, 8 and 12, respectively. All 88 patients who completed the study attained target LDL-C levels and 52 (59%) of patients achieved the target goal at the starting dose of atorvastatin 10 mg/day. In this group the differences between baseline and post-treatment values for LDL-C were 4.390.7 mmol/l (166926 mg/dl) versus 2.290.4 mmol/l (87914 mg/dl) (PB0.0001), respectively, a decrease of 47%. Similar trends were observed for total cholesterol, triglycerides, very low-density lipoprotein cholesterol and apolipoprotein B levels. The safety profile of atorvastatin in these patients was highly favorable and similar to those reported with other statins. Only one patient withdrew due to a possible drug-related adverse event. These data confirm the marked efficacy and safety of atorvastatin in type 2 diabetic patients with hyperlipidemia and the efficacy of atorvastatin 10 mg in helping patients attain their LDL-C goal. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Atorvastatin; Type 2 diabetes mellitus; Hypercholesterolemia; Low-density lipoprotein cholesterol; Triglyceride
www.elsevier.com/locate/atherosclerosis
1. Introduction
Type 2 diabetes is frequently associated with elevations of plasma low-density lipoprotein cholesterol (LDL-C) and triglycerides, and reduced levels of high-density lipoprotein cholesterol (HDL-C) [1,2]. Although elevated
levels of LDL-C are found with equal frequency in diabetic and non-diabetic patients, LDL particle compo-sition is altered unfavourably in diabetics and is more likely to be atherogenic.These lipid abnormalities are recognized as major risk factors for the development of coronary heart disease (CHD) and underlie the 2 – 4-fold greater prevalence of CHD, cerebrovascular disease and peripheral vascular disease in diabetic compared with non-diabetic patients [3]. It has been estimated that 75 – 80% of deaths among the diabetic population are attributable to cardiovascular disease, particularly is-chemic heart disease [4,5].
* Corresponding author. Present address: Departamento De Dia-betes y Metabolismo de Lipidos, Vasco de Quiroga 15, Mexico City 14000, Mexico. Tel.:+52-5-5130002.
E-mail address:[email protected] (C.A. Aguilar-Salinas).
A number of measures are recommended for the treatment of hyperlipidemia associated with diabetes. These include improved glycemic control, diet and weight modification, and control of other CHD risk factors, such as smoking and alcohol intake. If non-pharmacological strategies are not sufficient to control the lipid abnormalities, introduction of drug therapy may be warranted. Agents that are used to treat hyper-cholesterolemia or hypertriglyceridemia in non-diabetic patient groups, such as niacin and bile acid seques-trants, may not be suitable in patients with type 2 diabetes due to the possibility of aggravation of hyper-triglyceridemia and/or insulin resistance [6]. In diabetic patients, drugs of choice to reduce LDL-C are the hydroxymethylglutaryl coenzyme A (HMG-CoA) re-ductase inhibitors (statins) (some statins also reduce triglyceride levels), because they are generally well toler-ated and do not adversely affect glycemic control. Data from subgroup analyses in recent cholesterol-lowering clinical trials using statin therapy indicate that the benefits of cholesterol lowering are at least as great in diabetic as in non-diabetic groups [7,8].
The results of a recent study have suggested that diabetic patients without CHD have as high a risk of a myocardial infarction as non-diabetic patients with CHD [9]. These data provide a rationale for treating cardiovascular risk factors in diabetic patients as ag-gressively as non-diabetic subjects in the high-risk CHD category. For diabetic patients without pre-existing CHD, the current American Diabetic Association (ADA) goal for LDL-C is B2.6 mmol/l (100 mg/dl)
[3,10]. The recommendations of the ADA for the treat-ment of elevated LDL-C generally follow the guidelines of the NCEP [11], which state that diabetic subjects with clinical CHD and an LDL-C level of \2.6 mmol/l (100 mg/dl) after behavioural and glucose interventions should be treated with pharmacological agents. Despite the recommendations recent data suggest that only a small proportion of type 2 diabetic patients achieve and sustain target LDL-C levels of B3.4 mmol/l (130 mg/ dl) to impact on cardiovascular morbidity [12 – 14].
The objectives of this study were to assess the efficacy and safety of 10 – 80 mg atorvastatin for the treatment of hyperlipidemia in type 2 diabetes patients with LDL-C levels ]3.4 mmol/l (130 mg/dl). Atorvastatin is a synthetic HMG-CoA reductase inhibitor which has been shown to be highly effective at lowering LDL-C and triglyceride levels in non-diabetic patients. In hy-perlipidemic subjects, doses of atorvastatin from 10 – 80 mg once-daily have been shown to reduce LDL-C levels by 41 – 61% [15] and triglycerides by 23 – 45% [16].
2. Materials and methods
2.1. Patients
All patients were previously diagnosed with type 2 diabetes according to the criteria of the ADA and had
to have HbA1CB10 for at least 4 weeks prior to
screening for the trial. Patient characteristics at baseline are detailed in Table 1. The trial included men and post-menopausal or non-pregnant women aged between
18 and 80 years who had a Body Mass Index of 530.
Plasma LDL-C levels in these patients were \3.4
mmol/l (130 mg/dl) after the initial 6 weeks of
isocaloric diet.
Patients were excluded if they had type 1 diabetes, uncontrolled hypertension, severe renal dysfunction, nephrotic syndrome or dysproteinemias, fasting plasma triglycerides \10 mmol/l, aspartate aminotransferase
(AST) or alanine aminotransferase (ALT) levels \
1.5×the upper limit of normal (ULN), or if their
creatine phosphokinase (CPK) levels were \3×ULN.
Consumption of any lipid-altering drug within the pre-vious 4 weeks (6 months for probucol) prevented entry into the study. None of the study subjects had tendi-nous xanthomata.
The protocol was approved by an Ethics Committee of every Institution and every patient provided wit-nessed, written, informed consent prior to entering the study.
2.2. Study design
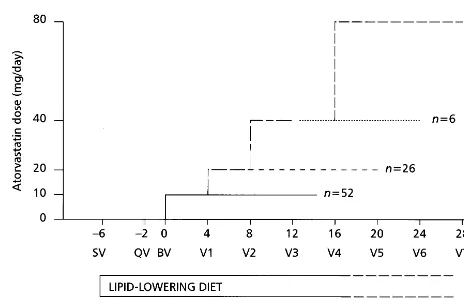
This was a multicenter, open-label, 34-week dose-ti-tration study (Fig. 1). Patients attended an initial
Table 1
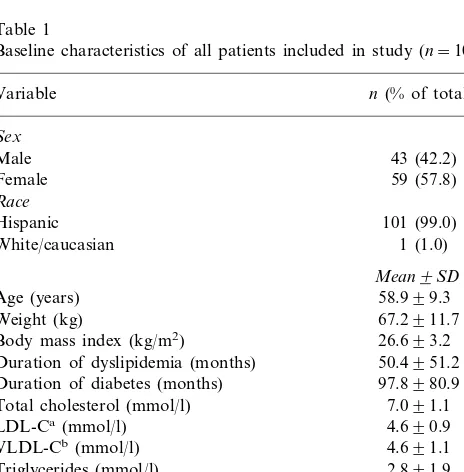
Baseline characteristics of all patients included in study (n=102)
n(% of total)
Weight (kg) 67.2911.7
Body mass index (kg/m2) 26.693.2
Duration of dyslipidemia (months) 50.4951.2 97.8980.9
Triglycerides (mmol/l) 2.891.9 Apolipoprotein B (g/l) 1.490.28 Fasting plasma glucose (mmol/l) 7.691.9 Fructosamine (mmol/l) 242.4962.5
HbA1C(%) 7.691.2
Fig. 1. Schematic figure of study design showing timings of patient visits, diet and dose titration periods.
The primary efficacy parameters of the study were percentage changes in LDL-C and triglycerides from baseline. Secondary efficacy parameters included the
percentage changes in total cholesterol, HDL-C,
VLDL-C, and apolipoproteins A1 and B from baseline.
2.5. Safety e6aluation
Before entering the study patients underwent a com-plete physical examination with clinical laboratory
eval-uation including blood count, measurement of
thyroid-stimulating hormone (TSH) and CPK levels, urinalysis, liver function tests (ALT and AST), glycemic
profile (fasting plasma glucose, HbA1C and
fruc-tosamine) and a pregnancy test. These tests were re-peated at the end of the study. At each visit, liver function tests, glycemic profile and CPK levels were measured. Clinically important events were defined as:
CPK \5×ULN at two consecutive measurements 1
week apart accompanied by muscle pain, tenderness or
weakness; CPK \10×ULN at any time; ALT or AST
\3×ULN at two consecutive measurements 1 week
apart. Patients were excluded from the study if they developed severe hyperglycemia or any other significant deviation from safety tests. Other reasons for dismissal were lack of compliance to the drug or diet.
2.6. Laboratory analyses
The laboratory of the Departamento de Diabetes y Metabolismo de Lı´pidos of the Instituto Nacional de la Nutricio´n in Mexico performed all lipid and clinical laboratory measurements using standardized proce-dures. This laboratory is certified for standardization of tests by the External Comparative Evaluation of Labo-ratories Program of the College of American Patholo-gists. Blood samples were taken after an overnight fast (]9 h). All laboratory analyses were performed with commercially available standardized methods. Glucose was measured using the glucose oxidase method and
HbA1C using latex immunoagglutination inhibition
(Bayer laboratories) [17]. Total serum cholesterol and triglycerides were measured using an enzymatic method
(SERA-PAK®) (CV 3.3%). HDL-C levels were assessed
using phosphotungstic acid and Mg2+ (CV 2.5%).
LDL-C concentration was estimated by the Friedewald formula [18]. Direct LDL-C was determined by ultra-centrifugation (bquantification) at Week 0, on comple-tion of 16 weeks of treatment and in every patient in whom triglyceride levels were \4.5 mmol/l (400 mg/dl) [19]. Apolipoprotein B concentration was measured by an immunonephelometric method. Fructosamine con-centration was measured using a reduction test with nitroblue tetrazolium (Bohringer – Mannheim).
screening visit followed by a qualifying visit 4 weeks later in which compliance with diet was assessed and blood samples were taken. Within 2 weeks patients returned for a baseline visit during which further blood samples were tested and drug treatment was initiated. Changes in lipid profile after periods of treatment with atorvastatin were compared against the mean labora-tory parameters obtained during the qualifying (week −2) and baseline (week 0) visits.
Throughout the trial, including the pre-treatment period of 6 weeks, patients were required to comply with an isocaloric, standard lipid-lowering diet defined by a registered dietician of 50% carbohydrates, 20% protein, 30% fat and 30 g/day fiber. Dietary advice was given at the initial visit and compliance with the diet assessed at every subsequent visit (at 4-week intervals) using a 3-day food record. Drug compliance and effi-cacy, as well as laboratory parameters were also mea-sured at every visit.
2.3. Dose titration
The starting dose of 10 mg/day was doubled every 4
weeks until either the desired LDL-C level (52.6
mmol/l [100 mg/dl]) or a maximum dose of 80 mg
atorvastatin once daily were achieved. Once patients had achieved their target LDL-C level, they maintained the same dose regimen for a further 16 weeks of treatment.
2.4. Efficacy parameters
2.7. Statistical analysis
Statistical analysis was performed with the SAS Statis-tical Package version 6.12 TS020. All differences between groups were evaluated using two-tailed paired t-tests.
3. Results
3.1. Patients
One hundred and ninety-four patients were screened for the study. The characteristics and baseline measure-ments of the patients who entered the study are shown in Table 1. A total of 102 patients satisfied the inclusion/ exclusion criteria and entered the trial. Eighty-eight patients completed the study; 14 patients were not considered in the final analysis due to lack of compliance (n=10), withdrawal due to adverse events (n=2) or administration difficulties (n=2).
3.2. Efficacy
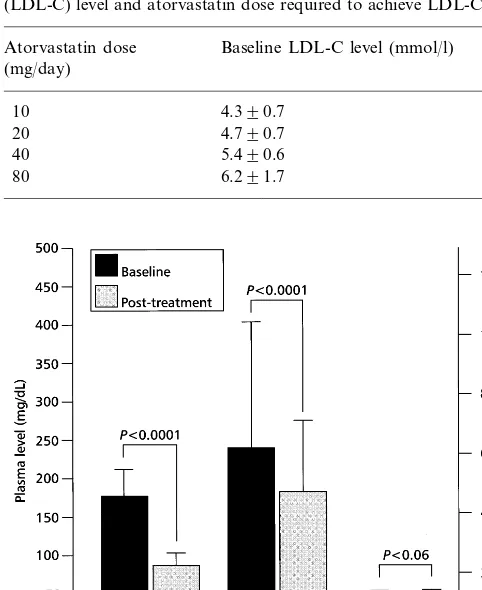
All 88 patients who completed the study attained the target LDL-C level of52.6 mmol/l (100 mg/dl). Of these patients, 52 (59%) achieved their LDL-C goal with the 10 mg/day atorvastatin dose, 26 (29.5%) with the 20 mg dose, six (7%) with the 40 mg dose and four (4.5%) with the 80 mg dose. As expected the main determinant of the dosage needed to achieve the LDL-C goal was baseline LDL-C level (Table 2). When patients were stratified by their baseline LDL-C levels, 67 (76%) patients who finished the study had a baseline LDL-C ofB5.2 mmol/l (200 mg/dl). Of these patients, 47 (70.1%) achieved their LDL-C goal with the 10 mg/day atorvastatin dose, 17 (25.4%) with the 20 mg/day dose and only three (4.5%) required a 40 mg dose or greater. Of the 21 patients
finishing the study whose baseline LDL-C was ]5.2
mmol/l (200 mg/dl), 14 (66.7%) required 20 mg/day or less to achieve the LDL-C goal. The highest dose (80 mg/day) was required in only three cases.
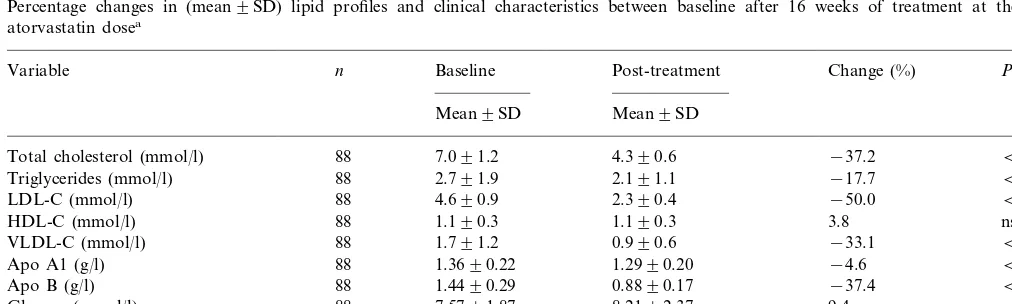
A 16-week treatment period of atorvastatin (10, 20, 40 or 80 mg) in 88 patients with type 2 diabetes resulted in a mean reduction from baseline in plasma LDL-C of 50% (4.690.9 mmol/l [177934 mg/dl] to 2.290.4 mmol/l [87916 mg/dl], PB0.0001) and a mean reduction in triglyceride levels of 18% (2.791.8 mmol/l [2409164 mg/dl] to 2.191.1 mmol/l [183995 mg/dl],PB0.0001) (Fig. 2). During this period there was no significant change in Body Mass Index. The majority of patients achieved the target level of 52.6 mmol/l (100 mg/dl) at the 10 mg dose of atorvastatin (n=52, 59.1%) (Fig. 3). A total of 26 (29.5%) patients required titration to 20 mg/day; six (6.8%) required titration to 40 mg/day, and only four (4.5%) required titration to 80 mg/day.
The effect of atorvastatin on the lipid profile showed a dose-dependent trend although this was not tested statistically (Table 3). After 16 weeks of treatment at the effective dose, patients showed mean decreases in plasma LDL-C of 47.1, 53.0, 59.9 and 53.0% at daily
Table 2
Relationship between baseline low-density lipoprotein cholesterol (LDL-C) level and atorvastatin dose required to achieve LDL-C goal
Atorvastatin dose Baseline LDL-C level (mmol/l) (mg/day)
10 4.390.7
20 4.790.7
40 5.490.6
80 6.291.7
Fig. 2. Bar chart showing change (mean9SD) in low-density protein cholesterol (LDL-C), triglycerides and high-density lipo-protein cholesterol (HDL-C) between baseline and after 16 weeks of treatment at the effective atorvastatin dose.
Table 3
Percentage change in the lipid profile and clinical characteristics between baseline and post-treatment valuesa
Percent change (%)
10 mg 20 mg 40 mg 80 mg
(n=26)
(n=52) (n=6) (n=4)
−39.4
Total cholesterol −34.5 −43.9 −47.3
Triglycerides −13.6 −19.3 −22.1 −52.4
LDL-C −47.1 −53.0 −59.9 −53.0
5.0 5.8
2.3 12.5
HDL-C
VLDL-C −29.3 −36.4 −32.1 −60.0
−4.4 −1.7
−5.5 2.2
Apo A1
−34.9
Apo B −40.0 −42.1 −45.6
7.0
Glucose 11.5 11.6 23.4
9.0 9.7
2.4 −0.7
HbA1
16.0
Fructosamine 26.4 12.5 20.1
aTitration to the higher dose only occurred in non-responders. LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein
cholesterol; VLDL-C, very low-density lipoprotein cholesterol; Apo A1, apolipoprotein A1; Apo B, apolipoprotein B.
Table 4
Percentage changes in (mean9SD) lipid profiles and clinical characteristics between baseline after 16 weeks of treatment at the effective atorvastatin dosea
Baseline Post-treatment
Variable n Change (%) P
Mean9SD Mean9SD
7.091.2 4.390.6
Total cholesterol (mmol/l) 88 −37.2 B0.0001
Triglycerides (mmol/l) 88 2.791.9 2.191.1 −17.7 B0.0001
LDL-C (mmol/l) 88 4.690.9 2.390.4 −50.0 B0.0001
1.190.3 1.190.3
88 3.8
HDL-C (mmol/l) ns
VLDL-C (mmol/l) 88 1.791.2 0.990.6 −33.1 B0.0001
1.3690.22 1.2990.20
88 −4.6
Apo A1 (g/l) B0.0001
1.4490.29 0.8890.17
Apo B (g/l) 88 −37.4 B0.0001
7.5791.87 8.2192.37
88 9.4
Glucose (mmol/l) B0.001
HbA1 (%) 88 7.791.3 7.991.5 4.7 ns
242.8957.1 288.4956.6
88 22.3
Fructosamine (mmol/l) B0.0001
aBaseline=mean of qualifying and baseline visits; post-treatment=mean of Weeks 12 and 16 of treatment period at effective atorvastatin dose;
ns, not significant; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; Apo A1, apolipoprotein A1; Apo B; apolipoprotein B.
doses of 10, 20, 40 and 80 mg atorvastatin, respectively. At the same doses plasma triglyceride levels were re-duced by 13.6, 19.4, 22.2 and 52.4%, respectively. The variable effects of the 80 mg dose may be explained in part by the low sample size (n=4).
Mean values for the secondary efficacy parameters revealed that VLDL-C was significantly reduced from baseline by 33.1% from 1.791.2 mol/l to 0.990.6 mol/l (PB0.0001) (Table 4). Apolipoprotein B levels were reduced from baseline by 37.4% from 1.4490.29 g/l to 0.8890.17 g/l (PB0.0001). Patients showed a small but significant decrease in apolipoprotein A1
levels (4.6%, PB0.0001). There was a trend toward
increased plasma HDL-C (3.8%), but this did not reach significance in this patient group (PB0.06). Impor-tantly, the lack of effect on HbA1C indicates that the
patients remained under adequate diabetic control.
3.3. Safety
The safety analysis included all patients entering the
study (n=102). Adverse events that may have been
related to drug treatment were mild-to-moderate and reported by 8 (7.8%) patients. Two patients withdrew from the study because of adverse events. Only one was possibly attributable to the study drug in a patient who experienced a mild generalized rash after 4 weeks of 10
mg/day atorvastatin. A brain tumor was diagnosed
4. Discussion
Lipid abnormalities are common in patients with type 2 diabetes. However, dyslipidemias are inade-quately controlled in a substantial proportion of type 2 diabetic patients, suggesting that few achieve and sus-tain lipid goals recommended by international guideli-nes. This is a serious concern as it is well established that type 2 diabetics are at a significantly greater risk of CHD or arteriosclerotic vascular conditions than pa-tients without diabetes. The strong association of in-creased concentrations of LDL-C with coronary artery disease in diabetic patients [12] suggests that physicians should adopt a more aggressive lipid-lowering strategy in this patient population. This approach is supported by a recent Finnish population-based study which has demonstrated that type 2 diabetic patients without a history of myocardial infarction have a similar progno-sis to non-diabetic patients with a prior myocardial infarction [9].
Statins reduce hepatic cholesterol synthesis by inhibi-tion of HMG-CoA reductase, a major enzyme involved in synthesizing cholesterol in the liver. Consequent depletion of hepatic cholesterol results in up-regulation of LDL receptors and increased hepatic removal of LDL-C. The long half-life of atorvastatin compared with other statins results in impressive cholesterol-low-ering efficacy. Atorvastatin also significantly reduces VLDL-C, increased levels of which are one of the characteristics of diabetic dyslipidemia.
In non-diabetic populations, previous studies have demonstrated that atorvastatin is a highly effective and safe drug for the treatment of dyslipidemia. In the CURVES study treatment with atorvastatin 10 – 40 mg resulted in significantly greater reductions in plasma LDL-C than simvastatin, pravastatin, lovastatin, and fluvastatin [20]. In addition, a study comparing the cost-effectiveness of statin therapies showed that signifi-cantly greater proportions of patients achieve their NCEP LDL-C targets with atorvastatin [21]. In the study of patients with CHD, or risk factors for CHD, 90% of atorvastatin-treated patients reached their NCEP goal compared with 79% for simvastatin and lovastatin, and 55% for fluvastatin-treated patients
(PB0.05) at median doses of 10, 40, 80 and 40 mg,
respectively [21].
In the current study treatment with atorvastatin re-sulted in a mean reduction in LDL-C of 50% across all of the doses (PB0.0001). In the majority of patients (59%), target LDL-C levels of 52.6 mmol/l (100 mg/ dl) were achieved at the starting dose of 10 mg atorvastatin.
These data support the findings of previous studies [15,16]. A retrospective, pooled analysis of 21 studies has demonstrated that atorvastatin 10 mg/day reduced
LDL-C by 36% in type 2 diabetic patients (n=156)
[22]. Triglyceride and apolipoprotein B levels were also reduced by 21 and 29%, respectively. The authors of this pooled analysis concluded that atorvastatin can be used effectively in the diabetic patient population [22]. An open-label study has directly compared the efficacy parameters of atorvastatin 10 mg versus simvastatin 10 mg over a 4-week period in 25 type 2 diabetic patients [6]. Atorvastatin led to significantly greater reductions in total cholesterol (−30 vs. −24%) and LDL-C (− 39 vs. −30%) compared with simvastatin [6].
Analysis of secondary efficacy parameters has
demonstrated that atorvastatin caused a 33% reduction in plasma VLDL-C. Atorvastatin also markedly re-duced triglyceride levels by 18%. In previous studies atorvastatin has been shown to have significantly greater triglyceride-lowering properties than other statins at starting doses (23 – 25). In the three, 1-year, double-blind clinical studies comparing the effects of atorvastatin (10 mg/day) versus either lovastatin (20 mg/day), pravastatin (20 mg/day) or simvastatin (10 mg/day) in patients with primary hypercholesterolemia, atorvastatin resulted in a significantly greater reduction in triglycerides: −16 versus −8% [23], −17 versus −9% [24] and −23% versus −15% [25], respectively. As hypertriglyceridemia is one of the most common lipid imbalances in type 2 diabetic patients, atorvastatin may therefore offer greater benefits than other statins in this patient group.
The decrease in triglycerides may be explained by a reduction in both VLDL- and LDL-triglycerides. The data suggest that atorvastatin not only reduces the number of LDL particles but also decreases VLDL secretion. Other mechanisms to explain the triglyceride reduction are unlikely as the statins do not affect the activity of the lipolytic enzymes or the remnant recep-tor activity [26]. This interpretation is in agreement with a recent report in which atorvastatin decreased
both plasma cholesterol in familial
The safety analysis of this study demonstrates that atorvastatin has a good tolerability profile [22,6]. Ad-verse events reported that were potentially related to drug use were all mild-to-moderate in intensity with headache and inferior extremity pain being the most common. The incidence of adverse events was unrelated to dosage. Only one patient withdrew from the study due to a possible atorvastatin-related adverse event. No patients in this study experienced statistically relevant elevations of CPK. Furthermore, atorvastatin did not effect glycemic control in these patients. Although a small but statistically significant increase in fasting glu-cose was observed, the increase was not dose-related and there was no increase in HbA1C.
Although the most frequent lipoprotein abnormali-ties in this type of diabetes are an increase in triglyce-ride-rich lipoproteins and a decrease in high-density lipoproteins, hypercholesterolemia is as powerful a pre-dictor of CHD risk in diabetic patients as in non-dia-betic subjects. In spite of this knowledge, there is to date no solid evidence to indicate whether correction of dyslipoproteinemia in order to reduce CHD risk in patients with type 2 diabetes is more, equally, or less beneficial than it is in non-diabetic subjects [31]. The only currently available data come from post-hoc sub-group analyses of the Helsinki Heart Study [32] and the Scandinavian Simvastatin Survival Study [7].
Long-term morbidity and mortality studies with ator-vastatin have not yet been completed. However, in order to investigate the benefits of aggressive lipid-low-ering in the prevention of CHD in patients with type 2 diabetes, several clinical endpoint trials are underway. The Atorvastatin Study for the Prevention of Coronary Heart Disease Endpoints in patients with non-insulin-dependent diabetes mellitus (ASPEN) will compare treatment with once-daily atorvastatin 10 mg versus placebo over 4 years. Centers in the USA, Canada, Europe, South Africa and Australia will study approxi-mately 2250 diabetics. In the UK, the Collaborative AtoRvastatin Diabetes Study (CARDS) will follow 2100 diabetic patients without a history of myocardial infarction who are being treated with either once-daily atorvastatin 10 mg or placebo over a 5-year period. Lipid entry criteria for both ASPEN and CARDS are LDL-C 54.1 mmol/l (160 mg/dl) and triglycerides
56.8 mmol/l (600 mg/dl).
In conclusion, atorvastatin is a well tolerated and very efficacious member of the statin class of drugs. In this trial significant reductions in total cholesterol (34%), LDL-C (47%) and apolipoprotein B (34%) were achieved with the lowest dose of atorvastatin (10 mg/ day). The 10 mg dose lowered LDL-C to 52.6 mmol/l (100 mg/dl) in 59% of patients. Even greater reductions are achievable with larger doses. These results clearly show that atorvastatin will enable the majority of type 2 diabetes hyperlipidemic patients to achieve primary
and secondary prevention goals for lipid reduction without the need for complex regimens of several drugs. In type 2 diabetes patients with a baseline LDL-C ofB5.2 mmol/l (200 mg/dl), atorvastatin 10 mg/day will allow the majority of patients to achieve the goals of an aggressive lipid-lowering therapy. The current study demonstrates that the beneficial effects of ator-vastatin can be extended to include type 2 diabetes patients. All patients who completed the 16-week trial attained the target LDL-C level of 52.6 mmol/l (100 mg/dl). The majority of patients (59%) achieved this level at the 10 mg starting dose of atorvastatin. At this dose atorvastatin was also effective at reducing triglyce-rides by 13.6% and VLDL-C by 29.3%. The lipid-lower-ing effects achieved at the 10 mg dose confirm that atorvastatin is a very useful agent for the treatment of lipid abnormalities in patients with type 2 diabetes.
References
[1] Vinik AI, Colwell JA. Effects of gemfibrozil on triglyceride levels in patients with NIDDM. Diab Care 1993;16(1):37 – 44. [2] Manzato E, Zambon A, Lapolla A, Zambon S, Braghetto L,
Crepaldi G, Fedele D. Lipoprotein abnormalities in well-treated Type II diabetic patients. Diab Care 1993;16(2):469 – 75. [3] American Diabetes Association. Clinical Practice
Recommenda-tions 1998. Diabetes Care 1998;21 (Suppl 1) 179 – 182. [4] Consensus Statement. Detection and management of lipid
disor-ders in diabetes. Diab. Care 1993;16 (2):106 – 112.
[5] Raskin P, Ganda OP, Schwartz S, Willard D, Rosenstock J, Lodewick PA, Cressman MD, Phillipson B, Weiner B, McGov-ern ME, et al. Efficacy and safety of pravastatin in the treatment of patients with type I or type II diabetes mellitus and hyperc-holesterolemia. Am J Med 1995;99:362 – 9.
[6] Best JD, Nicholson GC, O’Neal DN, Kotowicz MA, Tebbutt NC, Chan D-W, Sanders KM. Atorvastatin and simvastatin reduce elevated cholesterol in non-insulin dependent diabetes. Diab Nutr Med 1999;9:74 – 80.
[7] Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diab Care 1997;20(4):614 – 620.
[8] Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR. Braunwald E for the cholesterol and recurrent events trial investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. New Engl J Med 1996;335:1001 – 9.
[9] Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New Engl J Med 1998;339:229 – 34. [10] National Cholesterol Education Program (NCEP) Expert Panel:
Summary of the second report of the NCEP expert panel on detection, evaluation and treatment of high blood cholesterol (Adult treatment panel II). J Am Med Assoc 1993;209:3015 – 3023.
[11] American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diab. Care 1998;21:179 – 182.
disease in non-insulin dependent diabetes mellitus: United King-dom prospective diabetes study (UKPDS: 23). Br Med J 1998;316:823 – 8.
[13] Prihoda JS, Illingworth DR. Drug therapy of hyperlipidemia. Curr Probl Cardiol 1992;17:547 – 605.
[14] Nawrocki J, Shurzinske L, Black DM. Effectiveness of atorvas-tatin in treating patients to LDL-C goals compared with lovas-tatin, pravastatin and simvastatin. Atherosclerosis 1997;134:120. [15] Nawrocki JW, Weiss SR, Davidson MH, Sprecher DL, Schwartz SL, Lupien PJ, Jones PH, Haber HE, Black DM. Reduction of LDL cholesterol by 25 to 60% in patients with primary hyperc-holesterolemia by atorvastatin, a new HMG-CoA reductase in-hibitor. Arterioscler Thromb Vasc Biol 1995;15(5):678 – 82. [16] Schrott H, Gmerek A, Fereshetian A, Knopp RH, Bays H,
Jones PH, Littlejohn TW, McLain R, Black DM. A multicenter, placebo-controlled, dose-ranging study of atorvastatin. J Cardio-vasc Pharmacol Therapeut 1998;3(2):119 – 24.
[17] Hiar CE. Performance of the DCA 2000™ hemoglobin A1c system. Miles Diagnostic division, 1992.
[18] Friedewald WT, Levy IR, Fredrickson DS. Estimation of the concentration of low density lipoproteins cholesterol in plasma without the use of the ultracentrifuge. Clin Chem 1972;18:449 – 502.
[19] Lipid and lipoprotein analysis. In: Manual of laboratory Opera-tions: Lipid Research Clinics Program Report. Washington DC: 1974. US Department of Health, Education and Welfare publi-cation NIH 75-628, Vol 1.
[20] Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravas-tatin, lovaspravas-tatin, and fluvastatin in patients with hypercholes-terolemia (the CURVES study). Am J Cardiol 1988;81:582 – 7. [21] Koren MJ, Smith DG, Hunninghake DB, Davidson MH,
McKenney JM, Weiss SR, Schrott HG, Henley Jr RW, Tresh P, McLain RW, Bakker-Arkema RG, Black DM. The cost of reaching National Cholesterol Education Panel (NCEP) goals in hypercholesterolaemic patients. A comparison of atorvastatin, simvastatin, lovastatin and fluvastatin. Pharmacoeconomics 1998;14:59 – 70.
[22] Black DM, Bakker-Arkema R, Heinonen T, Nawrocki JW. Efficacy and safety of atorvastatin in hyperlipidemic patients with concurrent hypertension or non-insulin-dependent diabetes mellitus. Presented at the International Society of Hypertension, 7 – 11 June 1998, Amsterdam.
[23] Davidson M, McKenney J, Stein E, Schrott H, Bakker-Arkema R, Fayyad R, Black D. Comparison of one-year efficacy and safety of atorvastatin versus lovastatin in primary hypercholes-terolemia. Atorvastatin Study Group I. Am J Cardiol 1997;79(11):1475 – 81.
[24] Bertolini S, Bon GB, Campbell LM, Farnier M, Langan J, Mahla G, Pauciullo P, Sirtori C, Egros F, Fayyad R, Nawrocki JW. Efficacy and safety of atorvastatin compared to pravastatin in patients with hypercholesterolemia. Atherosclerosis 1997;130(1-2):191 – 7.
[25] Dart A, Jerums G, Nicholson G, d’Emden M, Hamilton-Craig I, Tallis G, Best J, West M, Sullivan D, Bracs P, Black D. A multicenter, double-blind, one-year study comparing safety and efficacy of atorvastatin versus simvastatin in patients with hyper-cholesterolemia. Am J Cardiol 1997;80(1):39 – 44.
[26] Schonfeld G, Aguilar-Salinas CA, Nizar E. Role of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) in fa-milial combined hypercholesterolemia. Am J Cardiol 1998;81(4A):43B – 6B.
[27] Couture P, Gaudet D, Brun D, Bergeron J, Cantin L, Black O, Gagne´ C. Effect of atorvastatin on serum levels of lipoproteins in French Canadians with homozygous or compound het-erozygous familial hypercholesterolemia. Atherosclerosis 1997;134:54 – 9.
[28] Marais AD, Naoumova RP, Firth JC, Penny C, Neuwirth CK, Thompson GR. Decreased production of low density lipoprotein by atorvastatin after apheresis in homozygous familial hyperc-holesterolemia. J Lipid Res 1997;38(10):2071 – 8.
[29] Data on file. Parke-Davis Pharmaceutical Research Division. Warner-Lambert Company, Ann Arbor, Michigan.
[30] Raal F, Pilcher G, Illingworth R, Pappu A, Stein E, Laskarzewski P, Mitchel Y, Melino M. Expanded-dose simvas-tatin is effective in homozygous familial hypercholesterolemia. Atherosclerosis 1997;135:249 – 56.
[31] Pyorala K, Steiner G. Will correction of dyslipoproteinaemia reduce coronary heart disease risk in patients with non-insulin-dependent diabetes? Need for trial evidence. Ann Med 1996;28(4):357 – 62.
[32] Manninen V, Elo MO, Frick MH, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. J Am Med Assoc 1988;260:641 – 51.