Nervous System Responses to the Cold Pressor Test
in Alzheimer’s Disease
Marcella Pascualy, Eric C. Petrie, Kayla Brodkin, Elaine R. Peskind,
Charles W. Wilkinson, and Murray A. Raskind
Background: Increased basal activity of the
hypothalam-ic–pituitary–adrenocortical (HPA) axis has been
repeat-edly demonstrated in Alzheimer’s disease (AD), and some
studies suggest increased basal activity of the sympathetic
nervous system (SNS) in this disorder; however, the effects
of AD on HPA axis or SNS responses to a standardized
aversive stressor have not been examined. The
neuroen-docrine response to aversive stress may be relevant to the
pathophysiology of AD.
Methods: Plasma adrenocorticotropic hormone (ACTH),
cortisol, norepinephrine (NE), and epinephrine responses
to a 1-min cold pressor test (CPT) were measured in nine
medically healthy AD outpatients (age 76
6
2 years) and
nine age- and gender-matched medically healthy
cogni-tively normal older subjects (age 76
6
1 year).
Results: The cortisol response to CPT was increased in
the AD group but the ACTH response did not differ
between groups. Basal NE concentrations were higher in
the AD group. Although NE responses to CPT did not
differ between groups, the blood pressure response to CPT
was higher in the AD subjects.
Conclusions: These results suggest increased HPA axis
responsiveness to CPT at the level of the adrenal cortex in
AD. The results also suggest increased basal
sympatho-neural activity and increased cardiovascular
responsive-ness to sympathoneural stimulation in AD under the
conditions of this experimental protocol. Increased SNS
stimulatory modulation of the adrenal cortex is a possible
mechanism contributing to the observed enhanced cortisol
response to CPT in these AD subjects. Biol Psychiatry
2000;48:247–254 ©
2000
Society
of
Biological
Psychiatry
Key Words: Alzheimer’s disease, cortisol, ACTH,
nor-epinephrine, nor-epinephrine, stress
Introduction
T
he activity of the
hypothalamic–pituitary–adrenocor-tical (HPA) axis and both the sympathoneural and
sympathoadrenomedullary components of the sympathetic
nervous system (SNS) may be increased in Alzheimer’s
disease (AD). If increased activity of these stress
respon-sive neuroendocrine systems does occur in AD, it could
contribute to the pathophysiology of this disorder.
Increased cortisol concentrations could impair memory
(de Quervain et al 1998; Newcomer et al 1999) and even
accelerate the neuropathology of AD by lowering the
threshold for hippocampal neuronal damage (Sapolsky
et al 1985). Increased SNS activity could contribute to
sleep disturbance, cognitive impairment, and agitation
in AD (Eisdorfer and Cohen 1978; Peskind et al 1995;
Prinz et al 1979), and high concentrations of
norepi-nephrine (NE) could exacerbate
b
-amyloid peptide
neurotoxicity in AD (Fu et al 1998).
Evidence supporting increased activity of the HPA
axis in AD is substantial and includes reports of
increased basal concentrations of cortisol in plasma,
urine, and cerebrospinal fluid (CSF; Davis et al 1986;
Hartmann et al 1997; Maeda et al 1991; Martignoni et al
1990; Masugi et al 1989; Swaab et al 1994); decreased
sensitivity of the HPA axis to feedback inhibition by
dexamethasone (Hatzinger et al 1995; Jenike and Albert
1984; Molchan et al 1990; Raskind et al 1982); and
increased cortisol (but not adrenocorticotropic hormone
[ACTH]) responses to exogenous corticotropin
releas-ing factor (CRF; Hatzreleas-inger et al 1995; Na¨sman et al
1996); physostigmine (Peskind et al 1996); a glucose
load (de Leon et al 1988); and hypoglycemia (O’Brien
et al 1994). Studies assessing effects of AD on SNS
activity are few and results inconsistent. Some studies
have reported increased basal plasma norepinephrine
From Northwest Network Mental Illness Research, Education and Clinical Center (ECP, ERP, MAR) and Geriatric Research, Education and Clinical Center (MP, KB, CWW), Veterans Affairs Puget Sound Health Care System, and the Department of Psychiatry and Behavioral Sciences, University of Washington (MP, ECP, ERP, CWW, MAR), Seattle, Washington.
Address reprint requests to Murray A. Raskind, M.D., VA Puget Sound Health Care System, Mental Health Service (S-116), 1660 S. Columbian Way, Seattle WA 98108.
Received September 20, 1999; revised February 25, 2000; accepted February 25, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
(NE; Ahlskog et al 1996; Raskind et al 1984) and
epinephrine (EPI; Peskind et al 1998) in AD, whereas
others have found no effect of AD on basal plasma
catecholamines (Vitiello et al 1993). Results of studies
addressing AD effects on SNS responses to nonaversive
pharmacologic or physical stimuli have also been
mixed. The plasma NE response to upright posture was
unaffected by AD (Vitiello et al 1993); the plasma NE and
EPI responses to the
a
-2 adrenergic antagonist yohimbine
are greater in AD than in young subjects but equivalent to
those of normal older subjects (Peskind et al 1995, 1998);
and plasma NE responses either to a mental performance
task or thyrotropin releasing hormone infusion were
blunted in AD (Borson et al 1989; Lampe et al 1989).
The effects of AD on either HPA axis or SNS responses
to a standardized aversive stressor have not been
exam-ined. Cortisol elevation in response to aversive stress may
be particularly relevant to the pathophysiology of
hip-pocampal neurodegeneration in AD. In nonhuman
pri-mates, endogenous cortisol elevations in response to
aversive stressors have been associated with hippocampal
neurodegeneration (Magarin˜os et al 1996; Sapolsky et al
1990). In contrast, we were unable to demonstrate any
neurodegenerative effects of chronic high dose exogenous
cortisol in unstressed older macaques (Leverenz et al 1999).
Here we estimated the effects of AD on both HPA axis
and SNS responses to the brief immersion of one hand in
ice water (the “cold pressor test”). The cold pressor test
(CPT) is a well-tolerated brief aversive stressor that
increases SNS and HPA axis activity by activation of both
thermal and nociceptor afferents (Bullinger et al 1984;
Ebert et al 1992; Edelson and Robertson 1986; Kelly and
Cooper 1998; Lindheim et al 1994). Because the
sympa-thoneural component of the SNS exerts stimulatory
mod-ulation of the adrenocortical response to ACTH (Bornstein
and Chrousos 1999), elevated SNS activity may contribute
to the reported increased cortisol but unaltered or reduced
ACTH response in AD to pharmacologic and metabolic
stimuli (de Leon et al 1988; Hatzinger et al 1995; Na¨sman
et al 1996; O’Brien et al 1994; Peskind et al 1996). A
stimulus like the CPT that simultaneously stimulates the
SNS and HPA axis allows an assessment of the
relation-ship between both basal and stimulated SNS activity and
the cortisol response to stress.
In subjects with AD and age-comparable cognitively
normal community volunteers, we measured plasma ACTH,
cortisol, NE, and EPI responses to the CPT. We hypothesized
plasma concentrations of these “stress hormones” (Selye
1973) in response to CPT would be increased in AD.
Methods and Materials
These studies were approved by the University of Washington Human Subjects Review Committee, Seattle, WA. Informed
consent was obtained from all cognitively normal subjects and from the legal representatives of the subjects with AD (in each case, the AD subject’s spouse). The procedure and consent forms were reviewed with all AD subjects, and all verbally agreed to the procedure and signed the consent form. Given the inherent uncertainty of a cognitively impaired AD subject’s completely comprehending the study, the AD subject’s spouse and a neutral clinician observer not affiliated with the study were present during the study to help interpret the wishes of the AD subject.
Subjects
Subjects included 9 persons with AD (4 men and 5 women, mean age5 766 2 years [mean 6 SEM] and 9 older cognitively normal persons (4 men and 5 women, mean age57661 year). All subjects were nonsmokers in good general health and had been free of psychotropic, antihypertensive, or other medications known to affect the SNS or HPA axis for at least 1 month prior to study. All were normotensive (blood pressure less than 150 mmHg systolic and 90 mmHg diastolic) and within 125% of ideal body weight (Metropolitan Life Insurance Company 1983). Body mass index (BMI) was significantly greater in cognitively normal older subjects (2661.3 SEM) than in AD subjects (236
0.5). All subjects were free of past or present major psychiatric or neurologic disorders (other than AD), alcohol or drug abuse, renal or hepatic disease, diabetes mellitus, or symptomatic cardiovascular disease. AD subjects were outpatients who met criteria for probable AD of the National Institute of Neurologic and Communicative Disorders and Stroke (McKhann et al 1984). All AD subjects were cooperative with the experimental proce-dures and free of disruptive agitation on the mornings of the study. In addition, AD subjects were selected for participation only if a careful history from the caregiver and observation of the patient by clinical personnel revealed a pattern of cooperation with the caregiver and no history of disruptive agitation, delu-sions, hallucinations or major depressive episode. It was felt that selection of such AD subjects was necessary to increase the likelihood that AD subjects would be able to complete the protocol. Normal older subjects had a Mini Mental State Exam (MMSE; Folstein et al 1975) score of 29 or 30 (maximum score530) and no history or evidence of cognitive decline. The mean MMSE score of the AD patients at prestudy screening evaluation was 1761 SEM, with a range of 8 –23.
Experimental Procedures
Studies were conducted in a clinical research unit at the VA Puget Sound Health Care System. Subjects fasted from midnight prior to study and were maintained at bed rest from 8:00 AM
throughout the study. At 8:30AM, an intravenous catheter was placed in an antecubital vein for blood sampling and kept patent with a slow normal saline drip at 50 mL/hour. At 9:05 and 9:10
CPT without difficulty. Blood samples were obtained at 5, 10, and 15 min after the beginning of hand immersion and then every 15 min for a total sampling period of 135 min. Blood pressure and heart rate were measured automatically (Dinamap, Critikon, Tampa, FL) following the baseline samples, at the end of the 1-min CPT and then following each blood sample collection. Mean arterial blood pressure (MAP) was calculated as diastolic BP plus (systolic BP minus diastolic BP divided by 3).
Chemical Assays
Blood was collected into prechilled tubes containing ethylene-bis-b-aminoethyl ether N,N1 tetra-acetic acid (EGTA) and re-duced glutathione for NE and EPI determination, and ethylene diamine-tetra-acetic acid (EDTA) for ACTH and cortisol deter-mination. Blood samples were placed on ice and cold centrifuged at 4°C within 1 hour of collection. Plasma was stored at270°C until assay. Plasma NE and EPI were determined within 1 month of sample collection by a sensitive single isotope radioenzymatic assay as previously described (Evans et al 1978). The intraassay coefficient of variation is less than 5% for both NE and EPI. The interassay coefficient is 6.5% in the greater than 300 pg/mL range and 12% in the 100 pg/mL range for NE and EPI. ACTH was measured by a double antibody sandwich radioimmunomet-ric assay (Nichols Institute Diagnostics, San Juan Capistrano, CA). The detection limit for ACTH was 2 pg/mL. Intra- and inter-assay coefficients of variation were 6.3% and 12.8%, respectively. Cortisol was measured in unextracted plasma as described previously (Wilkinson et al 1997). The detection limit for cortisol was 5 ng/mL. Intra- and inter-assay coefficients of variation were 4.6% and 10.2%, respectively. All samples from an individual subject and equal numbers of subjects from each group were assayed in a single assay.
Subjective Discomfort Assessments
Immediately following the CPT, subjects rated their subjective discomfort during the procedure using 100 mm Likert scales for pain and for anxiety.
Statistical Analysis
Differences in catecholamine, ACTH, and cortisol concentra-tions and cardiovascular parameters among groups and over time were evaluated for statistical significance by analysis of variance (ANOVA) with repeated measures. ACTH, NE, and cortisol differences from baseline to 5 min (maximum response times for ACTH and NE) or 15 min (maximum response time for cortisol) were calculated, and differences in these “deltas” between groups were analyzed by t test. MAP and heart rate differences from baseline to the 1-min sample point were calculated and analyzed in a similar manner. In addition, area under the curve (AUC) following CPT was calculated for each endocrine parameter by the trapezoidal method and compared between groups by Stu-dent’s t test. Because of a possible noradrenergic facilitatory effect on cortisol release directly at the adrenal cortex (Bornstein and Chrousos 1999), correlations were performed between both basal plasma NE and plasma NE delta and cortisol AUC.
Because some studies have suggested relationships between dementia severity and both SNS activity (Elrod et al 1997; Peskind et al 1998) and HPA axis activity (Gurevich et al 1989; Jenike and Albert 1984; Molchan et al 1990), the relationships between dementia severity and endocrine responses to CPT within AD subjects were evaluated by correlating MMSE scores with deltas for each endocrine parameter. Variables are ex-pressed as mean6SEM. Standard errors of the mean were used to facilitate graphic presentation of the data. Standard deviations can be calculated by multiplying SEMs by=n.
Results
HPA Axis Parameters
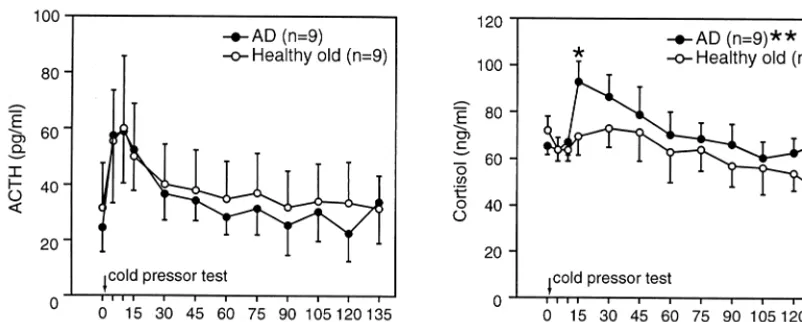
Corticotropin and cortisol concentrations are presented in
Figure 1. Corticotropin concentrations and the ACTH
response to CPT did not differ between groups. There was
a significant overall effect for time [F(11,176)
5
3.24, p
,
.001] with equivalent ACTH increases by 5 min after CPT
in both groups. The correlation between MMSE and delta
ACTH for AD subjects was not significant (r
5 2
.34).
The cortisol response to CPT was greater in AD subjects
than in normal older subjects. The cortisol AUC following
CPT was greater in AD subjects than in normal older
subjects (p
,
.05). Furthermore, only in AD subjects was
there a significant cortisol increase at 15 min following
CPT (p
,
.01). In addition, there was a significant
correlation within AD subjects between basal plasma NE
and cortisol AUC (r
5
.69, p
,
.05). The correlation
between delta NE and cortisol AUC was not significant.
Within AD subjects, there was a significant inverse
correlation between MMSE score and delta cortisol (r
5
2
.74, p
,
.05), suggesting a greater cortisol response in
the more severely demented subjects.
Catecholamines
Norepinephrine and EPI concentrations are presented in
Figure 2. Norepinephrine was significantly higher in AD
subjects [group main effect, F(1,176)
5
8.41, p
,
0.01],
but the acute NE response to CPT evident at 5 min did not
differ between groups. Within the AD subjects, the
corre-lation between MMSE score and delta NE was not
significant (r
5 2
.14). In contrast to NE, there was no
acute EPI response to CPT, nor did EPI concentrations
differ between groups [group main effect, F(1,176)
5
1.63, p
5
ns).
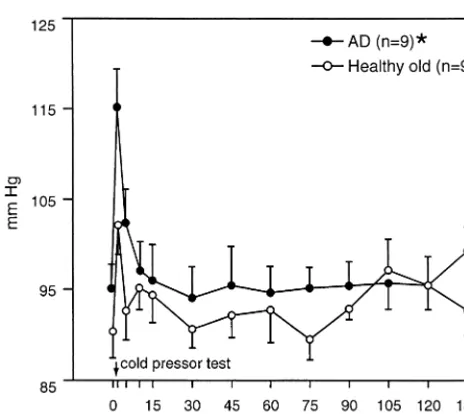
Cardiovascular Parameters: Mean Arterial
Pressure and Heart Rate
interaction [F(12,192)
5
2.20, p
5
0.01]. Heart rate
increased significantly from baseline to 1 min in AD
subjects (61
6
2 bpm to 66
6
2 bpm; t
5
4.19, p
,
.01).
Although heart rate increased in control subjects (63
6
3
bpm to 65
6
4 bpm), this increase was not significant; the
changes in heart rate from baseline to 1 min did not differ
between AD and control groups.
Subjective Discomfort Ratings
There were no significant differences between AD and
healthy older control subjects for ratings of pain (59
6
12
vs. 42
6
10) or anxiety (18
6
8 vs. 20
6
6). Within AD
subjects there were no significant correlations between
delta cortisol and either pain or anxiety ratings or between
MMSE score and either pain or anxiety ratings.
Discussion
This is the first report of the effects of AD on
neuroendo-crine responses to a standardized aversive stressor. The
effects of AD on basal hormone concentrations and
hormonal responses to CPT varied among the HPA axis,
the sympathoneural component of the SNS, and the
sympathoadrenomedullary component of the SNS. The
results provide support for increased adrenocortical but
not pituitary corticotroph responsiveness in AD to this
Figure 1. Plasma adrenocorticotropic hormone (ACTH) and cortisol responses to cold pressor test in nine Alzheimer’s disease (AD) patients and nine age- and gender-matched older normal subjects. *Greater than baseline, p,.05. **Area under the curve greater in AD than in older normal subjects, p,.05.aversive stressor. The elevated plasma NE concentrations
in AD suggested increased basal sympathoneural activity
during the experimental procedure, but these NE
eleva-tions appeared independent of the acute CPT stimulus.
HPA Axis
The enhanced adrenocortical but not pituitary corticotroph
response to CPT in AD is consistent with results of several
recent studies. Na¨sman et al (1996) and Hatzinger et al
(1995) reported enhanced cortisol but unaltered or even
reduced ACTH response to direct stimulation of the
pituitary corticotroph by exogenous CRF in AD subjects.
They also are consistent with our report of enhanced
cortisol but unaltered ACTH responses to physostigmine
infusion in AD subjects (Peskind et al 1996), and an
enhanced cortisol but blunted ACTH response in AD to
hypoglycemia (O’Brien et al 1994). The increased stress
responsiveness of cortisol but not ACTH in the current
study may have been a function of increased tonic
sym-pathoneural activity (as suggested by increased basal
plasma NE concentrations) in the AD subjects. In addition
to the primary stimulatory regulation by ACTH of cortisol
synthesis and release from the adrenal cortex, these
processes are under stimulatory modulation by
sympatho-neural innervation of the adrenal cortex (Bornstein and
Chrousos 1999). The observed positive correlation
be-tween basal NE and cortisol AUC following CPT in AD
subjects is consistent with this possibility. Studying HPA
axis responses to suprapituitary, pituitary, and
adrenocor-tical stimuli in the same subject with concurrent
evalua-tions of sympathoneural activity would clarify further the
effects of AD on HPA axis regulation.
The significant inverse correlation between the cortisol
response to CPT and MMSE score (lower MMSE
indi-cates more severe dementia) suggests greater HPA axis
response to CPT in the more advanced stages of AD, and
is consistent with several studies noting greater resistance
of the HPA axis to dexamethasone suppression in more
advanced AD subjects (Gurevich et al 1989; Jenike and
Albert 1984; Molchan et al 1990). This relationship also is
consistent with the possibility that elevated cortisol
re-sponsiveness may contribute to the pathophysiology of the
hippocampal neurodegenerative processes that contribute
to the clinical expression of AD. This latter possibility is
further supported by Lupien et al’s report of greater
hippocampal atrophy and declarative memory
deteriora-tion in AD patients whose plasma cortisol increased over
time (Lupien et al 1998), and de Leon and colleagues’
report of greater cortisol responses to oral glucose in AD
patients with more severe hippocampal atrophy (de Leon
et al 1988).
It is possible that the greater cortisol response to CPT in
AD subjects could have been a result of their cognitive
impairment, making the discomfort of the CPT a more
novel or otherwise more distressing experience. Previous
investigators have reported significant relationships
be-tween subjective pain appraisal and the cortisol response
to CPT in normal volunteers (Bullinger et al 1984);
however, the equivalent pain and anxiety ratings between
AD and control subjects in the current study argue against
a differential psychological appraisal of the CPT
account-ing for the observed differences in cortisol responses
between groups.
The reason for the absence of a significant cortisol
increase in the healthy older subjects following CPT is not
clear. Because this study was performed in the morning, a
modest cortisol response to CPT may have been obscured
by the decline in cortisol concentrations that occurs in the
morning as part of the normal plasma cortisol diurnal
rhythm (Wilkinson 1989). Cortisol measurements during a
resting morning condition without CPT would have been
necessary to evaluate this possibility.
Sympathetic Nervous System
Plasma NE concentrations were higher in AD subjects
throughout the experimental protocol, but the NE
re-sponses to CPT did not differ between groups. These
findings are consistent with increased basal
sympathoneu-ral activity but unaltered responsiveness to CPT. We
previously reported increased resting plasma NE
concen-trations in AD subjects with advanced dementia (Elrod et
Figure 3. Mean arterial blood pressure (MAP) responses to coldal 1997; Raskind et al 1984) and others have reported
increased resting plasma NE in AD subjects with mild to
moderate dementia (Ahlskog et al 1996).
Body mass index, a measure of overall adiposity, was
greater in the normal older subjects than in the AD
subjects. In healthy persons, BMI is positively correlated
with both sympathetic nerve discharge and urinary NE
excretion (Scherrer et al 1994; Troisi et al 1991; Ward et
al 1996). The effect of AD on plasma NE might have been
even greater if BMI had been equal between groups.
In a separate sample of AD outpatient subjects studied
in our laboratory, basal and yohimbine-stimulated plasma
dihydroxyphenylacetic
acid
(DOPA)
concentrations
tended to be higher in AD than nondemented older
comparison subjects (Raskind et al 1999). Because plasma
DOPA provides an estimate of NE biosynthesis (Goldstein
et al 1987; Kvetnansky et al 1992) these plasma DOPA
data suggest a tendency toward increased NE biosynthesis
in AD subjects during the conditions of that study. Using
another approach to assess SNS activity, Aharon-Peretz et
al (1992) demonstrated that power spectrum analysis of
electrocardiographic recordings suggested increased
car-diac sympathetic stimulation in AD. The higher MAP
response to CPT in these AD subjects leaves open the
possibility of a subtly enhanced sympathoneural response
to CPT that was not detectable using plasma NE
measure-ments or a greater end organ responsiveness to
sympatho-neural stimulation. NE kinetic studies as well as
concur-rent measurements of plasma NE together with its
precursor DOPA and its intraneuronal metabolite
dihy-droxyphenylglycol (which provides an estimate of NE
turnover [Li et al 1983; Eisenhofer et al 1992]) would
provide a more comprehensive assessment of AD effects
on basal and stress responsive sympathoneural activity
(Esler et al 1988).
Other studies are not consistent with increased basal
sympathoneural activity in AD. Vitiello et al (1993) found
no difference in plasma NE or EPI concentrations between
a large sample of AD subjects and age-comparable
non-demented comparison subjects, and reduced systolic blood
pressure responses to upright posture in AD subjects,
particularly those manifesting depressive symptoms. The
discrepancies between the current data and the findings of
Vitiello et al (1993) possibly reflect differences in
exper-imental settings. Their AD subjects (but not their older
healthy comparison subjects) were residents of the
exper-imental unit in which the study was conducted, and
perhaps had become acclimated to this environment. In
contrast, the subjects in the current study came to an
unfamiliar research setting directly from home.
Further-more, they were informed as part of the consent process
that the CPT was likely to be uncomfortable, albeit for a
brief period of time. The higher plasma NE concentrations
in the AD subjects participating in the current study may
have reflected their greater SNS responsiveness to a novel
setting or greater arousal in anticipation of an aversive
stimulus.
These results suggest increased responsiveness in AD of
several classic stress-sensitive neuroendocrine systems to
at least some types of stressors. Aversive physical stimuli
and/or encountering an unfamiliar environment may be
types of stressors to which persons with AD are
particu-larly responsive. If these results can be confirmed in larger
numbers of AD subjects, they could have implications for
the management of AD (Brady et al 1991). For example,
several antidepressant drugs have been demonstrated in
preclinical studies to reduce the HPA axis response to
stress (Barden 1999; Dellbende et al 1991) and the
associated aversive stress-induced hippocampal pyramidal
cell dendritic atrophy (Magarin˜os et al 1999). If the
hypothesized involvement of enhanced neuroendocrine
responses to stress in the pathophysiology of AD
(Sapol-sky 1992) can be confirmed, clinical trials to assess the
effects of drugs that reduce these responses on AD
symptomatology and progression would be warranted.
This investigation was supported by the Department of Veterans Affairs, Washington, District of Columbia; Grants Nos. AG08419 and AG05136 from the National Institutes of Health, Bethesda, Maryland; and the Alhadeff Alzheimer’s Research Fund, Seattle, Washington.
The authors thank Susan Martin for manuscript preparation.
References
Aharon-Peretz J, Harel T, Revach M, Ben-Haim SA (1992): Increased sympathetic and decreased parasympathetic cardiac innervation in patients with Alzheimer’s disease. Arch Neurol 49:919 –922.
Ahlskog JE, Uitti RJ, Tyce GM, O’Brien JF, Peterson RC, Kokmen E (1996): Plasma catechols and monoamine oxidase metabolites in untreated Parkinson’s and Alzheimer’s dis-eases. J Neurol Sci 136:162–168.
Barden N (1999): Regulation of corticosteroid receptor gene expression in depression and antidepressant action. J
Psychi-atr Neurosci 24:25–39.
Bornstein SR, Chrousos GP (1999): Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: Neural and immune inputs. J Clin Endocrinol Metab 84:1729 –1736.
Borson S, Barnes RF, Veith RC, Halter JB, Raskind MA (1989): Impaired sympathetic nervous system response to cognitive effort in early Alzheimer’s disease. J Gerontol 44:M8 –M12. Brady LS, Whitfield HJ Jr, Fox RJ, Gold PW, Herkenham M (1991): Long-term antidepressant administration alters corti-cotropin-releasing hormone, tyrosine hydroxylase, and min-eralocorticoid receptor gene expression in rat brain: therapeu-tic implications. J Clin Invest 87:831– 837.
relationships to subjective pain appraisal and coping.
Psychi-atry Res 12:227–233.
Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathe AA, Johns CA, Horvath TB (1986): Cortisol and Alzheimer’s disease I. Basal studies. Am J Psychiatry 143:300 –305. de Leon MJ, McRae T, Tsai JR, George AE, Marcus DL,
Freedman M, et al (1988): Abnormal cortisol response in Alzheimer’s disease linked to hippocampal atrophy. Lancet 8:391–392.
Dellbende C, Contesse V, Mocaer E, Kamoun A, Vaudry H (1991): The novel antidepressant, tianeptine, reduces stress-evoked stimulation of the hypothalamo-pituitary-adrenal axis.
Eur J Pharmacol 202:391–396.
de Quervain DJ, Roozendaal B, McGaugh JL (1998): Stress and glucocorticoids impair retrieval of long-term spatial memory.
Nature 394:787–790.
Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ (1992): Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol 263:H798 –H803. Edelson JT, Robertson GL (1986): The effect of the cold pressor
test on vasopressin secretion in man.
Psychoneuroendocrinol-ogy 11:307–316.
Eisdorfer C, Cohen D (1978): Autonomic activity in senile dementia of the Alzheimer type: A pilot study. In: Katzman R, Terry RD, Bick KL, editors. Alzheimer’s Disease, Senile
Dementia and Related Disorders. New York: Raven, 233–
240.
Eisenhofer G, Esler MD, Meredith IT, Dart A, Cannon RO, Quyyumi AA, et al (1992): Sympathetic nervous function in human heart as assessed by cardiac spillover of dihydroxy-phenylglycol and norepinephrine. Circulation 8:1775–1785. Elrod R, Peskind ER, DiGiacomo L, Brodkin K, Veith RC,
Raskind MA (1997): Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine. Am J Psychiatry 154: 25–30.
Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, et al (1988): Assessment of human sympathetic nervous system activity from measurements of norepinephrine turn-over. Hypertension 11:3–20.
Evans MI, Halter JB, Porte D (1978): Comparison of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem 24:567–570. Folstein MF, Folstein SE, McHugh PR (1975): Mini-Mental
State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189 –198. Fu W, Luo H, Parthasarathy S, Mattson MP (1998):
Cat-echolamines potentiate amyloid beta-peptide neurotoxicity: Involvement of oxidative stress, mitochondrial dysfunction, and perturbed calcium homeostasis. Neurobiol Dis 5:229 – 243.
Goldstein DS, Udelsman R, Eisenhofer G, Keiser HR, Kopin IJ (1987): Neuronal source of plasma dihydroxyphenylalanine.
J Clin Endocrinol Metab 64:856 – 861.
Gurevich D, Siegel B, Dumlao M, Perl E, Chaitin P, Bagne C, et al (1989): The relationship between cognitive impairment, plasma cortisol levels and HPA responsivity to dexametha-sone in dementia. Prog Clin Biol Res 317:175–187. Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I
(1997): Twenty-four hour cortisol release profiles in patients
with Alzheimer’s and Parkinson’s disease compared to nor-mal controls: Ultradian secretory pulsatility and diurnal vari-ation. Neurobiol Aging 18:285–289.
Hatzinger M, Z’brun A, Hemmeter U, Seifritz E, Baumann F, Holsboer-Trachsler E, et al (1995): Hypothalamic-pituitary-adrenal system function in patients with Alzheimer’s disease.
Neurobiol Aging 16:205–209.
Jenike MA, Albert MS (1984): The dexamethasone suppression test in patients with presenile and senile dementia of the Alzheimer’s type. J Am Geriatr Soc 32:441– 444.
Kelly CB, Cooper SJ (1998): Plasma norepinephrine response to a cold pressor test in subtypes of depressive illness.
Psychi-atry Res 81:39 –50.
Kvetnansky R, Armando I, Weise VK, Holmes C, Fukuhara K, Deka-Starosta A, et al (1992): Plasma DOPA responses during stress: dependence on sympathoadrenal activity and tyrosine hydroxylation. J Pharmacol Exp Ther 261:899 –909. Lampe TH, Veith RC, Plymate RC, Risse SC, Kopeikin H, Cubberley L, et al (1989): Pressor, norepinephrine, and pituitary responses to two TRH doses in Alzheimer’s disease and normal older men. Psychoneuroendocrinology 14:311– 320.
Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER (1999): Effect of chronic high-dose cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci 19:2356 –2361.
Li PP, Warsh JJ, Godse DD (1983): Rat brain norepinephrine metabolism: Substantial clearance through 3,4-dihydroxyphe-nylethyleneglycol formation. J Neurochem 41:1065–1071. Lindheim SR, Legros RS, Morris RS, Wong IL, Tran DQ, Vijod
MA, et al (1994): The effect of progestins on behavioral stress responses in postmenopausal women. J Soc Gynecol Investig 1:79 – 83.
Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, et al (1998): Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1:69 –73.
Maeda K, Tanimoto K, Terada T, Shintani T, Kakigi T (1991): Elevated urinary free cortisol in patients with dementia.
Neurobiol Aging 12:161–163.
Magarin˜os A, Deslandes A, McEwen BS (1999): Effects of antidepressants and benzodiazepam treatments on the den-dritic structure of CA3 pyramidal neurons after chronic stress.
Eur J Pharmacol 371:113–122.
Magarin˜os AM, McEwen BS, Flugge G, Fuchs E (1996): Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci16:3534 –3540.
Martignoni E, Petraglia F, Costa A, Bono G, Genazzani AR, Nappi G (1990): Dementia of the Alzheimer type and hypothalamus-pituitary-adrenocortical axis: Changes in cere-brospinal fluid corticotropin releasing factor and plasma cortisol levels. Acta Neurol Scand 81:452– 456.
Masugi F, Ogihara T, Sakaguchi K, Otsuka A, Tsuchiya Y, Morimoto S, et al (1989): High plasma levels of cortisol in patients with senile dementia of the Alzheimer’s type.
Meth-ods Find Exp Clin Pharmacol 11:707–710.
Metropolitan Life Insurance Company Tables (1983): New
York: Metropolitan Life Insurance Company.
Molchan SE, Hill JL, Mellow AM, Lawlor BA, Martinez R, Sunderland T (1990): The dexamethasone suppression test in Alzheimer’s disease and major depression: Relationship to dementia severity, depression and CSF monoamines. Int
Psychogeriatr 2:99 –122.
Na¨sman B, Olsson T, Fagerlund M, Eriksson S, Viitanen M, Carlstro¨m K (1996): Blunted adrenocorticotropin and increased adrenal steroid response to human corticotropin-releasing hor-mone in Alzheimer’s disease. Biol Psychiatry 39:311–318. Newcomer JW, Selke G, Melson AK, Hershey T, Craft S,
Richards K, et al (1999): Decreased memory performance in healthy humans induced by stress-level cortisol treatment.
Arch Gen Psychiatry 56:527–533.
O’Brien JT, Schweitzer I, Ames D, Mastwyk M, Colman P (1994): The function of the hypothalamic-pituitary-adrenal axis in Alzheimer’s disease. Br J Psychiatry 165:650 – 657. Peskind ER, Elrod R, Dobie DJ, Pascualy M, Petrie E, Jensen C,
et al (1998): Cerebrospinal fluid epinephrine in Alzheimer’s disease and normal aging. Neuropsychopharmacology 19: 465– 471.
Peskind ER, Raskind MA, Wingerson D, Pascualy M, Thal LJ, Dobie DJ, et al (1996): Hypothalamic-pituitary-adrenocorti-cal axis responses to physostigmine: Effects of Alzheimer’s disease and gender. Biol Psychiatry 40:61– 68.
Peskind ER, Wingerson D, Murray S, Pascualy M, Dobie DJ, Le Corre P, et al (1995): Effects of Alzheimer’s disease and normal aging on cerebrospinal fluid norepinephrine responses to yohim-bine and clonidine. Arch Gen Psychiatry 52:774 –782. Prinz P, Halter J, Benedetti C, Raskind M (1979): Circadian
variation of plasma catecholamines in young and old men: Relation to rapid eye movement and slow wave sleep. J Clin
Endocrinol 49:300 –304.
Raskind M, Peskind E, Rivard M-F, Veith R, Barnes R (1982): Dexamethasone suppression test and cortisol circadian rhythm in primary degenerative dementia. Am J Psychiatry 139:1468 –1471.
Raskind MA, Peskind ER, Halter JB, Jimerson DC (1984): Norepinephrine and MHPG levels in CSF and plasma in Alzheimer’s disease. Arch Gen Psychiatry 41:343–346.
Raskind MA, Peskind ER, Holmes C, Goldstein DS (1999): Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzhei-mer’s disease. Biol Psychiatry 46:756 –765.
Sapolsky RM (1992): Stress, the Aging Brain, and the
Mecha-nisms of Neuronal Death. Cambridge, MA: MIT Press.
Sapolsky RM, Krey LC, McEwen BS (1985): Prolonged glu-cocorticoid exposure reduces hippocampal neuron number: Implications for aging. J Neurosci 5:1221–1226.
Sapolsky RM, Uno H, Rebert CS, Finch CE (1990): Hippocam-pal damage associated with prolonged glucocorticoid expo-sure in primates. J Neurosci 10:2897–2902.
Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P (1994): Body fat and sympathetic nerve activity in healthy subjects. Circulation 89:2634 –2640.
Selye H (1973): The evolution of the stress concept. Am Scientist 61:692– 699.
Swaab DF, Raadsheer FC, Endert E, Hofman MA, Kamphorst W, Ravid R (1994): Increased cortisol levels in aging and Alzheimer’s disease in postmortem cerebrospinal fluid.
J Neuroendocrinol 6:681– 687.
Troisi RJ, Weiss ST, Parker DR, Sparrow D, Young JB, Landsberg L (1991): Relation of obesity and diet to sympa-thetic nervous system activity. Hypertension 17:669 – 677. Vitiello B, Veith RC, Molchan SE, Martinez RA, Lawlor BA,
Radcliffe J, et al (1993): Autonomic dysfunction in patients with dementia of the Alzheimer’s type. Biol Psychiatry 34:428 – 433.
Ward KD, Sparrow D, Landsberg L, Young JB, Vokonas PA, Weiss ST (1996): Influence of insulin, sympathetic nervous system activity, and obesity on blood pressure: The Norma-tive Aging Study. J Hypertens 14:301–308.
Wilkinson CW (1989): Endocrine rhythms and the pineal gland. In: Patton HD, Fuchs AF, Hille B, Scher AM, Steiner RA, editors. Textbook of Physiology, Vol 2, 21st ed. Philadelphia: Saunders, 1239 –1261.