Growth, soluble carbohydrates, and aloin concentration of
Aloe
6
era
plants exposed to three irradiance levels
Alejandra Paez
a, G. Michael Gebre
b, Maria E. Gonzalez
a,
Timothy J. Tschaplinski
c,*
aLaboratorio de Ecofisiologia.Dept.Biologia,Facultad de Ciencias,Uni6ersidad del Zulia,Maracaibo,Venezuela bDepartment of Bioagricultural Sciences and Pest Management,Colorado State Uni6ersity,Fort Collins,CO80523-1177,USA
cEn6ironmental Sciences Di6ision,Oak Ridge National Laboratory,P.O.Box2008,Oak Ridge,TN37831-6422,USA Received 17 June 1999; received in revised form 6 June 2000; accepted 8 June 2000
Abstract
Research was conducted on Aloe6era, a traditional medicinal plant, to investigate the effects of light on growth, carbon allocation, and the concentrations of organic solutes, including soluble carbohydrates and aloin. The plants were vegetatively propagated and grown under three irradiances: full sunlight, partial (30% full sunlight), and deep shade (10% full sunlight) for 12 – 18 months. After 1 year of growth, five plants from each treatment were harvested to determine total above- and below ground dry mass. Four plants from the full sunlight and the partial shade treatments were harvested after 18 months to assess the soluble carbohydrate, organic acid and aloin concentrations of the clear parenchyma gel and the yellow leaf exudate, separately. Plants grown under full sunlight produced more numerous and larger axillary shoots, resulting in twice the total dry mass than those grown under partial shade. The dry mass of the plants grown under deep shade was 8.6% that of plants grown under full sunlight. Partial shade increased the number and length of leaves produced on the primary shoot, but leaf dry mass was still reduced to 66% of that in full sunlight. In contrast, partial and deep shade reduced root dry mass to 28 and 13%, respectively, of that under full sunlight, indicating that carbon allocation to roots was restricted under low light conditions. When plants were sampled 6 months later, there were only minor treatment effects on the concentration of soluble carbohydrates and aloin in the leaf exudate and gel. Soluble carbohydrate concentrations were greater in the gel than in the exudate, with glucose the most abundant soluble carbohydrate. Aloin was present only in the leaf exudate and higher irradiance did not induce a higher concentration. Limitation in light availability primarily affected total dry mass production and allocation, without substantial effects on either primary or secondary carbon metabolites. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Aloe6era; Aloin; Carbon allocation; Partitioning; Irradiance; Carbohydrates
www.elsevier.com/locate/envexpbot
* Corresponding author. Tel.: +1-865-5744597; fax: + 1-865-5769939.
E-mail address:[email protected] (T.J. Tschaplinski).
1. Introduction
Aloe 6era, a member of the Liliaceae plant family, is a common crop on Margarita Island, Venezuela, and a wild species in other xerophytic regions of Venezuela (Hoyos, 1985). A. 6era gel has been used as a traditional medicine to induce wound healing, and as an anti-cancer, and anti-vi-ral agent (Maze et al., 1997). Some of the medici-nal properties of aloe have been attributed to aloin, also known as barbaloin, a C-glycoside derivative of anthraquinone (Reynolds, 1985). Al-though aloe is widely cultivated and valued for its medicinal properties (Grindlay and Reynolds, 1986; Maze et al., 1997), few studies have been conducted to determine the effects of various growing conditions on plant dry mass and the production of aloin.
An increase in leaf thickness of aloe plants with moisture and a corresponding increase in gel production have been reported (Genet and van Schooten, 1992). McCarthy and van Rheede van Oudtshoorn (1966) reported seasonal varia-tion in the concentravaria-tion of aloin from leaf exu-date of two aloe species in South Africa. The concentration of aloin increased from winter to summer and the authors suggested that this change may have been due to temperature-in-duced changes in metabolic processes. Since light regimes influence growth and physiological responses of all plants (Nobel, 1976; Givnish, 1988), the growth response of A. 6era and its aloin production may also be influenced by light.
The present study was undertaken to deter-mine the effect of irradiance on growth, carbon allocation (distribution), and carbon partitioning (chemical fractionation) of assimilated carbon into soluble carbohydrates, organic acids and aloin in tissues of aloe. Leaves acclimated to high irradiance levels generally have higher pho-tosynthetic rates (Givnish 1988). It has also been suggested that high irradiation can lead to higher concentrations of phenolic compounds as a result of the increased carbon production (Shure and Wilson 1993). We hypothesized that the highest concentrations of aloin would be achieved under the high irradiance given the
in-creased availability of carbon precursors and stimulation of secondary carbon metabolism. The study specifically addressed the main effects of shading to evaluate the growth potential of
A. 6era in response to light and its potential to produce aloin in relation to soluble carbohy-drate availability.
2. Material and methods
The research was conducted under field condi-tions in an area adjacent to the Facultad de Ciencias, Zulia University, Maracaibo, Venezuela. A total of 60 A. 6era L. plants were vegetatively propagated in 30 kg plastic pots and grown under three irradiance regimes: full sunlight, partial (30% full sunlight) and deep shade (10% full sunlight). The irradiance re-ceived by the plants on a typical sunny day was determined with a LiCor Li-188b radiometer (LiCor, Lincoln, NE). The range of readings av-eraged between 1340 and 1860 mmol m−2 s−1
(full sunlight), 420 – 670 mmol m−2 s−1 (partial
shade), and 130 – 190 mmol m−2 s−1 (deep
shade). Plants were irrigated every morning with tap water. Nutrients were applied at a rate of 100 kg N, 50 kg P, and 50 kg K ha−1 every 3
months.
After 1 year of growth, destructive and non-destructive measurements were conducted during the summer (July). The non-destructive measure-ments included the number, width and length of leaves; number, width and length of lateral branches; and total shoot length. Five plants per treatment were harvested to assess dry mass production. The plants were cut at the root col-lar and separated into leaves, stems and roots. The roots were washed and all tissues dried in an oven at 60°C for 2 weeks for dry mass deter-mination.
liquid exudate, produced by the bundle sheath cells of outer margin of the leaf, has a characteris-tic smell and bitter taste (Grindlay and Reynolds, 1986). In contrast, the gel is the inner, colorless and tasteless parenchyma tissue. The exudate was separated from the clear parenchyma gel by al-lowing it to drip into a container and the gel was excised with a scalpel as a filet from the core of the leaf lamina. All samples were prepared for soluble carbohydrate, organic acid and aloin analyses, as follows. Samples were freeze dried for 48 h, weighed, and placed directly into the deriva-tizing reagent. Approximately 10 – 20 mg of dried exudate and 10 – 25 mg of gel were subjected to analysis. Soluble carbohydrates, organic acids and aloin were analyzed as trimethylsilyl derivatives by dissolving and heating samples with 2 ml of Tri-Sil ‘Z’ (Pierce Chemical, Rockford, IL) for 45 min with samples left overnight before analysis the next day (Tschaplinski et al., 1993). Samples were then analyzed using capillary gas chromatog-raphy – mass spectrometry (GC – MS), which was also used to confirm the identity of solutes mea-sured. A total of 1ml of each sample was injected into a HP 5972 GC – MS (Hewlett-Packard, Avondale, PA). Operating conditions of the GC – MS were as described elsewhere (Gebre et al., 1998). External standards of known carbohy-drates were injected to determine the concentra-tion in plant samples. The data were subjected to analysis of variance, and treatment means were compared using Student’st-tests at a significance level of P50.05. However, probabilities of
PB0.10 are reported, and those of P\0.10 are designated not significant (ns).
3. Results
3.1. Carbon production and allocation
At the end of 1 year of growth,A. 6eraplants that were exposed to partial shade (30% full sun-light) produced 27% more leaves that were 21% longer relative to the leaves of plants under full sunlight. Overall, partial shading increased shoot length (Table 1). However, plants grown under full sunlight produced wider leaves and had more and larger axillary shoots than partially shaded plants (Table 1), resulting in twice as much total dry mass as those plants grown under partial shade and more than 11× that of plants grown under deep shade (Table 2). The allocation of carbon within plants grown under full sunlight was 53% to leaves and 28% to roots. Partial shade increased allocation to leaves to 70%, but de-creased allocation to roots to 13%. A similar trend was observed under deep shade with the corresponding values being 70% for leaves and 21% for roots. Root dry mass of plants in the full sunlight treatment was 4× and 15× that of plants under partial shade and deep shade, respectively.
3.2. Carbon partitioning
3.2.1. Soluble carbohydrates and organic acids
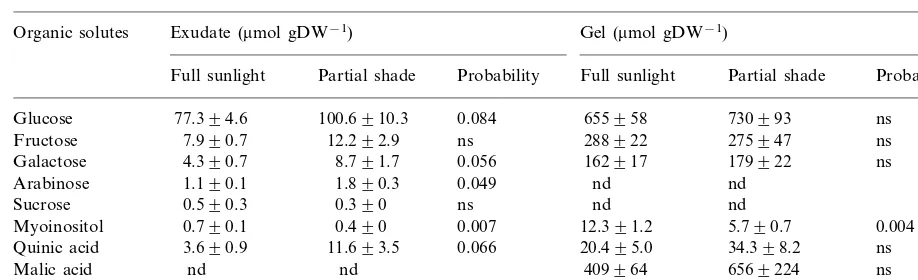
After 18 months of growth, partial shade re-duced the myoinositol concentration of the gel to 47% of that of plants grown in full sun (Table 3). Otherwise, there was no effect of shading on the concentration of the major solutes measured in
Table 2
Dry biomass ofAloe6eraplants grown under three irradiances: full sunlight, partial and deep shade (30 and 10% of full sunlight, respectively) after 1 yeara
Leaf DW (g) Stem DW (g)
Irradiance Axillary shoot DW (g) Root DW (g) Total DW (g)
12.791.3a
12.191.1a 36.293.4a 130.395.7a
69.3094.1a Full sunlight
Partial shade 46.092.8b 6.990.3b 4.190.4b 8.690.7b 65.593.8b 11.491.1c 2.490.2c
1.090.c 0c 8.090.7c
Deep shade
Table 3
Mean (9SE) concentrations of soluble carbohydrates, organic acids, and aloin ofAloe6eraplants grown under full sunlight and partial shade (30% full sunlight) treatments after 18 months (n=4 exceptn=3 for full sunlight treatment of gel)a
Exudate (mmol gDW−1)
Organic solutes Gel (mmol gDW−1)
Partial shade Probability Full sunlight
Full sunlight Partial shade Probability
Glucose 77.394.6 100.6910.3 0.084 655958 730993 ns 7.990.7
Fructose 12.292.9 ns 288922 275947 ns
8.791.7 0.056 162917
4.390.7 179922
Galactose ns
1.190.1
Arabinose 1.890.3 0.049 nd nd
0.590.3
Sucrose 0.390 ns nd nd
0.490 0.007 12.391.2
0.790.1 5.790.7
Myoinositol 0.004
11.693.5 0.066 20.495.0 34.398.2
Quinic acid 3.690.9 ns
nd 409964
nd 6569224
Malic acid ns
20079543
Aloin 20969129 ns nd nd
and, solute was not detected in the tissue analyzed.
the gel. Partial shade tended to increase the con-centrations of some of the major solutes including glucose, galactose and quinic acid. However, these changes were only significant at PB0.10. Partial shade reduced the myoinositol concentration of leaf exudate to 54% of that observed in full sun. Partial shade also increased the arabinose concen-tration of the exudate by 1.66×, but both of these changes involved minor constituents. Glu-cose was the major soluble carbohydrate mea-sured in both the exudate and the gel samples (Table 3), constituting 81 – 84% of the total solu-ble carbohydrates (excluding phenolic glucosides) in the exudate and 59 – 61% of the total in the gel. In contrast, the sucrose concentration was low, which is not surprising given the nature of the tissues analyzed (i.e. neither chlorophyll-contain-ing tissues nor conductchlorophyll-contain-ing tissues were analyzed). With the exception of aloin, most of the solutes measured were at higher concentrations in the gel than in the exudate (Table 3). For example, the concentrations of fructose and galactose were much higher (20 – 40×) in the gel than the exu-date. The main exudate constituents were aloin and glucose, followed by low concentrations of fructose, galactose, arabinose, myoinositol, and sucrose. Quinic acid was the major organic acid in the exudate, but it was detected at low concentra-tions ranging from 4 to 12 mmol gDW−1, in
contrast with the higher concentrations of 20 – 34
mmol gDW−1in the gel. Although malic acid was
not detected in the exudate, it was the major organic acid in the gel.
3.2.2. Aloin
Aloin was only detected in the exudate with no differences between sun and shade plants (Table 3). Any limitation in carbon availability due to lower light levels was largely evident in dry mass differences, rather than in the concentrations of soluble carbohydrates or secondary carbon com-pounds. The concentration of aloin in the exudate ranged from 2007 to 2096mmol gDW−1,
account-ing for 93.7 – 95.6% of the major organic solutes determined in the exudate. Although there was no significant effect of treatment, our samples showed high variability in the aloin concentration of partially shaded plants (Table 3).
4. Discussion
retention of carbon in shoots at the expense of roots. A decrease in the allocation of carbon to roots in plants grown under shade has also been reported in other species such as cogongrass (Im
-perata cylindrica) (Patterson, 1980).
Whereas glucose was the major soluble carbo-hydrate in aloe leaves, shading did not affect its concentration in the leaf gel. Christopher and Holtum (1996) also reported that glucose was the major soluble storage carbohydrate in A. 6era, peaking at 350 mmol gDW−1 at 1500 h,
fol-lowed by sucrose at 275 mmol gDW−1, and
fructose at 125 mmol gDW−1. The somewhat
higher concentrations reported for the gel in our study, relative to the concentrations reported for the whole leaf, is not unexpected because the parenchymatous gel tissue has less inactive dry residue that would dilute the concentrations ob-served. In addition to glucose accumulation, aloe is known to accumulate starch (Christopher and Holtum, 1996) and acetylated mannans (aceman-nan) as storage polysaccharides in protoplasts of parenchymatous cells (Femenia et al., 1999).
Many factors contribute to the fluctuation in the concentrations of the major organic solutes observed. Given that leaves were sampled in the late morning, the relatively high concentrations of malate were expected. Malate concentrations in aloe leaves typically peak at 06:00 h at 250
mmol gDW−1 and reach their minimum,
negligi-ble concentrations at 15:00 h (Christopher and Holtum, 1996). Again, the low amount of inactive residue of the parenchymatous tissue produce the somewhat higher concentrations in our study compared to studies where the whole leaf is ex-tracted. Our sampling occurred in the winter (dry season, daytime temperature 28 – 30°C) with leaves older than when growth measurements were taken. However, Yaron (1993) reported that although irrigation ofA.6eraaffected the concen-tration of carbohydrates, leaf age had no signifi-cant effect. Season of harvest also had no effect on dry matter content. The extract in that study contained 1% dry matter with soluble carbohy-drates constituting 0.2 – 0.3% and polysaccharides 0.1 – 0.2%.
There are few reports on the effect of shading on aloin concentration, but Chauser-Volfson and
Gutterman (1998) noted that the concentrations of aloin (barbaloin), homonataloin, and nataloin were higher in A. mutabilis plants growing in the shade than in the direct sun light. Such a report coupled with our findings suggest reduced light availability does not restrict the production of aloin or glucose, its conjugated moiety. Aloin was only detected in the exudate, which was similar to that reported by Grindlay and Reynolds (1986), with no differences between sun and shade plants. In Aloe ferox, van Wyk et al. (1995) found the contribution of aloin, aloeresin A and aloesin was 70 – 97% of total dry weight of leaf exudate with a geographical variation in the aloin content alone ranging from 9.5 to 31.2%. A difference in aloin concentration between leaves within the same plant has also been reported with the highest concentration just below the apex of the plant (younger leaves) and lowest at the base (Okamura et al., 1996; Chauser-Volfson and Gutterman, 1998). All leaves in our study were collected from the same position on each plant. The concentra-tion of aloin and other closely-related anthrone C-glycosides have also been shown to be highest in the top third of a leaf and lowest at its base in
Aloe mutabilis and A. hereroensis (Chauser-Volfson and Gutterman, 1997, 1998). There are many potential sources of variability reported in the literature. McCarthy and van Rheede van Oudtshoorn (1966) found seasonal variation in aloin concentration with an increase during the summer corresponding to an increase in tempera-ture. They also suggested that wind affects aloin production by shrinking the leaf. Park et al. (1998) reported that aloin content determined from total leaf weight (gel and exudate combined) was variable throughout the season.
5. Conclusions
in light availability primarily affected total dry mass production and carbon allocation, without substantial effects on soluble carbohydrates. There were only minimal treatment effects in or-ganic solute concentrations of exudate and the gel after 18 months growth. Soluble carbohydrates were more abundant in the gel than in the exu-date. Aloin was present only in the exudate and shading did not affect its concentration. The hy-pothesis that the higher irradiance would induce higher glucose concentrations in tissues, and con-sequently, higher aloin concentrations was not substantiated.
Acknowledgements
The authors wish to express their gratitude to CONDES (Universidad del Zulia) and CONICIT for supporting part of this research conducted in Venezuela. The research was also funded, in part, by the Bioenergy Feedstock Development Pro-gram, US Department of Energy, at Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the US Department Energy under con-tract DE-AC05-00OR22725. Publication No. 5006, Environmental Sciences Division, Oak Ridge National Laboratory. The second author was also supported by an appointment to the Oak Ridge National Laboratory Postdoctoral Re-search Associates Program administered jointly by the Oak Ridge National Laboratory and the Oak Ridge Institute for Science and Education.
References
Christopher, J.T., Holtum, J.A.M., 1996. Patterns of carbon partitioning in leaves of Crassulacean acid metabolism species during deacidification. Plant Physiol. 112, 393 – 399. Chauser-Volfson, E., Gutterman, Y, 1997. Content and distri-bution of the secondary phenolic compound homonataloin inAloe hereroensisleaves according to leaf part, position, and monthly changes. J. Arid Environ. 37, 115 – 122. Chauser-Volfson, E., Gutterman, Y, 1998. Content and
distri-bution of anthrone C-glycosides in the South African arid plant speciesAloe mutabilisgrowing in the direct sunlight and the shade in the Negev Desert of Israel. J. Arid
Environ. 40, 441 – 451.
Femenia, A., Sanchez, E.S., Simal, S., Rossello, C., 1999. Compositional features of polysaccharides fromAloe6era (Aloe barbadensisMiller) plant tissues. Carbohydr. Polym. 39, 109 – 117.
Gebre, G.M., Tschaplinski, T.J., Tuskan, G.A., Todd, D.E., 1998. Clonal and seasonal differences in leaf osmotic po-tential and organic solutes of five hybrid poplar clones grown under field conditions. Tree Physiol. 18, 645 – 652. Genet, W.B.M., van Schooten, C.A.M., 1992. Water
require-ments ofAloe6erain a dry Caribbean climate. Irrig. Sci. 13 (2), 81 – 85.
Givnish, T.J., 1988. Adaptation of sun and shade: a whole plant perspective. Aust. J. Plant Physiol. 15, 63 – 92. Grindlay, D., Reynolds, T., 1986. TheAloe6eraphenomenon:
A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 16 (2-3), 117 – 152. J. Hoyos, 1985. Flora de la Isla de Margarita. Venezuela. Soc
y Fund. La Salle de Ciencias Naturales, Monografia No. 34, Caracas, Venezuela.
Maze, G., Terpolilli, R.N., Lee, M., 1997. Aloe6eraextract prevents aspirin-induced acute gastric mucosal injury in rats. Med. Sci. Res. 25, 765 – 766.
McCarthy, T.J., van Rheede van Oudtshoorn, M.C.B., 1966. The seasonal variation of aloin in leaf juice from Aloe feroxandAloe marlothii. Planta Med. 14, 62 – 65. Nobel, P.S., 1976. Photosynthetic rates of sun versus shade
leaves ofHyptis emoryiTorr. Plant Physiol. 58, 218 – 223. Okamura, N., Asai, M., Hine, N., Yagi, A., 1996. High-per-formance liquid chromatographic determination of pheno-lic compounds in aloe species. J. Chromatogr. A. 746, 225 – 231.
Park, M.K., Park, J.H., Kim, N.Y., et al., 1998. Analysis of 13 phenolic compounds in aloe species by high performance liquid chromatogrphy. Phytochem. Anal. 9, 186 – 191. Patterson, D.T., 1980. Shading effects on growth and
parti-tioning of plant biomass in cogongrass (Imperata cylin -drica) from shaded and exposed habitats. J. Weed Sci. 28 (6), 735 – 740.
Reynolds, T., 1985. Observations on the phytochemistry of the aloe leaf exudate compounds. Bot. J. Linn. Soc. 90 (3), 179 – 200.
Shure, D.J., Wilson, L.A., 1993. Patch-size effects on plant phenolics in successional openings of the southern Ap-palachians. Ecology 74, 55 – 67.
Tschaplinski, T.J., Norby, R.J., Wullschleger, S.D., 1993. Re-sponses of loblolly pine seedling to elevated CO2 and fluctuating water supply. Tree Physiol. 13, 283 – 296. van Wyk, B.-E., van Rheede van Oudtshoorn, M.C.B., Smith,
G.F., 1995. Geographical variation in the major com-pounds ofAloe feroxleaf exudate. Planta Med. 61, 250 – 253.
Yaron, A, 1993. Characterization ofAloe6eragel before and after autodegradation, and stabilization of the natural fresh gel. Phytother. Res. 7, 11 – 13.