Brain Research 886 (2000) 224–236
www.elsevier.com / locate / bres
Interactive report
The intrinsic function of a motor system — from ion channels to
1
networks and behavior
*
´
S. Grillner , L. Cangiano, G.-Y. Hu, R. Thompson, R. Hill, P. Wallen
Nobel Institute for Neurophysiology, Department of Neuroscience, Karolinska Institutet, SE-171 77 Stockholm, Sweden Accepted 25 October 2000
Abstract
The forebrain, brainstem and spinal cord contribution to the control of locomotion is reviewed in this article. The lamprey is used as an experimental model since it allows a detailed cellular analysis of the neuronal network underlying locomotion. The focus is on cellular mechanisms that are important for the pattern generation, as well as different types of pre- and postsynaptic modulation. This experimental model is bridging the gap between the molecular and cellular level to the network and behavioral level. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Locomotion; Networks; Ion channel; Modulation; Modeling; Behavior
1. Introduction
mals also requires accurate foot placement.
Locomo-tion can also be combined with other motor tasks.
All vertebrates, without exception, depend on
locomo-tion, which often is the most complex motor behavior
Substantial information is now available concerning the
performed in a given species. It involves the coordination
neural subsystems responsible for the equilibrium control
of a large number of muscles (often more than a hundred),
[51,21] and for accurate foot placement during locomotion
each of which is coordinated in a specific motor pattern
in demanding terrain [22,36]. In this article, however, we
repeated within each locomotor cycle. In principle, three
will deal only with the neural machinery underlying
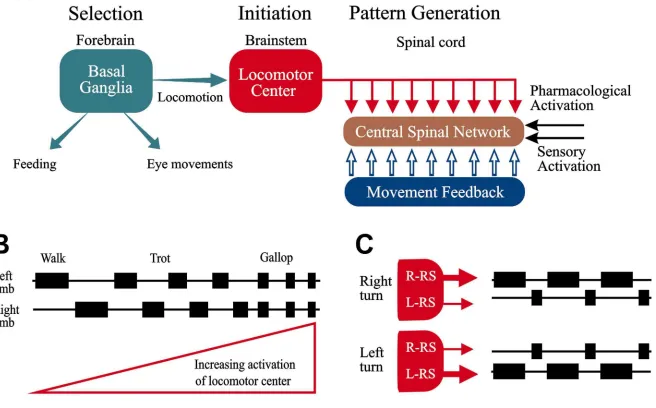
different types of control systems are involved (see Fig. 1).
propulsion.
1. The motor system which produces the propulsive
movements, be it the leg movements of tetrapods or
2. The propulsive neural control system — an
bipeds, wing movements of birds, or undulatory trunk
overview
movements of fish or snakes.
2. The postural motor system, which maintains the
The propulsive system generating the stereotypic
move-appropriate body orientation during ongoing locomo-
ments characteristic of locomotion is composed of a
tion.
supraspinal part, which is responsible for the initiation of
3. The goal directed aspect of the locomotor behavior,
locomotion and for maintaining a certain degree of drive to
which brings the animal to the goal of the locomotor
the spinal networks which generate the motor pattern
episode, while avoiding all objects that may impede
[30–32,37,64], be it swimming, walking or flying (see Fig.
the locomotion. In most cases this is achieved by
1). The spinal networks are composed of excitatory and
visuomotor coordination, which in the case of mam-
inhibitory interneurons which activate the different groups
of motoneurones in the appropriate sequence. Stimulation
of two areas in the brain stem elicits locomotion by
1
activating the spinal networks [37]. One area is located at
Published on the World Wide Web on 22 November 2000.
the meso-pontine border and referred to as the
mesence-*Corresponding author. Tel.:146-8-728-6900; fax:146-8-349-544.
E-mail address: [email protected] (S. Grillner).
phalic locomotor region MLR [64] and another area is
Grillner
et
al
.
/
Brain
Research
886
(2000
)
224
–
236
225
226 S. Grillner et al. / Brain Research 886 (2000) 224 –236
located in the ventral thalamus (lamprey [25]) or a
a simple experimentally advantageous model, which
re-corresponding area in mammals [50,65].
tains the basic vertebrate features — the lamprey CNS
Simple (e.g. 30 Hz) electrical stimulation of the
[60,35]. The lamprey, a cyclostome, diverged from the
locomotor areas at a certain strength may elicit locomotion
main vertebrate evolutionary line at a stage before
elas-by
activating
the
spinal
motor
pattern
generators
mobranchs and teleosts, and has since remained
compara-[37,64,65]. If the stimulation strength is increased (every-
tively unchanged over more than 400 million years. The
thing else being unchanged), the animal will increase the
brainstem–spinal cord can be maintained in vitro over
speed of locomotion (Fig. 1B). In a mammal, the pattern
several days (Fig. 2A), and the motor pattern underlying
of locomotion may change from slow walking to trot, and
locomotion can be evoked in the isolated nervous system.
finally gallop. In this case the step cycle of each limb will
This condition has allowed a detailed study of the neural
gradually shorten in duration, but in addition the pattern of
mechanisms of the network underlying locomotion (see
coordination between the two hind- or forelimbs will
Ref. [35]).
change from the strict alternation of walk and trot to the
approximate in phase coordination of gallop or bound. In
the case of fish swimming, the situation is simpler in that
3. The lamprey model — forebrain control
the alternating movements progressively increase in
fre-quency from low to top speed [39]. This type of brain-
Goal-directed locomotion can be elicited by visual or
stem–spinal cord organization simplifies the control task
olfactory stimuli [72,73,43]. Afferents from the optic nerve
for the brain, which only needs to decide when to locomote
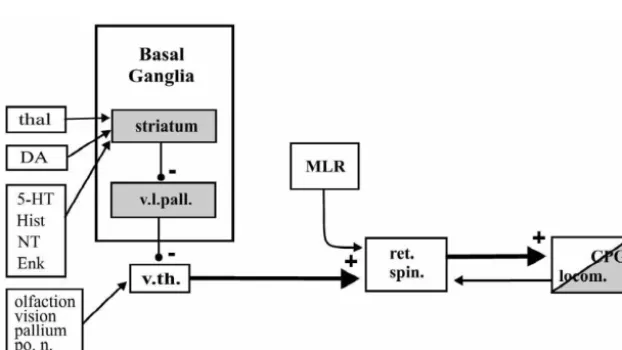
or the olfactory bulb project to the ventral thalamus (VTH
and the general level of activity, leaving the spinal cord
in Fig. 2), which in turn projects to and activates
re-network to faithfully generate the complex pattern of
ticulospinal neurons [25]. Reticulospinal neurons, in turn,
muscle activity required to generate the locomotor move-
activate
spinal
cord
locomotor
networks
[48].
An
ments.
oligosynaptic pathway activated by visual or olfactory
In legged animals, including humans, there is a separate
stimuli may then elicit behaviorally relevant locomotor
network for each limb that can be further subdivided in
activity.
network units involved in the control of single joints and
The basal ganglia represent an area that is important for
groups of muscles [31] (cf. Ref. [66]). The networks for
the control of motor behavior. The lamprey basal ganglia
the different limbs can be combined in different ways to
appear to be organized in a way similar to that of higher
produce the different gaits from walk, pace and trot to the
vertebrates [57,58] (Fig. 2). The striatum is comprised of
different types of gallops.
spiny neurons, and contains GABAergic and presumed
In all vertebrates, it appears that corresponding brain-
cholinergic neurons. It has a dense dopaminergic input
stem areas are involved in the supraspinal control of
from an area analogous to the ventral tegmental area, and
locomotion [37] — while the spinal networks have been
also 5-HT, enkephalin, galanin, tachykinin and neurotensin
adapted to the particular type of coordination of a given
inputs (Fig. 2) [57,58,7,5]. Striatal GABAergic neurons
species. For a number of years, we have chosen to work on
project to an area in the ventrolateral pallium (possibly
corresponding to the ventral pallidum of mammals). This
provide inhibition of the neurons on the contralateral side
area, in turn, provides GABAergic projections to the
(Fig. 3B; Refs. [15,16,13], and unpublished). The network
ventral thalamus. The basal ganglia may then control the
produces one half cycle of excitation in motoneurones and
output of the ventral thalamus — a type of gate control
interneurons of one side followed by one half cycle of
determining whether, for example, visual or olfactory
inhibition when the contralateral side is active [61]. Using
stimuli should or should not result in locomotor activity
biophysically realistic modelling it has been shown that
[25].
pools of model interneurons, with similar properties to
The basal ganglia appear to have a similar overall role in
those found experimentally, can produce the alternating
lamprey and mammals including man, since MPTP, a
motor pattern (see below; Ref. [41]).
‘toxin’ which destroys the DA supply to striatum, gives
rise to the same general class of symptoms in man and
lamprey. In the latter case, locomotor episodes are initiated
5. Importance of cellular properties for the pattern
more rarely, and each episode becomes much briefer.
generation
Moreover, the time lag from the initial signs of locomotor
initiation to actual effective locomotion is prolonged, and
Not only the connectivity but also the membrane
upon cessation of locomotor activity an after-discharge
properties of different cell types are of critical importance.
may be present in the trunk muscles [70]. These symptoms
Calcium currents as well as the activation of calcium
concur with the motor symptoms of Parkinson’s disease,
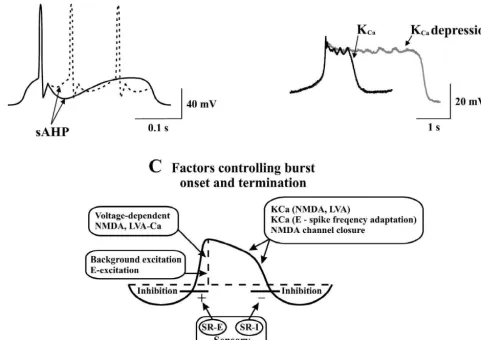
dependent potassium channels (K
) have a key role. The
which also are produced by MPTP intoxication in primates
Capost-spike afterhyperpolarisation is the main determinant
including man.
of the frequency regulation including frequency adaptation
in all network neurons (Fig. 4A; Ref. [26]). During
network activity the short range spike frequency adaptation
4. The lamprey model — brainstem–spinal cord
is one important factor. In most cases only a few spikes are
circuitry
generated within each burst. K
Cacurrents are also
im-portant in relation to other processes that cause increased
By stimulation in the locomotor areas of the lamprey
21
Ca
levels in the dendrites or cell body. These include the
brainstem, locomotor-like activity can thus be elicited in
activation of NMDA receptors which give rise to
plateau-the spinal cord [48]. The alternating segmental burst
like depolarizations (Fig. 3B; Ref. [77]). The termination
activity is coordinated along the spinal cord, generally with
of these plateaus is caused by the activation of K
a rostro-caudal phase lag, which corresponds to the
Cacurrents (Fig. 4B,C), and presumably also by an activation
coordination in the swimming lamprey in which an
21
of low voltage activated Ca
currents [67]. These lamprey
undulatory wave pushes the animal forward through the
21
neurons express Ca
channels of the N subtype, which
water (cf. Refs. [29,78]). Also in the isolated spinal cord
are mainly responsible for the activation of K
channels
locomotor coordination can be elicited by elevating the
Caunderlying the afterhyperpolarisation, and for the synaptic
excitability of the spinal cord by administering excitatory
release of transmitter [80,23,76,24].
L-channels are less
amino acid receptor agonists like NMDA, kainate, AMPA,
abundant but contribute, however, to the NMDA induced
and
D-glutamate to the bath [20,38,9] (Fig. 3A, cf. Ref.
plateau depolarizations.
[59]). Pharmacological analysis has indicated that the
Low voltage-activated (LVA) calcium channels are also
network essentially depends on excitatory glutamatergic,
present in network neurons. They are activated when the
and inhibitory glycinergic synaptic transmission [40,8]. In
cells are depolarized from a comparatively hyperpolarized
addition, a number of modulatory transmitters modify
level, and open below the threshold for the action
po-neuronal and thereby network activity, which will be dealt
21
tential. The LVA Ca
channels can thus boost the
with separately below.
membrane depolarization enabling it to reach the threshold
The spinal cord networks are thus responsible for the
for an action potential. The calcium entry through LVA
motor pattern produced. They are activated from the
21
Ca
thus provides a post-inhibitory rebound. This can
brainstem via reticulospinal axons (Figs. 2, 3B) that excite
contribute to the stability of rhythmic network activity [67]
spinal
excitatory
and
inhibitory
interneurons
and
21
motoneurones via both NMDA and AMPA / kainate re-
since in modelling experiments a blockade of LVA Ca
ceptors (cf. Ref. [35]). The reticulospinal system thus
channels may cause a change from a strict reciprocal
21
drives the spinal cord networks and determines the level of
pattern to more irregular activity. Thus, both LVA Ca
activity in a burst range from 0.2 to 10 Hz. The excitatory
channels and voltage-dependent NMDA channels may
spinal interneurons have ipsilateral axons and excite
contribute to burst stability.
motoneurones and inhibitory interneurons with ipsilateral
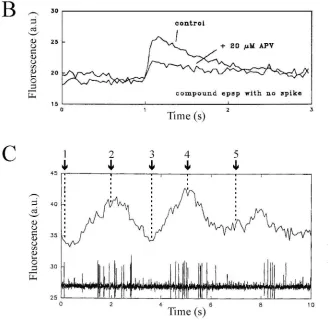
In calcium imaging experiments in the spinal cord of the
and contralateral axons [14]. In principle, the ipsilateral
lamprey [3] it was shown that in both soma and dendrites
21
excitatory interneurons excite all types of cells on the same
of the network, there is entry of Ca
ions during
21228
S
.
Grillner
et
al
.
/
Brain
Research
886
(2000
)
224
–
236
Fig. 4. Spike frequency regulation, NMDA-plateau potentials and control of burst termination. (A) The amplitude of the slow afterhyperpolarization (sAHP) will determine whether one or several action potentials will occur during the phase of synaptic excitation in locomotor cycle. A large and
21 1
long-lasting sAHP will make locomotor bursts shorter. (B) Ca -dependent K channels (K ) not only cause the sAHP but will also promote theCa
termination of NMDA-receptor induced plateau potentials. The control plateau (solid trace) is markedly prolonged in the presence of the K -channelCa
blocker apamin (dotted trace). (C) Several different factors contribute to the initiation of the depolarizing phase, its maintenance, and its termination. In addition to the cell through these channels, cause activation of K , and thereby a progressive hyperpolarization leading to closure of the NMDA channels.Ca
The initiation of the depolarizing phase is facilitated by activation of ipsilateral excitatory stretch receptor neurons(SR-E), while the termination of the depolarized phase is partially a result of activation of contralateral inhibitory stretch receptor neurons (SR-1). Abbreviation: E, excitatory interneuron.
21
occurs in both soma and dendrites and during synaptic
effects may result from the entry of Ca
. In the latter case
21activation (sub-threshold EPSPs) there are local increases
a Ca
entry occurs both via NMDA receptors and through
21of calcium in the dendrites at the synaptic region (Fig. 5B).
either low voltage activated Ca
channels or through
21 21
The Ca
entry during the glutamatergic EPSPs was due to
AMPA receptors of a subtype that is Ca
permeable. One
21 21
both NMDA channels (50%) and to LVA Ca
channels,
distinct possibility would be that Ca
activates K
Caand possibly AMPA channels (Fig. 5B). During locomotor
channels, which in that case would give rise to a local
21
activity there is a phasic Ca
entry occurring in the
conductance increase in the dendritic region where the
21dendrites in neurons, even in the absence of action
synapse is located. Since the Ca
increase outlasts the
21potentials. This synaptically driven Ca
entry peaks
EPSP with several hundreds of milliseconds, it would in
during the ipsilateral burst and reaches a minimum during
that case be expected to give rise to a shunting of
the trough (Fig. 5C).
subsequent EPSPs. If so, the first EPSP elicited by a train
21
The fact that a Ca
entry occurs in dendrites during
of action potentials in the presynaptic axon should be
ongoing locomotion, and also during stimulation of re-
expected to be larger than the subsequent EPSPs (due to
21
230
S
.
Grillner
et
al
.
/
Brain
Research
886
(2000
)
224
–
236
a significant activation of K . This appears, however, not
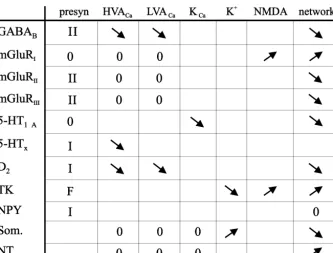
Calar, cellular, and overall network effects. Table 1 shows
to be the case, since the EPSPs remain at the same
some of the actions mediated by different modulators that
amplitude, and they are not modified if apamin-sensitive
act via different receptor subtypes. The leftmost column
K
Cachannels are blocked [18].
shows presynaptic actions. In most cases there is a
21
These results thus suggest that the Ca
entry, due to
presynaptic inhibitory effect (I), but with regard to
tach-EPSPs evoked from a reticulospinal axon does not cause a
ykinins (substance P-like peptides) synaptic transmission is
significant K
Caactivation. This may be due to either that
instead facilitated (F) [53,55]. Presynaptic interactions
21
the Ca
levels do not reach a sufficiently high con-
occur at all levels in the locomotor control system, namely
centration to activate local K
Cachannels, and / or that K
Caon sensory inputs, inhibitory and excitatory network
channels are located at some distance from the gluta-
synapses, and descending reticulospinal axons. Moreover,
21
matergic synapses, where the Ca
concentration will be
during locomotor activity there is a phase-dependent gating
too low. These findings suggest that the dendritic process-
of synaptic transmission in both interneuronal and sensory
ing is more predictable than may otherwise have been case.
axons [1,28], making synaptic transmission more effective
The question of whether the amplitude of the locomotor
in one or other phase of the locomotor cycle. Other
drive potentials in a given neuron represents just the sum
modulators (5-HT, dopamine, substance P, mGluR, CCK,
of the excitatory and inhibitory input occurring during
PYY) provide a tonic gating of synaptic transmission, for
fictive locomotion, or whether other factors such as voltage
example of glutamatergic synaptic transmission from
dependent processes in the dendrites also contribute to the
reticulospinal axons [16,44,2,53,81].
net membrane potential oscillations have been addressed
The five middle columns in Table 1 show modulator /
by Hu et al. ([42], and unpublished observations). In
receptor-mediated actions on different types of ion
chan-21 1
lampreys, the compound QX-314 causes a blockade of
nels (Ca
channel subtypes, K , K , NMDA). It is
Ca 1Na
channels present in dendrites, and at a much higher
important to note that the effects are often specific to a
21concentration (10-fold) also of Ca
channels. During
given cell type or synapse, and that it is thus unfortunately
fictive locomotion QX-314 was injected through the
not possible to extrapolate from one type of neuron to
recording microelectrode. It caused a depression by 20–
another. For example, GABA receptors mediate powerful
B30% of the peak-to-peak amplitude of the synaptic drive
presynaptic inhibition of sensory and interneuronal axons
potential (peak EPSP to peak IPSP). The most likely
but not reticulospinal axons [19,1,2]. The converse is true
explanation for this depression is that voltage dependent
for dopamine, which acts on the latter synapse [79] but not
1
Na
channels present in dendrites amplify the synaptic
on the sensory level [27]. In addition to different effects at
excitatory drive potentials recorded in the soma. The
different levels in the spinal cord, modulatory effects are in
contribution of other voltage-dependent processes can,
many cases also neuron- and synapse-specific within the
however, not be ruled out. The results show, however, that
locomotor network itself (see Refs. [53,54]). It is thus
active properties of dendrites play a significant role.
imperative that individual network neurons are identified if
a satisfactory understanding of network effects is to be
achieved.
6. Modulator systems — action on ion channels
The column to the right in Table 1 shows the overall
manifested on the network-behavioral level
network effects exerted by the different modulator
232 S. Grillner et al. / Brain Research 886 (2000) 224 –236 Table 1
a
Spinal modulation involving G-protein-coupled receptors
a
Metabotropic amino acid, aminergic and peptidergic g-protein-mediated modulation of ion channel, synaptic, cellular and network activity in the lamprey spinal cord. The table summarizes the results of a number of studies (see text as well). The effects of different transmitters and receptors on different targets are listed in the columns on the right. The presynaptic actions can be targeted to sensory afferents, excitatory or inhibitory interneurons and descending reticulospinal axons (see Refs. [1,2,27,52]). Different transmitters have selective actions on different cellular targets (I indicates presynaptic inhibition and F facilitation). The locomotor network modulates phasically, in each cycle, the synaptic transmission from sensory afferents and
1
interneurons. The modulation of HVA , LVA , K , KCa Ca Ca and NMDA channels is indicated with a downward arrow for depression and an upward arrow for facilitation (cf. Refs. [41,56,45,63,47]). Again, the effects may be specific to particular cell types. Finally, the effects on the network level have been studied on the background of locomotor activity (arrows relate to locomotion burst frequency), and in related modelling experiments [63,67–69] 5-HT, 5-hydroxytryptamine (serotonin) receptor; D , type 2 dopamine receptor; HVA, high voltage activated; mGluR, metabotropic glutamate receptor; NPY,2
neuropeptide Y; NT, neurotensin; TK, tachykinin.
understanding of the mechanisms of modulator effects
range. It had five different compartments, axon hillock,
from the molecular to behavioral levels.
soma and three dendritic compartments, each of which
could be given different properties. In response to
simu-lated current injections these model neurons behaved as
their biological counterparts. They were subsequently
7. Modeling on the cellular, network and behavior
equipped with conductance increase EPSPs and IPSPs
2
levels
(Cl
equilibrium potential). In addition, voltage dependent
NMDA channels were simulated [23,12,71]. The inhibitory
In order to evaluate the experimental data which indeed
synapses were placed closer to the soma and the excitatory
is very extensive, it was important to utilize mathematical
ones more distally as indicated by the biology.
Sub-modelling on a cellular and network level, as an interactive
sequently, the inhibitory and excitatory neurons of the
analysis tool. So many interactive processes operate in
network were connected in a way similar to that
estab-parallel, at the cell and network level, that it is virtually
lished experimentally. Simulation of segmental networks
impossible to intuitively deduce the net outcome. We
driven from the brainstem could produce alternating burst
therefore modeled each type of neuron (Fig. 6A) with
activity in populations of model neurons of each cell type
1 1 21
voltage dependent Na , K
and Ca
currents of different
as referred to above (Fig. 6B).
subtypes,
using
Hodgkin–Huxley
formalism
[23,67].
The burst range produced by the segmental network
21Moreover, K
Cachannels activated by the Ca
entry
model could cover the normal biological frequency range.
during the action potential are responsible for the post-
The properties of the NMDA channels were found to be of
spike afterhyperpolarisation. The latter is a major deter-
particular importance for maintaining regular burst activity
minant of frequency regulation in these cells. Each type of
in a lower frequency range. Including low voltage
acti-21
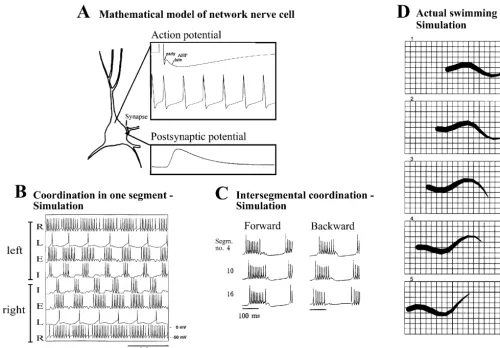
Fig. 6. Mathematical modelling of the lamprey locomotor network-simulations at neuronal, network and behavioral levels. (A) Neurons of the network
1 1 21 21 1
were simulated in a realistic fashion, with the different voltage-dependent (Na , K , Ca ), Ca -dependent K channels, and ligand-gated channels (AMPA / kainate, NMDA, glycine). Action potentials with early and late afterhyperpolarisation (AHP), and spike frequency adaptation, can be simulated, together with postsynaptic potentials occurring in different compartments. (B) Simulation of the segmental network using a pool of excitatory (E) and inhibitory (I) interneurons and lateral (L) interneurons. The activity is driven by excitatory reticulospinal neurons (R). Activity on the left and right sides alternates. (C) Pattern of intersegmental coordination, produced by a simulated network of 60 segments. This circuitry will produce a rostro-caudal phase lag along the simulated spinal cord, and this lag can be reversed if the excitability is increased in the caudal end, which results in backward locomotion. (D) Simulation of actual swimming movements using a neuro-mechanical model. Frames show steady-state swimming at 4 Hz, resulting from tonic excitation of the network, with the model lamprey moving forwards at a speed of 0.73 m / s. Time interval between frames is 50 ms (modified from Ref. [35]).
simulating a number of segments along the spinal cord, an
network model and the myotome into a ventral and a
intersegmental lag from rostral to caudal could be pro-
dorsal compartment, has allowed a separate control of
duced (Fig. 6C; Refs. [35,41]). The simulations show that
these two parts, and thereby swimming movements with a
with the available information on interneurons and their
superimposed steering in 3-D [23] (see Ref. [33]).
connectivity and membrane properties we can largely
account for the output of the locomotor network, at least to
a first approximation.
8. Concluding remarks
Ekeberg et al. [23] simulated the visco-elastic properties
234 S. Grillner et al. / Brain Research 886 (2000) 224 –236
¨ [11] L. Brodin, E. Theodorsson, J. Christensson, S. Cullheim, T. Hokfelt,
modify cellular properties in a given way that in turn will
J.C. Brown, A. Buchan, P. Panula, A.A.J. Verhofstad, M. Goldstein,
produce changes in network activity. We can thus now
Neurotensin-like peptides in the CNS of lampreys: chromatographic
bridge from the molecular to the behavioral level.
characterization and immunohistochemical localisation withrefer-An understanding of neuronal microcircuits, like the
ence to aminergic markers, Eur. J. Neurosci. 2 (1990) 1095–1109.¨
˚ ´ ´
[12] L. Brodin, H.G. Traven, A. Lansner, P. Wallen, O. Ekeberg, S.
locomotor CPG or cortical columns, is one major
require-Grillner, Computer simulations of N-methyl-D-Aspartate (NMDA)
ment for us to be able to utilize the extensive progress at
receptor induced membrane properties in a neuron model, J.
the molecular and cellular levels towards a better under-
Neurophysiol. 66 (1991) 473–484.standing of neuronal systems and the cellular bases of
[13] J.T. Buchanan, Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions andbehavior. The progress until 2010 hopefully will make us
morphology, J. Neurophysiol. 47 (1982) 961–975.
bridge this gap, which currently in most experimental
[14] J.T. Buchanan, S. Grillner, Newly identified ‘glutamate
inter-models appears insurmountable.
neurons’ and their role in locomotion in the lamprey spinal cord,Science 236 (1987) 312–314.
[15] J.T. Buchanan, S. Grillner, A new class of small inhibitory interneurones in the lamprey spinal cord, Brain Res. 438 (1988)
Acknowledgements
404–407.[16] J.T. Buchanan, S. Grillner, 5-hydroxytryptamine depresses
re-Support from the Swedish MRC (3026), the Science
ticulospinal excitatory postsynaptic potentials in motoneurones of the lamprey, Neurosci. Lett. 112 (1991) 71–74.Research Council and the Wallenberg Foundation is
grate-`
[17] N. Bussieres, A. El Manira, GABA(B) receptor activation inhibits
fully acknowledged.
N- and P/ Q-type calcium channels in cultured lamprey sensory neurons, Brain Res. 847 (1999) 175–185.
´
[18] L. Cangiano, P. Wallen, S. Grillner, Role of dendritic changes in
21
Ca levels and KCa activation during locomotor related synaptic
References
transmission in the lamprey spinal cord, Acta Physiol. Scand. A19 (2000), in press.
[1] S. Alford, J. Christenson, S. Grillner, Presynaptic GABAA and [19] J. Christenson, S. Alford, S. Grillner, T. Hokfelt, Co-localized¨ GABAB receptor-mediated phasic modulation in axons of spinal GABA and somatostatin use different ionic mechanisms to hy-motor interneurons, Eur. J. Neurosci. 3 (1991) 107–117. perpolarize target neurons in the lamprey spinal cord, Neurosci. Lett. [2] S. Alford, S. Grillner, The involvement of GABAB receptors and 134 (1991) 93–97.
´
coupled G-proteins in spinal GABAergic presynaptic inhibition, J. [20] A.H. Cohen, P. Wallen, The neural correlate of locomotion in fish: ‘Fictive swimming’ induced in an in vitro preparation of the Neurosci. 11 (1991) 3718–3726.
´ lamprey spinal cord, Exp. Brain Res. 41 (1980) 11–18.
[3] B.J. Bacskai, P. Wallen, V. Lev-Ram, S. Grillner, R.Y. Tsien,
´
[21] T.G. Deliagina, G.N. Orlovsky, S. Grillner, P. Wallen, Vestibular Activity-related calcium dynamics in lamprey motoneurons as
control of swimming in lamprey. II. Characteristics of spatial revealed by video-rate confocal microscopy, Neuron 14 (1995)
sensitivity of reticulospinal neurons, Exp. Brain Res. 90 (1992) 19–28.
¨ 489–498.
[4] F. Bongianni, J. Christenson, T. Hokfelt, S. Grillner, Neuropeptide
[22] T. Drew, Motor cortical activity during voluntary gait modifications Y-immunoreactive spinal neurons make close appositions on axons
in the cat. I. Cells related to the forelimbs, J. Neurophysiol. 70 of primary sensory afferents, Brain Res. 523 (1990) 337–341.
¨ (1993) 179–199.
[5] L. Brodin, J.T. Buchanan, T. Hokfelt, S. Grillner, J.F. Rehfeld, P.
¨ ´ ˚ ´
Frey, A.A.J. Verhofstad, G.J. Dockray, J.H. Walsh, Immuno-histo- [23] O. Ekeberg, P. Wallen, A. Lansner, H. Traven, S. L Brodin, Grillner, chemical studies of cholecystokinin-like peptides and their relation A computer based model for realistic simulations of neural net-to 5-HT, CGRP, and bombesin immunoreactivities in the brainstem works. I. The single neuron and synaptic interaction, Biol. Cybernet. and spinal cord of lampreys, J. Comp. Neurol. 271 (1988) 1–18. 65 (1991) 363–384.
¨ `
[6] L. Brodin, J.T. Buchanan, T. Hokfelt, S. Grillner, A.A.J. Verhofstad, [24] A. El Manira, N. Bussieres, Calcium channel subtypes in lamprey A spinal projection of 5-hydroxytryptamine neurons in the lamprey sensory and motor neurons, J. Neurophysiol. 78 (1997) 1334–1340. brainstem; evidence from combined retrograde tracing and immuno- [25] A. El Manira, M.A. Pompal, S. Grillner, Diencephalic projection to histochemistry, Neurosci. Lett. 67 (1986) 53–57. reticulospinal neurons involved in the initiation of locomotion in ¨ adult lampreys Lampetra fluviatilis, J. Comp. Neurol. 389 (1997) [7] L. Brodin, E. Christenson, J. Christenson, S. Cullheim, T. Hokfelt,
603–616. J.C. Brown, A. Buchanan, P. Panula, A.A.J. Verhofstad, M.
Golds-´
[26] A. El Manira, J. Tegner, S. Grillner, Calcium-dependent potassium tein, Neurotensin-like peptides in the CNS of lampreys:
chromato-channels play a critical role for burst termination in the locomotor graphic characterization and immunohistochemical localization with
network in lamprey, J. Neurophysiol. 72 (1994) 1852–1861. reference to aminergic markers, Eur. J. Neurosci. 2 (1989) 1095–
´
[27] A. El Manira, J. Tegner, S. Grillner, Locomotor-related presynaptic 1109.
modulation of primary afferents in the lamprey, Eur. J. Neurosci. 9 [8] L. Brodin, S. Grillner, The role of putative excitatory amino acid
(1997) 696–705. neurotransmitters in the initiation of locomotion in the lamprey
´
[28] A. El Manira, P. Wallen, Mechanisms of modulation of a neural spinal cord. I. The effects of excitatory amino acid antagonists,
network, News Physiol Sci. 15 (2000) 186–191. Brain Res. 360 (1985) 139–148.
[29] S. Grillner, On the generation of locomotion in the spinal dogfish, [9] L. Brodin, S. Grillner, C.M. Rovainen, NMDA, kainate and
quis-Exp. Brain Res. 20 (1974) 459–470. qualate receptors and the generation of fictive locomotion in the
[30] S. Grillner, Locomotion in vertebrates — Central mechanisms and lamprey spinal cord, Brain Res. 325 (1985) 302–306.
¨ reflex interaction, Physiol. Rev. 55 (1975) 247–304. [10] L. Brodin, A. Rawitch, T. Taylor, Y. Ohta, H. Ring, T. Hokfelt, S.
[31] S. Grillner, Control of locomotion in bipeds, tetrapods and fish, in: Grillner, L. Terenius, Multiple forms of pancreatic polypeptide
V.B. Brooks (Ed.), Handbook of Physiology, Sect 1. The Nervous related compounds in the lamprey CNS: partial characterization and
System II. Motor Contol, American Physiol. Soc, Waverly Press, immunohistochemical localization in the brain stem and spinal cord,
[32] S. Grillner, Neurobiological bases of rhythmic motor acts in reticulospinal synaptic inputs in the lamprey, J. Neurophysiol. 83 (2000) 2497–2507.
vertebrates, Science 228 (1985) 143–149.
[53] D. Parker, S. Grillner, Cellular and synaptic modulation underlying [33] S. Grillner, Neural networks for vertebrate for vertebrate
locomo-substance P-mediated plasticity of the lamprey locomotor network, tion, Sci. American 274 (1996) 64–69.
J. Neurosci. 18 (1998) 8095–8110. [34] S. Grillner, J.T. Buchanan, A. Lansner, Simulation of the segmental
[54] D. Parker, S. Grillner, Long-lasting substance P-mediated modula-burst generating network for locomotion in lamprey, Neurosci. Lett.
tion of NMDA-induced rhythmic activity in the lamprey locomotor 89 (1988) 31–35.
network involves separate protein and RNA synthesis-dependent ¨
[35] S. Grillner, T. Deliagina, O. Ekeberg, A. El Manira, R.H. Hill, A.
stages, Eur. J. Neurosci. 11 (1999) 1515–1522. ´
Lansner, G.N. Orlovsky, P. Wallen, Neural networks that co-ordinate
[55] D. Parker, S. Grillner, Activity-dependent metaplasticity of inhib-locomotion and body orientation in lamprey, Trends Neurosci. 18
itory and excitatory synaptic transmission in the lamprey spinal cord (1995) 270–279.
locomotor network, J. Neurosci. 19 (1999) 1647–1656. [36] S. Grillner, A.P. Georgopoulos, Visumotor coordination in reaching
[56] D. Parker, W. Zhang, S. Grillner, Substance P modulates NMDA and locomotion, Science 245 (1989) 1209–1210.
responses and causes long-term protein synthesis-dependent modula-[37] S. Grillner, A. Georgopoulos, L. Jordan, Selection and initiation of
tion of the lamprey locomotor network, J. Neurosci. 18 (1998) motor behavior, in: P. Stein, S. Grillner, A.I. Selverston, D.G. Stuart
4800–4813. (Eds.), Neurons, Networks and Motor Behavior, MIT Press, 1997,
[57] M.A. Pompal, A. El Manira, S. Grillner, Afferents of the lamprey pp. 3–19.
striatum with special reference to the dopaminergic system: A
´ ´
[38] S. Grillner, A. McClellan, K. Sigvardt, P. Wallen, M. Wilen,
combined tracing and immunohistochemical study, J. Comp. Neurol. Activation of NMDA-receptors elicits ‘fictive locomotion’ in
lam-386 (1997) 71–91.
prey spinal cord in vitro, Acta Physiol. Scand. 113 (1981). [58] M.A. Pompal, A. El Manira, S. Grillner, Organization of the ´
[39] S. Grillner, V.V. Smoljaninov, P. Wallen, S. Kashin, S. Rossignol, lamprey striatum — transmitters and projections, Brain Res. 766 Short communication. Locomotion in lamprey and trout: The (1997) 249–254.
relative timing of activation and movement, J. Exp. Biol. 143 (1989) [59] M.L.T. Poon, Induction of swimming in lamprey byL-DOPA and
559–566. amino acids, J. Comp. Physiol. 136 (1980) 337–344.
´
[40] S. Grillner, P. Wallen, Does the central pattern generation for [60] C.M. Rovainen, Neurobiology of lampreys, Physiol Rev. 59 (1979) locomotion in the lamprey depend on glycine inhibition?, Acta 1007–1077.
Physiol. Scand. 110 (1980) 103–105. [61] D.F. Russel, P. Wallen, On the control of myotomal motoneurones´ [41] J. Hellgren, S. Grillner, A. Lansner, Computer simulation of the during ‘fictive swimming’ in the lamprey spinal cord in vitro, Acta
segmental neural network generating locomotion in lamprey by Physiol. Scand. 117 (1983) 161–170.
using populations of network interneurons, Biol. Cybern. 68 (1992) [62] J.L. Schotland, O. Shupliakov, S. Grillner, L. Brodin, Synaptic and
1–13. nonsynaptic monoaminergic neuron systems in the lamprey spinal
´
[42] G.-Y. Hu, Z. Biro, S. Grillner, R.H. Hill, NMDA-induced membrane cord, J. Comp. Neurol. 372 (1996) 229–244.
potential oscillations in lamprey spinal neurons depend on non- [63] J. Schotland, O. Shupliakov, M. Wikstrom, L. Brodin, M.¨ synaptic inward currents, Acta Physiol. Scand. A19 (2000), in press. Srinivasan, Z. You, M. Herrera-Marschitz, W. Zhang, T. Hokfelt, S.¨ [43] H. Kleerekoper, J. Mogensen, Role of olfaction in the orientation of Grillner, Control of lamprey locomotor neurons by co-localized
Pertromyzon marinus. I. Response to a single amine in prey’s body monoamine transmitters, Nature 374 (1995) 266–268.
odor, Physiol. Zool. 36 (1963) 347–360. [64] M.L. Shik, G.N. Orlovsky, Neurophysiology of locomotor automat-[44] P. Krieger, A. El Manira, S. Grillner, Activation of pharmacological- ism, Physiol. Rev. 56 (1976) 465–501.
ly distinct metabotropic glutamate receptors depresses reticulos- [65] H.M. Sinamon, Preoptic and hypothalamic neurons and the initiation pinal-evoked monosynaptic EPSPs in the lamprey spinal cord, J. of locomotion in the anesthetized rat, Prog. Neurobiol. 41 (1993)
Neurophysiol. 76.6 (1996) 3834–3841. 323–344.
[45] P. Krieger, S. Grillner, A. El Manira, Endogenous activation of [66] P.S.G. Stein, J.C. Victor, E.C. Field, S.N. Currie, Bilateral control of metabotropic glutamate receptors contributes to burst frequency hindlimb scratching in the spinal turtle: Contralateral spinal circuitry regulation in the lamprey locomotor network, Eur. J. Neurosci. 10 contributes to the normal ipsilateral motor pattern of fictive rostral
(1998) 3333–3342. scratching, J. Neurosci. 15 (1995) 4343–4355.
[46] T. Matsushima, S. Grillner, Local serotonergic modulation of [67] J. Tegner, J. Hellgren-Kotaleski, A. Lansner, S. Grillner, Low´ calcium dependent potassium channels controls intersegmental voltage activated calcium channels in the lamprey locomotor coordination in the lamprey spinal cord, J. Neurophysiol. 67 (1992) network-simulation and experiment, J. Neurophysiol. 77 (1997)
1683–1690. 1795–1812.
´
[47] T. Matsushima, J. Tegner, R. Hill, S. Grillner, GABAB receptor [68] J. Tegner, A. Lansner, S. Grillner, Modulation of burst frequency by´ activation causes a depression of low- and high-voltage-activated calcium-dependent potassium channels in the lamprey locomotor
21)
(Ca currents, postinhibitory rebound, and postspike afterhyperpo- system: Dependence of the activity level, J. Comput. Neurosci. 5 larization in lamprey neurons, J. Neurophysiol. 70 (1993) 2606– (1998) 121–140.
2619. [69] J. Tegner, T. Matsushima, A. El Manira, S. Grillner, The spinal´
[48] A. McClellan, S. Grillner, Activation of ‘fictive’ swimming by GABA system modulates burst frequency and intersegmental electrical microstimulation of ‘locomotor command regions’ in the coordination in the lamprey: differential effects of GABAA and brainstem of the lamprey, Brain Res. 300 (1984) 357–361. GABABreceptors, J. Neurophysiol. 69 (1993) 647–657.
¨
[49] Y. Ohta, L. Brodin, S. Grillner, T. Hokfelt, J.H. Walsh, Possible [70] R. Thompson, J. Woolley, S. Grillner, Forebrain dopamine depletion target neurons of the reticulospinal cholecystokinin (CCK) projec- produces severe motor deficits in lamprey, Soc. for Neurosci. 30 tion to the lamprey spinal cord: Immunohistochemistry combined (2000) (abstr.)
¨
with intracellular staining with Lucifer Yellow, Brain Res. 44 (1988) [71] H. Traven, L. Brodin, A. Lansner, O. Ekeberg, P. Wallen, S.˚ ´ ´
400–403. Grillner, Computer simulations of NMDA and non-NMDA mediated
[50] G.N. Orlovsky, Work of reticulospinal neurons during locomotion, synaptic drive-sensory and supraspinal modulation of neurons and
Biofizika 15 (1970) 728–737. small networks, J. Neurophysiol. 70 (1993) 695–709.
´
236 S. Grillner et al. / Brain Research 886 (2000) 224 –236
´ ´
[73] F. Ullen, G. Orlovsky, T. Deliagina, S. Grillner, Role of dermal [77] P. Wallen, S. Grillner, N-Methyl-D-Aspartate receptor-induced, photoreceptors and lateral eyes in initiation and orientiation of inherent oscillatory activity in neurons active during locomotion in locomotion in lamprey, Behav. Brain Res. 54 (1993) 107–110. the lamprey, J. Neurosci. 7 (1987) 2745–2755.
¨
[74] P.A. van Dongen, T. Hokfelt, S. Grillner, A.A.J. Verhofstad, H.W.M. [78] P. Wallen, T. Williams, Fictive locomotion in the lamprey spinal´ Steinbusch, A.C. Cuello, L. Terenius, Immunohistochemical demon- cord ‘in vitro’ compared with swimming in the intact and spinal stration of some putative neurotransmitters in the lamprey spinal animal, J. Physiol. 347 (1984) 325–3259.
cord and spinal ganglia: 5-Hydroxytryptamine-, Tachykinin-, and [79] M. Wikstrom, Dopaminergic and Serotonergic Modulation of Cel-¨ Neuropeptide-Y-immunoreactive neurons and fibers, J. Comp. lular and Locomotor Network Properties in the Lamprey Spinal
Neurol. 234 (1985) 501–522. Cord, Doctoral thesis (1999) 1–66.
¨
[75] P.A. van Dongen, E. Theodorsson-Norheim, E. Brodin, T. Hokfelt, [80] M. Wikstrom, A. El Manira, Calcium influx through N- and P/ Q-¨ S. Grillner, A. Peters, A.C. Cuello, W.G. Forssman, M. Reinecke, type channels activate apamin-sensitive calcium-dependent potas-E.A. Singer, L. Laxarus, Immunohistochemical and chromatograph- sium channels generating the late afterhyperpolarization in lamprey ic studies of peptides with tachykinin-like immunoreactivity in the spinal neurons, Eur. J. Neurosci. 10 (1998) 1528–1532.
central nervous system of the lamprey, Peptides 7 (1986) 297–313. [81] M. Wikstrom, R. Hill, J. Hellgren, S. Grillner, The action of 5-HT¨ ¨
´ ˚ ´
[76] P. Wallen, O. Ekeberg, A. Lansner, L. Brodin, H. Traven, S. on calcium-dependent potassium channels and on the spinal Grillner, A computer based model for realistic simulations of neural locomotor network in lamprey is mediated by 5-HT1A-like receptors, networks. II. The segmental network generating locomotor Brain. Res. 678 (1995) 191–199.