Lp-PLA
2
activity and mass are associated with increased
incidence of ischemic stroke
A population-based cohort study from Malm¨o, Sweden
Margaretha Persson
a,∗, G¨oran Berglund
a, Jeanenne J. Nelson
c, Bo Hedblad
baThe Department of Clinical Sciences, Internal Medicine Research Group, Clinical Research Unit, University Hospital Malm¨o,
Lund University, Entrance 33, level 2, SE-205 02 Malm¨o, Sweden
bEpidemiological Research Group, University Hospital Malm¨o, Sweden cWorldwide Epidemiology, GlaxoSmithKline, Research Triangle Park, NC, USA Received 18 July 2007; received in revised form 26 October 2007; accepted 3 December 2007
Available online 21 February 2008
Abstract
Background: Data regarding the association between lipoprotein-associated phospholipase A2 (Lp-PLA2) level and incidence of cardiovas-cular (CV) events are conflicting. This prospective urban population-based study explored the relationship between baseline Lp-PLA2activity and mass, respectively, levels and incidence of first coronary heart disease (CHD) and ischemic stroke, respectively.
Methods: Lp-PLA2activity and mass were assessed in 5393 (60% women) subjects who participated in the Malm¨o Diet and Cancer Study cardiovascular program during 1991–1994.
Results: In all 347 subjects had an event (195 CHD and 152 ischemic strokes) during the follow-up period (mean 10.6±1.7 years). In an age-, sex- and CV risk factors-adjusted Cox regression analysis, comparing top to bottom tertile of Lp-PLA2activity, the relative risk [RR; 95% confidence interval (CI)] for incident CHD and ischemic stroke events were 1.48; 0.92–2.37 and RR: 1.94; 1.15–3.26, respectively. The corresponding figures for Lp-PLA2mass were 0.95; 0.65–1.40 and RR: 1.92; 1.20–3.10.
Conclusion: Elevated levels of Lp-PLA2activity and mass, respectively, were in this study, independently of established risk factors related to the incidence of ischemic stroke but after adjustment for lipids not significant related to incident CHD.
© 2007 Elsevier Ireland Ltd. All rights reserved.
Keywords: Myocardial infarction; Epidemiology; Inflammation; Lp-PLA2; Cardiovascular risk factors
1. Introduction
Atherosclerosis is widely recognized as a chronic inflam-matory process, with evidence of inflammation at all stages of disease, from initial plaque formation to destabiliza-tion and subsequent rupture[1,2]. Importantly, blood-borne cells (e.g., monocytes, macrophages, T-lymphocytes) play an important role in promoting the inflammatory processes [1], and epidemiology studies have explored the association between various inflammatory cells, mediators, or markers, and cardiovascular (CV) events (e.g., coronary heart disease
∗Corresponding author. Tel.: +46 40 332037; fax: +46 40 337081.
E-mail address:margaretha.persson@med.lu.se(M. Persson).
[CHD]-related events and ischemic stroke). Lipoprotein-associated phospholipase A2 (Lp-PLA2) is one such novel inflammatory marker associated with several cardiovascu-lar risk factors, low-density lipoprotein cholesterol (LDL), atherosclerotic disease and incident cardiovascular disease (CVD)[3–10].
The majority of epidemiological studies to date have revealed a positive association between Lp-PLA2 and car-diovascular risk that is independent of traditional (e.g., LDL, blood pressure, smoking, etc.) and emerging (e.g., C-reactive protein [CRP]) risk factors [11–15]. Of these, only two studies were population-based and included both men and women—the Atherosclerosis Risk in Communities (ARIC) and the Rotterdam studies [11,12,14]. A third study, the Monitoring of Trends and Determinants in Cardiovascular
Disease (MONICA) was also population-based, but included only men [13]. Plasma Lp-PLA2 mass was measured in the ARIC study, and there was only a non-significant 15% increase in CHD risk between the highest and lowest ter-tiles. However, among those with LDL-C levels below the median (130 mg/dL), there was more than a doubling of risk for CHD events between the highest and lowest ter-tiles, which was statistically significant [11]. In the ARIC study, Lp-PLA2masswas associated with nearly a two-fold statistically significant increase in ischemic stroke risk[12]. Lp-PLA2activitywas measured in the Rotterdam study, and those with levels in the highest tertile had nearly a two-fold increase for both CHD and stroke events compared with those in the lowest tertile, both statistically significant. In that study, no modification of effect by non-HDL-cholesterol was observed between Lp-PLA2activity and CVD related events [14].
A strong correlation between Lp-PLA2mass and activity has been reported in one study [16], but more recent data suggest the correlation may be considerably lower [3,10]. None of the population-based epidemiology studies to date have explored both Lp-PLA2activity and mass as markers of cardiovascular risk within the same cohort.
Therefore, the present study was conducted to investigate Lp-PLA2activity and mass, respectively, associations with incidence CHD or ischemic stroke, respectively, in a large prospective, urban population-based cohort from Malm¨o, Sweden.
2. Methods
2.1. Participants
The Malm¨o Diet and Cancer Study (MDCS) is a prospec-tive, population-based cohort study designed to explore the effects of diet on cancer risk[17]. All men and women 45–69 years old living in the city of Malm¨o, Sweden, were eligible for participation. Between October 1991 and February 1994, every other participant was invited to take part in a study of the epidemiology of carotid artery disease[18].
This cardiovascular cohort consisted of 6103 participants (3531 women and 2572 men). The age at baseline examina-tion was between 46 and 68 years. After an over night fasting, 5540 participants returned to donate blood samples, and of these, sufficient stores of plasma were available from 5393 (3162 women and 2231 men) for the purpose of measuring Lp-PLA2. The MDCS was approved by the Ethics Commit-tee of Lund University, Sweden. All participants provided an informed consent.
2.2. Baseline examination
Anthropometrics, supine blood pressure measurement, blood sampling and a self-administered questionnaire, ascer-taining heredity, previous and current diseases, medication,
and lifestyle factors (including smoking habits and physi-cal activity) were obtained at the baseline examination and have been described in detail previously[3,18]. Body mass index (BMI) was calculated as kg/m2. Fasting blood plasma samples were obtained for analysis of lipid profile and mea-sures of glucose status; aliquots of plasma were stored at
−80◦C.
Diabetes was defined as self-reported physician diag-nosis per questionnaire or current anti-diabetic treatment
or a fasting whole blood glucose ≥6.1 mmol/L. Smoking habits was classified as current, former- and never smoker. Blood pressure (mmHg) was measured once after a 10-min rest in the supine position. Hypertension was defined as self-reported diagnosis of hypertension or current use of anti-hypertensive treatmentora blood pressure≥140 or
≥90 mmHg. High alcohol consumption was characterized as consumption >30 g alcohol/day for women and >40 g alco-hol/day for men.
All participants were instructed to refrain from smok-ing, alcohol and food intake, over night fasting or at least 10 h before sample drawing. Blood samples were drawn for blood glucose, total and HDL-cholesterol and measured according to standard procedures at the Department of Clini-cal Chemistry, University Hospital Malm¨o. LDL-cholesterol concentration was calculated according to Friedewald’s for-mula. The high-sensitivity assay for CRP (hsCRP) was performed using the Tina-quant® CRP latex assay (Roche Diagnostics, Basel, Switzerland) on an ADVIA® 1650 Chemistry System (Bayer Healthcare, NY, USA). The aver-age coefficient of variation (CV) was 4.59%.
Test of distribution of Lp-PLA2and correlation between Lp-PLA2 and cardiovascular risk factors in this cohort has been previously reported [3]. The age- and sex-adjusted correlations between Lp-PLA2activity and mass with LDL-cholesterol are 0.48 and 0.33, respectively, and for hsCRP the corresponding figures were 0.02 and 0.10.
2.3. Measurement of Lp-PLA2activity and mass
Measurement of Lp-PLA2activity and mass, respectively, has been described in detail previously[3]. In short, Lp-PLA2 activity was measured using [3H]-platelet activating factor as substrate. The range of detection was 8–150 nmol/min/mL. All samples were tested in duplicate. Samples were retested if the replicate coefficient of variation was >20%. The average CV was 5.78%.
Lp-PLA2 mass measurements were performed using the second generation PLAQTMTest (diaDexus Inc., South San Francisco, CA, USA) commercially available enzyme-linked immunosorbant assay (ELISA) kit. All samples were analyzed in duplicate, and if a duplicate showed a coefficient of variance of more than 20%, the sample was reanalyzed. The average CV was 4.62% on random of 50 first subjects in the MDCS.
2.4. Classification of cardiovascular events
Record linkage with the Swedish Hospital Discharge Reg-ister[19], the Malm¨o Myocardial Register[20], the Stroke register of Malm¨o (STROMA) [21], and Cause of Death Register obtained information on morbidity and mortality from CHD and strokes in the MDCS. Information on case retrieval, validity and ascertainment of cases in the MDCS has been described in detail previously[18,21]. Underlying causes of death and hospitalization diagnosis, respectively, were coded in accordance with the 9th version of the Inter-national Classification of diseases (ICD). In short, all cases were followed from the baseline examination until first occurring CHD, ischemic stroke, emigration from Sweden, or death until December 31st 2003. A CHD event was defined as non-fatal myocardial infarction (MI) (ICD code 410) or death due to ischemic heart disease (ICD codes 410–414). Ischemic stroke (ICD code 434) was diagnosed when computed tomography, magnetic resonance imaging or autopsy could verify the infarction and/or exclude haemor-rhage and non-vascular disease. By definition patients with transient ischemic attacks were excluded. In order to find cases who moved out from the city after the screening examination, we also used the National Hospital Discharge Register[19]and the Swedish Cause of Death Register, using the same diagnosis validation procedures as for STROMA [21].
2.5. Statistical methods
SPSS 13.0 was used for the statistical analysis.T-test for continuous variables and chi-square for dichotomous vari-ables were used to test differences between mean levels or proportions comparing participants without or with incident CHD and ischemic strokes. Correlation coefficients between Lp-PLA2 activity and mass, and the correlation between Lp-PLA2 and cardiovascular risk factors at baseline have been presented previously[3]. Lp-PLA2 activity and mass distributions were normally distributed[3], however hsCRP was markedly skewed and therefore log-transformed before introduced in the multivariate analysis. Kaplan–Meier sur-vival plots were used to study the cumulative event-free survival in relation to tertiles of Lp-PLA2, a log–rank test was used to evaluate statistical differences between groups. Cox regression was used to investigate the incidence for CHD and ischemic stroke, respectively, in relation to tertiles (using the lowest tertile as the referent group) of Lp-PLA2 activity and mass, respectively, with adjustment for con-founding factors. To evaluate factors expected to attenuate the association between Lp-PLA2 and CV risk, sequen-tial modelling included: adjustment for age and gender; further adjustment for LDL, HDL and lipid-lowering treat-ment and finally adjusttreat-ment for BMI, smoking, systolic blood pressure (SBP), blood pressure lowering treatment, diabetes, hsCRP, alcohol consumption. Age, blood lipids, log hsCRP, BMI, SBP were introduced into the model as
continuous variables all other as dichotomous variables. In the first model 195 CHD and 152 ischemic strokes, respectively, were included. The second model included 186 and 147 cases and the final model 185 and 143 cases, respectively. The decrease in cases in the second model depended almost on missing information on LDL-C. We excluded 85 subjects with a history of myocardial infarc-tion in the analyses on CHD and 47 subjects with a history of stroke in the analyses on ischemic stroke. We calculated tolerance in order to assess collinearity between the inde-pendent variables. Tolerance was for Lp-PLA2activity and mass above 0.7 and 0.85, respectively, in the final model. Possible interaction between elevated Lp-PLA2 and LDL-cholesterol were analyzed by including interaction terms in the final model. To test if LDL levels modified the associa-tion between Lp-PLA2 mass and CHD or ischemic stroke, we split the population by the median LDL-cholesterol level (i.e. 4.1 mmol/L).
To analyze possible sex-specific difference on risk of incident ischemic stroke we constructed sex-specific tertiles of Lp-PLA2 activity and mass, respectively. Two-tailedP -values < 0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics
Baseline characteristics between participants who experi-enced a CHD event, an ischemic stroke and those who did not are shown inTable 1. As compared to those who remained event-free during the follow-up period, cases with ischemic strokes and CHD-related events, respectively, had signifi-cantly higher mean blood pressure (140, 152 and 152 mmHg, respectively), mean Lp-PLA2[both activity (45.0, 49.9 and 50.7 nmol/min/mL, respectively) and mass (268.2, 290.4 and 291.6 ng/mL, respectively] and higher prevalence of males, diabetes and smoking. Notably, those with a CHD event had statistically significant higher total and LDL-cholesterol as compared to ischemic stroke cases and to event-free partici-pants.
3.2. Baseline Lp-PLA2activity and mass in relation to
ischemic strokes and CHD events
Table 1
Baseline characteristics in subjects with incident CHD, with incident ischemic stroke and those who were event-free during the follow-up period
Variable Event-free subjects
(n= 4903)
Subjects with incident ischemic stroke (n= 152)
Subjects with incident CHD (n= 195)
Male sex (%) 39.4 53.3† 62.9‡
Age (years) 57.2±5.9 61.4±4.7‡ 60.4±5.3‡
BMI (kg/m2) 25.7±3.9 27.1±4.3† 26.8±3.8‡
SBP (mmHg) 140±19 152±19‡ 152±19‡
DBP (mmHg) 87±9 92±9‡ 91±10‡
Hypertension¶(%) 61.4 85.5‡ 84.5‡
BP lowering medication (%) 13.9 40.1‡ 24.2†
Cholesterol (mmol/L) 6.2±1.1 6.2±1.0 6.3±1.1*
LDL-C (mmol/L) 4.2±1.0 4.2±0.9 4.4±1.0‡
HDL-C (mmol/L) 1.4±0.4 1.2±0.4‡ 1.2±0.3‡
LDL-C/HDL-C ratio 3.2±1.2 3.6±1.1‡ 3.9±1.2‡
Triglycerides (mmol/L) 1.1 (0.9–1.6) 1.3 (1.0–2.0)‡ 1.3 (1.0–1.7)‡
Lipid lowering treatment (%) 1.7 4.6 3.1
Blood glucose (mmol/L) 5.1±1.3 5.7±1.9‡ 5.9±2.3‡
Diabetes¶(%) 7.3 18.4‡ 21.1‡
Glucose-lowering medication (%) 0.9 4.6‡ 6.7‡
hsCRP (mg/L) 1.3 (0.7–2.7) 2.0 (1.1–3.7)‡ 2.1 (1.0–4.1)‡
Lp-PLA2activity (nmol/min/mL) 45.0±12.9 49.9±13.6‡ 50.7±13.4‡
Lp-PLA2mass (ng/mL) 268.2±80.0 290.4±75.9‡ 291.6±81.2‡
Current smokers (%) 25.2 31.6* 36.1†
High alcohol consumption¶(%) 3.5 6.6 4.6
Use of HRT# (%) 20.4 14.7 11.5
Data are presented as mean±S.D., median with inter-quartile range or as percentages. BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; BP: blood pressure; LDL-C: low-density cholesterol; HDL-C: high-density cholesterol; hsCRP: high sensitive C-reactive protein; Lp-PLA2: lipoprotein-associated phospholipase A2; HRT: hormone replacement therapy.¶see Section2for definition.#Use of HRT among women (n= 3093).*P< 0.05; †P< 0.01 and‡P< 0.001 indicates statistical difference between subjects who experienced a CHD, an ischemic stroke or stayed event-free during the follow-up period.
3.2.1. Ischemic stroke
Both Lp-PLA2activity and mass was statistically signif-icantly associated with increased risk for incident ischemic strokes. In an age- and sex-adjusted model the relative risk (RR) associated with the upper compared to the bottom tertile of Lp-PLA2activity and mass were 1.79 (95% confidence interval, CI: 1.16–2.76) and 1.71 (1.12–2.62), respectively. This risk increase for incident ischemic stroke remained when age, sex, LDL, HDL, lipid lowering treatment, BMI, systolic blood pressure, blood pressure lowering treatment, diabetes mellitus, smoking, hsCRP and alcohol consumption was taken into account (RR: 1.94; 1.15–3.26 for Lp-PLA2 activity and RR: 1.92; 1.20–3.10 for Lp-PLA2mass, respec-tively) (Table 2).
No statistically significant multiplicative interaction was observed between Lp-PLA2 activity or mass, respectively, and LDL (P= 0.136 and 0.400, respectively) on incident stroke. However, among subjects with LDL-cholesterol above 4.1 mmol/L (e.g. the median value in the cohort) the top tertile of Lp-PLA2 activity and mass were in a fully adjusted model associated with a significant risk increase of incident ischemic stroke (RR: 2.32; 1.00–5.41 and RR: 2.19; 1.11–4.31, respectively). In subjects with LDL-cholesterol
≤4.1 mmol/L the corresponding RRs were: 1.69; 0.81–3.50 and 1.50; 0.72–3.12, respectively.
In additional analyses, and although based on a limited number of ischemic stroke events (79 men and 64 women),
the RR point estimates for incident ischemic stroke associated with the sex-specific upper tertile of Lp-PLA2activity and mass, respectively, were in a fully adjusted model rather sim-ilar between men and women (RR: 1.66; 0.88–3.14 and 2.59; 1.39–4.82 for men and 1.90; 0.96–3.80 and 1.72; 0.86–3.45 for women).
In an age- and sex-adjusted model comparing upper to lower tertile of hsCRP the RR for incident ischemic stroke was 1.98; 1.29–3.03.
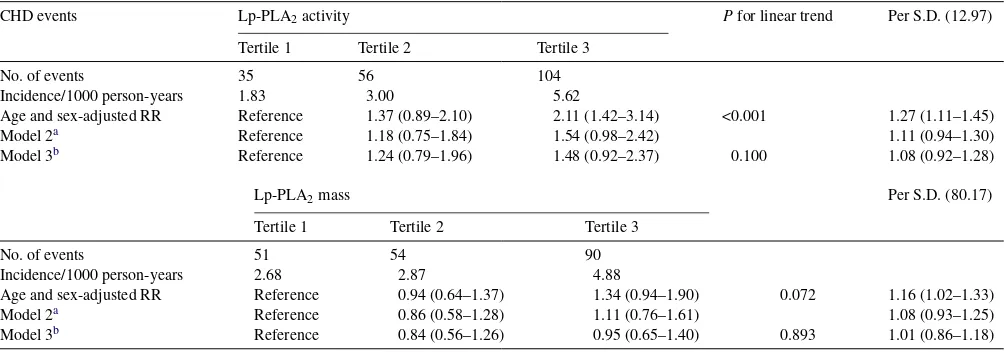
3.2.2. Coronary heart disease
The age- and sex-adjusted risk increase for incident CHD associated with the upper compared to the bottom tertile of Lp-PLA2activity and mass were 2.11; 1.42–3.14 and 1.34; 0.94–1.90, respectively. This risk increase was markedly attenuated and turned non-statistically significant for Lp-PLA2 activity (RR: 1.54; 0.98–2.42) after adjustment for LDL and HDL-cholesterol. The corresponding figure for Lp-PLA2mass was 1.11; 0.76–1.61 (Table 3). Further adjustment for other cardiovascular risk factors did only marginally affect the relationship. No evidence of a statistically significant multiplicative interaction was observed between Lp-PLA2 activity or mass, respectively, and LDL-cholesterol on inci-dence of CHD events (P= 0.239 and 0.547, respectively).
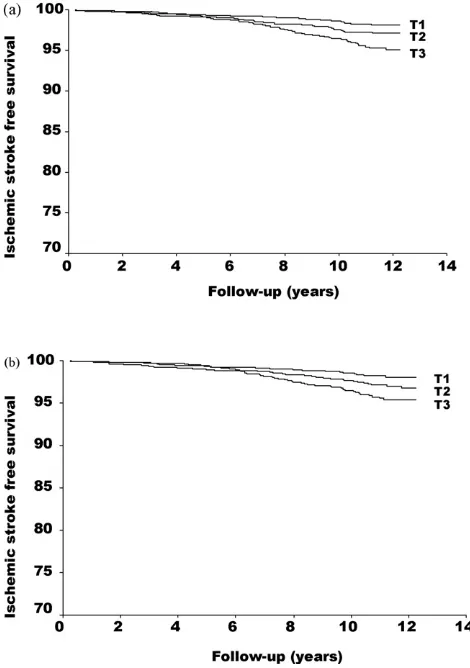
Fig. 1. First ischemic stroke free survival in relation to baseline Lp-PLA2 activity (a) and Lp-PLA2mass (b) levels in tertiles (T1: <38.8; T2: 38.8–49.5 and T3:≥49.5 mmol/min/mL for activity and T1: >227.7; T2: 227.7–295.9 and T3:≥296.0 ng/M for mass).Pfor trend <0.001 both for Lp-PLA2activity and mass.
4. Discussion
Clinical studies [10,22] have reported increased levels of Lp-PLA2 in patients with CHD, and population-based studies[11–14]the relationships between Lp-PLA2and inci-dence of cardiovascular disease. Few studies have explored whether increased levels of Lp-PLA2, as assessed as activity or mass within the same cohort, are related to the incidence of CHD and ischemic stroke. The present prospective urban population-based study, of apparently healthy middle-aged Caucasian men and women, shows that both elevated levels of Lp-PLA2activity and mass, even after taking blood lipids and other potential confounders into account, predict the incidence of ischemic stroke. However, Lp-PLA2was after adjustment for lipids not statistically significant associated to incident CHD.
The relationship between elevated Lp-PLA2 levels, whether assessed by activity or mass, and incident ischemic stroke from the present study are consistent with those from the ARIC [12] and the Rotterdam study [14]. In all three studies this risk increase was independent of potential con-founders, e.g. blood lipids, blood pressure, BMI, hsCRP and life style factors. In addition, we did not observe any evidence of a modification of effect of LDL-cholesterol on the association between Lp-PLA2 activity or mass, respec-tively, and ischemic stroke risk. This finding is in line with the Rotterdam Study [14] demonstrating a lack of significant interaction between Lp-PLA2 activity and non-HDL-cholesterol on ischemic stroke risk.
In contrast to the Rotterdam Study [14], the present study did not show any statistically significant independent relationship between Lp-PLA2activity and incident CHD. Similar to the findings in the ARIC study[11]elevated levels of Lp-PLA2mass was not, independently of LDL- and HDL-cholesterol, associated with an increased risk for CHD related
Table 2
Incidence and estimated covariate adjusted relative risk (RR) of first ischemic stroke event by baseline Lp-PLA2activity and mass levels in tertiles (T1 to T3) during 10 years of follow-up
Ischaemic stroke events Lp-PLA2activity Pfor linear trend Per S.D. (12.97)
Tertile 1 Tertile 2 Tertile 3
No. of events (n= 152) 31 48 73
Incidence/1000 person-years 1.62 2.55 3.90
Age and sex-adjusted RR Reference 1.36 (0.86–2.14) 1.79 (1.16–2.76) 0.007 1.24 (1.06–1.44)
Model 2a Reference 1.44 (0.90–2.31) 1.83 (1.11–3.01) 1.23 (1.02–1.47)
Model 3b Reference 1.44 (0.88–2.37) 1.94 (1.15–3.26) 0.012 1.23 (1.02–1.48)
Lp-PLA2mass Per S.D. (80.17)
Tertile 1 Tertile 2 Tertile 3
No. of events (n= 152) 32 51 69
Incidence/1000 person-years 1.66 2.69 3.74
Age and sex-adjusted RR Reference 1.45 (0.93–2.26) 1.71 (1.12–2.62) 0.014 1.24 (1.06–1.44)
Model 2a Reference 1.55 (0.98–2.44) 1.89 (1.21–2.97) 1.19 (1.01–1.40)
Model 3b Reference 1.65 (1.02–2.65) 1.92 (1.20–3.10) 0.008 1.23 (1.02–1.48)
aModel 2 adjusted for age, sex, LDL- and HDL-cholesterol and lipid lowering treatment. 166 subjects excluded due to missing values.
Table 3
Incidence and estimated covariate adjusted relative risk (RR) of first CHD by baseline Lp-PLA2 activity and mass level in tertiles (T1 to T3) during 10 years of follow-up
CHD events Lp-PLA2activity Pfor linear trend Per S.D. (12.97)
Tertile 1 Tertile 2 Tertile 3
No. of events 35 56 104
Incidence/1000 person-years 1.83 3.00 5.62
Age and sex-adjusted RR Reference 1.37 (0.89–2.10) 2.11 (1.42–3.14) <0.001 1.27 (1.11–1.45)
Model 2a Reference 1.18 (0.75–1.84) 1.54 (0.98–2.42) 1.11 (0.94–1.30)
Model 3b Reference 1.24 (0.79–1.96) 1.48 (0.92–2.37) 0.100 1.08 (0.92–1.28)
Lp-PLA2mass Per S.D. (80.17)
Tertile 1 Tertile 2 Tertile 3
No. of events 51 54 90
Incidence/1000 person-years 2.68 2.87 4.88
Age and sex-adjusted RR Reference 0.94 (0.64–1.37) 1.34 (0.94–1.90) 0.072 1.16 (1.02–1.33)
Model 2a Reference 0.86 (0.58–1.28) 1.11 (0.76–1.61) 1.08 (0.93–1.25)
Model 3b Reference 0.84 (0.56–1.26) 0.95 (0.65–1.40) 0.893 1.01 (0.86–1.18)
aModel 2 adjusted for age, sex, LDL- and HDL-cholesterol, and use of lipid lowering treatment. 166 subjects excluded due to missing values.
b Model 3 adjusted for age, sex, LDL- and HDL-cholesterol, use of lipid lowering treatment, BMI, hsCRP, smoking, diabetes, systolic blood pressure and high alcohol consumption. 256 subjects excluded due to missing values.
events. Furthermore, the present study found no evidence of an interaction between Lp-PLA2mass and LDL-cholesterol on incident CHD, whereas a prominent interaction between these two biomarkers on incident CHD was noted in the ARIC study[11].
Question has been raised of whether Lp-PLA2 is causally involved in the inflammatory processes that pro-mote atherosclerosis or whether the enzyme is a surrogate for LDL-cholesterol (e.g. because of the strong statistical correlation between LDL-cholesterol and Lp-PLA2, and that LDL-cholesterol acts as a functional reservoir for Lp-PLA2in the circulation). The association between Lp-PLA2(whether assessed as activity or mass) and CVD is in our study as in other population-based studies[11–14]markedly attenuated for CHD[11,13,14], but rather unchanged[12]or strength-ened[14]for ischemic stroke after adjustment for LDL- and HDL-cholesterol. It is well known that LDL-cholesterol is a major risk factor for CHD related events, however, seems to be less important for ischemic stroke [23,24]. The rea-son for this has been discussed extensively; however there is today no definite explanation. Although few studies have explored the relationship between Lp-PLA2and cardiovas-cular risk, this seems to be another molecule with different associations to incidence of CHD and cerebrovascular events such as ischemic stroke. Our findings are in agreement with those in the ARIC and Rotterdam studies[11,12,14], although the association between Lp-PLA2activity and incident CHD still reached significance after taking non-cholesterol into account in the Rotterdam study. Lp-PLA2activity and mass, respectively, are moderately correlated to LDL-cholesterol (r= 0.48 and 0.33, respectively, in the present study). How-ever, the unique variance in our study which is important in a multivariate analysis is substantial (e.g. 77 and 89%, respectively).
Another intriguing finding from the present cohort is the different associations between HDL-cholesterol and Lp-PLA2activity and mass, respectively (age-and sex-adjusted
r=−0.24 and−0.09, respectively)[3]. A moderate to strong, inverse correlation between HDL and Lp-PLA2 mass has been reported both in the Women’s Health Study[15]and in the ARIC study[11], but not in the WOSCOPS[7] and in the German MONICA study[13]. Similar to the present study HDL was weak to moderate inversely correlated to Lp-PLA2activity in the Rotterdam[14]study. The disparity in association between Lp-PLA2and HDL among the various studies could be explained, in part, by different study popula-tions and different methods used for assessment of Lp-PLA2 activity and mass.
(141 mg/dL)[11]was considerably lower than in the Malm¨o study (∼171 mg/dL) and the mean non-HDL-cholesterol in the Rotterdam study was∼215 mg/dL[14].
The correlation between Lp-PLA2activity and mass in the present study (r= 0.57) was higher than what was observed in a recent study involving post-acute coronary syndromes (ACS) patients (r= 0.36)[10], but was considerably lower than what was reported in a study by Caslake et al. (r= 0.86) that involved only 86 patients with documented CAD and 52 healthy control subjects matched by age[16]. The activity and mass assays used in the present study were identical to those used in the study involving post-ACS patients[10], but slightly different assays were used in the Caslake study[16]. Clearly, this observation speaks to the need for additional research to identify the factors (e.g. genetic or biomolecular) that may explain discrepancies between plasma mass and activity measurements. As previously reported there is dis-cordance for Lp-PLA2 activity and Lp-PLA2 mass among non-diabetic subjects within the Malm¨o study[8]. Only 6 of 10 subjects with elevated levels (e.g. the top tertile) of Lp-PLA2 activity had elevated levels of Lp-PLA2 mass. This matter might be one explanation for the different associa-tions observed in our study between Lp-PLA2 activity and mass, respectively, on incidence of CHD related events and ischemic stroke.
It has been suggested that better agreement between mass (monoclonal antibody-based) and activity (substrate-based) measurements can be achieved with more extensive solubi-lization of the enzyme in plasma and that the monoclonal antibody-based test and the substrate-based test may not quantify identical populations of the Lp-PLA2enzyme when it is in association with lipoprotein particles (Bob Wolfert, personal communication, 2006).
This study has several strengths, however, also limitations. National and regional registers were used to ascertain cases of CHD and ischemic stroke[19–21]. The validity of CHD and stroke cases from these registries has been shown to be very high[19,25]. From an epidemiologic viewpoint the most important advantage is the large urban population-based design in apparently healthy middle-aged subjects. Further-more, to our best knowledge this is the first population-based prospective study measuring both Lp-PLA2activity and mass within the same cohort. However, the results cannot be inferred outside this cohort of middle-aged European Cau-casian subjects. Another limitation is that only 42% of the eligible background population accepted participation in the Malm¨o Diet and Cancer Study, suggesting possible volunteer bias[26]. As a result, the associations observed in this study may underestimate the true relationship between Lp-PLA2 and cardiovascular risk. Additionally, although the number of events in the present study for incident CHD were somewhat higher than for incident ischemic stroke (195 and 152 cases, respectively) we cannot rule out the possibility that lack of statistical significant association between elevated Lp-PLA2 and incident CHD might be explained by limited number of CHD related events.
A consistent finding in all population-based studies is that women tend to have lower plasma Lp-PLA2mass and activity compared with men[3,11,13,14]. Thus, an intrigu-ing question is whether the association between Lp-PLA2 and cardiovascular risk is different among men and women. Although based on a limited number of ischemic stroke events (79 men and 64 women), the RR point estimates for inci-dent ischemic stroke associated with the sex-specific elevated Lp-PLA2in our study was rather similar between sexes. How-ever, clearly there is a need to identify the factors that regulate the expression of Lp-PLA2in men and women.
Despite the limitations, the findings from this study adds further evidence to the opinion that elevated levels of Lp-PLA2,whether measured as activity or mass, and inde-pendently of traditional CV risk factors, is associated with increased incidence of ischemic stroke [12,14]. Additional experimental and observational studies are necessary to clar-ify certain issues (particularly the factors that influence mass and activity levels) before Lp-PLA2 should be used rou-tinely in clinical practice. However, there is a considerable unmet need, particularly among individuals considered as an intermediate risk category, for improved risk stratifica-tion [24]. Inflammatory markers, including Lp-PLA2, that can be demonstrated to improve current risk assessment may be helpful in addressing this unmet need.
In conclusion, in this prospective population urban-based middle-aged cohort elevated levels of Lp-PLA2activity and mass, respectively, are independent of other cardiovascular risk factors related to ischemic strokes. After adjustment for lipids, no similar statistically significant relationship was observed between Lp-PLA2and incident CHD.
Acknowledgements
This study was supported by grants from the Swedish Medical Research Council, The Swedish Cancer Society, The Swedish Heart and Lung Foundation, GlaxoSmithKline, and the Region of Skane.
References
[1] Hansson GK. Inflammation, atherosclerosis, and coronary artery dis-ease. N Engl J Med 2005;352:1685–95.
[2] Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Cir-culation 2002;105:1135–43.
[3] Persson M, Nilsson JA, Nelson JJ, Hedblad B, Berglund G. The epidemiology of Lp-PLA(2): distribution and correlation with car-diovascular risk factors in a population-based cohort. Atherosclerosis 2007;190:388–96.
[4] McPhee CH, Moores K, Boyd H, et al. The lipoprotein-associated phos-pholipase A2 generates two bioactive products during the oxidation of low density lipoprotein. Studies using a novel inhibitor. Biochem J 1999;338:479–87.
[6] Karabina SA, Liapikos TA, Grekas G, Goudevenos J, Tselepis AD. Dis-tribution of PAF-acetylhydrolase activity in human plasma low-density lipoprotein subfractions. Biochem Biophys Acta 1994;1213:34–8. [7] Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated
phospholipase A2 as an independent predictor of coronary heart dis-ease. West of Scotland Coronary Prevention Study Group. N Engl J Med 2000;343:1148–55.
[8] Persson M, Hedblad B, Nelson JJ, Berglund G. Elevated Lp-PLA2 lev-els add prognostic information to the metabolic syndrome on incidence of cardiovascular events among middle-aged non-diabetic subjects. Arterioscler Thromb Vasc Biol 2007;27:1411–6.
[9] Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coro-nary artery disease, and major adverse events at follow-up. Eur Heart J 2005;26:137–44.
[10] O’Donoghue M, Morrow DA, Sabatine MS, et al. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation 2006;113:1745–52.
[11] Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2004;109:837–42.
[12] Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med 2005;165:2479–84.
[13] Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation 2004;110:1903–8.
[14] Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 2005;111:570–5.
[15] Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol 2001;38:1302–6.
[16] Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis 2000;150:413–9.
[17] Berglund G, Elmstahl S, Janzon L, Larsson SA. Design and feasibil-ity. The Malmo Diet and Cancer Study. J Intern Med 1993;233:45– 51.
[18] Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis 2005;179:325–31.
[19] The National Board of Health and Welfare. Evaluation of quality of diagnosis of acute myocardial infarction, inpatient register 1997 and 1995. Stockholm, Sweden; Socialstyrelsen, 2000. (In Swedish). [20] Tyden P, Hansen O, Janzon L. Intra-urban variations in incidence
and mortality in myocardial infarction. A study from the myocar-dial infarction register in the city of Malm¨o, Sweden. Eur Heart J 1998;19:1795–801.
[21] Khan FA, Zia E, Janzon L, Engstrom G. Incidence of stroke and stroke subtypes in Malmo, Sweden, 1990–2000: marked differences between groups defined by birth country. Stroke 2004;35:2054–8.
[22] Blankenberg S, Stengel D, Rupprecht HJ, et al. Plasma PAF-acetylhydrolase in patients with coronary artery disease: results of a cross-sectional analysis. J Lipid Res 2003;44:1381–6.
[23] Bots ML, Elwood PC, Nikitin Y, et al. Total and HDL cholesterol and risk of stroke: EUROSTROKE. A collaborative study among research centres in Europe. J Epidemiol Community Health 2002;56:19i– 24i.
[24] Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). J Am Med Assoc. 2001;285(19).
[25] Engstr¨om G, Jerntorp I, Pessah-Rasmussen H, Hedblad B, Berglund G, Janzon L. Geographic distribution of stroke incidence within an urban population: relations to socio-economic circumstances and prevalence of risk factors. Stroke 2001;25:923–31.