Summary We examined interrelationships between crop load, nitrogen (N), phosphorus (P), and potassium (K) uptake, and root growth in mature, alternate-bearing pistachio ( Pis-tacia vera L.) trees. Pistachio trees bear heavy (on-year) and light (off-year) fruit crops in alternate years. Uptake and parti-tioning of N, P, and K among tree parts were determined during (a) spring flush (mid-March to late May), (b) nut fill (late May to early September), and (c) postharvest--leaf senescence (late September to early December). Nutrient uptake occurred pri-marily during nut fill in both on-year and off-year trees. In on-year trees, N and K uptake increased by 35 and 112%, respectively, during nut fill compared with off-year trees. Dur-ing this period, nutrients were allocated largely to embryo development in on-year trees and to storage in perennial tissues in off-year trees. Nutrient uptake was negligible between har-vest and leaf senescence. Although root growth was reduced during nut fill in on-year trees compared with off-year trees, there was no relationship between root growth and the uptake of N, P or K from the soil. Our data support the hypothesis that sink demand regulates the uptake and distribution of N, P, and K in pistachio trees.
Keywords: crop yield, nutrient use efficiency, sink demand.
Introduction
Knowledge of seasonal patterns of N, P, and K uptake in mature trees is an important component of fertilizer manage-ment and can be used to increase nutrient use efficiency (i.e., nutrient recovered/nutrient applied) by matching fertilizer ap-plications with periods of high nutrient uptake capacity. Little is known, however, about the seasonal patterns of nutrient uptake in mature trees. In pistachio (Pistacia vera L.), nutrient uptake is influenced by: (1) nutrient storage in perennial tree parts over winter (Rosecrance 1996); and (2) differential crop load (Weinbaum et al. 1994b, Brown et al. 1995). Pistacia vera trees are pronounced alternate bearers (Crane and Iwakiri 1981), producing yields that are 3 to 5 times larger in heavy-fruiting on-years than in light-heavy-fruiting off-years (Johnson and Weinbaum 1987).
Studies with trees grown in pots or sand culture have been conducted to determine the periodicity of N uptake capacity in citrus (Legaz et al. 1982, Dasberg et al. 1983), peaches (Stas-sen et al. 1981, Munoz et al. 1993), apples (Han(Stas-sen 1971b, 1973, Millard and Neilsen 1989), and grapes (Conradie 1992). High N uptake rates during fruiting were observed in some studies (Legaz et al. 1982, Conradie 1992, Munoz et al. 1993), but not in others (Hansen 1971b, 1973). The restricted rooting volumes, light fruit loads, and small N reserves, which are characteristics of potted and sand-grown trees but not of ma-ture, field-grown trees, may account for the discrepancies. Furthermore, it is unlikely that nutrient uptake by mature trees can be extrapolated from studies performed using young, non-bearing or potted trees because total nutrient uptake (Smith et al. 1988) and storage reserves (Miller 1995) change as trees mature. Thus, there is a need for field studies with mature trees. Fruiting is reported to depress root growth in apple (Maggs 1963, Head 1969, Hansen and Grauslund 1978, Heim et al. 1979), peach (Chalmers and van den Ende 1975, Williamson and Coston 1989, Nii 1993), and citrus (Smith 1976, Golomb and Goldschmidt 1987). Little is known, however, about the relationship between root growth and nutrient uptake in mature trees.
Alternate bearing strongly influences tree demand for nitro-gen and phosphorus, as indicated by the severely reduced nutrient content in perennial tissues of mature fruit trees fol-lowing heavy fruiting (Golomb and Goldschmidt 1987, Wein-baum et al. 1994b, Brown et al. 1995). However, it is not known how alternate bearing influences N, P, and K uptake or how uptake capacity varies seasonally in alternate-bearing trees. We hypothesized that N, P, and K demand (primarily determined by fruit growth) regulates N, P, and K uptake in pistachio. The objectives of this study were: (1) to determine seasonal patterns of N, P, and K uptake by measuring whole-tree N, P, and K accumulation and isotopically labeled fertil-izer N uptake over the alternate-bearing cycle; and (2) to relate the seasonal patterns of root growth in heavy-fruiting (on-year) and light-fruiting (off-year) pistachio trees with seasonal pat-terns of N, P, and K uptake. We also assessed the role of sink demand and root growth on the patterns of N, P, and K uptake in pistachio.
Assessment of nitrogen, phosphorus, and potassium uptake capacity
and root growth in mature alternate-bearing pistachio (
Pistacia vera
)
trees
R. C. ROSECRANCE, S. A. WEINBAUM and P. H. BROWN
Pomology Department, University of California Davis, Davis, CA 95616, USA
Received November 1, 1995
Materials and methods
Plant material and experimental manipulation
Forty-two 20-year-old Kerman pistachio trees on Pistacia at-lantica Desf. rootstock were selected in 1992, an on-year. In May 1992, 21 of the trees were defruited to transform them to off-year trees. Thus, environmental effects were standardized by comparing on-year and off-year trees in the same year. Tree yields were measured in 1992 and 1993. Of the 42 trees, 18 (nine off- and nine on-year trees) were selected for 15N appli-cation and subsequent tree excavation and 12 (six off- and six on-year trees) were selected for root growth measurements based on similar fruit yields and trunk cross-sectional areas. Trees were spaced 3 m apart within rows and 10 m between rows with a population of 212 female trees ha−1. The trial was located in a commercial orchard (S & J Ranch, Madera, CA). The Ramona series soils at the site were coarse textured and well drained, with a pH of 6.0. Trees were kept well watered with a micro-sprinkler irrigation system.
Root growth measurements
Root growth was determined in root observation boxes (rhi-zotrons). Six rhizotrons were installed in February 1994. Each rhizotron was located between an on-year and an off-year tree, about 1 m from each tree trunk. Each rhizotron had two glass windows, one facing an on-year tree and the other facing an off-year tree. The windows were 61 cm wide and 92 cm high and installed in wood boxes that were 1.0 m long by 0.75 m wide and 1.5 m deep. Rhizotron covers were constructed from 10.5-cm thick Reflectix (Rockland, CA) insulating material. Some back filling of soil was necessary to ensure good contact between the soil and the windows. The area around the rhi-zotrons was kept weed free to ensure that all roots at the rhizotron faces were pistachio roots.
Roots of 12 trees (six fruiting and six nonfruiting) were measured every two weeks between fruit set and leaf senes-cence (April 15 to November 15, 1994). Visible roots were traced onto clear acetate sheets; the tracings were digitized and the lengths computed. Cumulative root growth was determined by the difference in root lengths from one measurement period to the next. Root growth rate was calculated from the cumula-tive root growth data divided by the number of intervening days (usually 14 days). Both cumulative root growth and root growth rate data are expressed per square centimeter of win-dow. The number of newly initiated, white roots growing against the rhizotron windows was also counted.
Determination of nitrogen uptake
Tree phenology and recovery of 15N fertilizer Isotopically la-beled N fertilizer ((15NH4)2SO4, 0.01 atom% 15N) was applied to both on- and off- year pistachio trees during three periods in 1994: (1) spring flush and pericarp development, March 17 to May 24, 1994; (2) nut fill, July 8 to September 8; and (3) postharvest--leaf senescence, September 25 to December 13, 1994. At the end of the spring flush (mid-May), the fruit pericarp had attained full size, and shoot extension growth and leaf area development were completed (Crane and Iwakiri
1981). Following spring flush, embryo development occurred between mid-June and September. Nutrient resorption and leaf senescence occurred between harvest (mid-September) and leaf abscission (mid-November).
Three on-year and three off-year trees were fertilized with 15N fertilizer (applied as a 40% (w/v) (15NH
4)2SO4 solution) at the start of each of the three study periods (March 17, July 8 and September 25 and 29, 1994). Two circular holes, approxi-mately 25 cm deep and 1 m in diameter, were dug 1 m away from either side of the trees. Isotopic N was applied to the holes at a rate of 970 g N per tree. After application, the holes were refilled with soil to minimize ammonia volatilization, and the trees were immediately irrigated. In commercial orchards, pistachio trees are typically fertilized with 1.0 to 1.5 kg N per tree per year (200 to 300 kg N ha−1 year−1) (L. Ferguson, personal communication).
Application of ammonium sulfate fertilizer on July 8 (nut fill) resulted in severe leaf burn and so these trees were ex-cluded from the study. Four nonlabeled trees (two on-year and two off-year) were selected several days later and excavated in September. Nitrogen uptake by these nonlabeled trees was calculated by the difference method (see below). During post-harvest--leaf senescence, the application of 15N was split; half the fertilizer was applied on September 25 and the other half was applied on September 29. No leaf burn was observed during this period.
Trees were excavated with a backhoe two months after isotope application (May 24, September 8 and December 13, 1994) and each tree was separated into eight fractions: (1) fine roots < 1 cm diameter; (2) roots > 1 cm diameter; (3) rootstock; (4) trunk; (5) canopy branches; (6) current-year wood; (7) leaves; and (8) fruit (cropping trees only). The various tree fractions were weighed and perennial tree parts were mechani-cally chipped. Subsamples were weighed fresh, dried to a constant weight at 60 °C, reweighed, ground in a Wiley mill to pass a 30-mesh screen, and analyzed for nutrient content. Because of the small amounts of N, P, and K in the fine roots and rootstock fractions, the three root fractions were com-bined.
Total N concentration was determined in subsamples by macro-Kjeldhal following the procedures of Weinbaum and Neumann (1977). Potassium in subsamples was extracted with acetic acid and measured by flame emission with a Varian Techtron AA 120 atomic absorption spectrometer (Sunnyvale, CA). To determine phosphorus concentrations, 1-g subsamples were ashed overnight (560 °C), extracted with 1 N HN03 and measured by plasma emission spectrometry (Model 3510, Ap-plied Research Laboratories, Sunland, CA). Duplicate samples were run for each tree fraction.
to determine the amount of 15N recovered. Total N was deter-mined by macro-Kjeldahl as decribed above.
The difference method: sequential excavations and N, P, and K analyses of mature trees Tree N, P, and K accumulation during the spring flush, nut fill, and postharvest--leaf senes-cence periods were determined by difference following se-quential tree excavations and N, P, and K analyses. Trees were paired according to their crop yields determined in 1992 and 1993 to reduce variance due to differences in tree size. In addition, there was a significant linear relationship between trunk cross-sectional area (TCA) and tree biomass (r2 = 0.56). Thus, TCA was used to normalize trees of varying sizes, follow-ing procedures similar to Kappel (1991). Leaf dry weight, for example, was normalized using the following formula:
Leafadj= Leafi× TCAavg/ TCAi ,
where Leafi and TCAi denote the measured tree part. Other variables were adjusted similarly. After correcting for tree size, no significant differences were found in total tree weight between on- and off-year trees in May or September; however, in December, off-year trees were 8% heavier than on-year trees.
Determination of nutrient removal in leaf litter
Annual N, P, and K removal in abscised leaves of both on- and off-year trees was calculated as the product of average leaf N, P, and K concentrations of eight sampled branches and the estimated leaf biomass per tree at the time of leaf abscission. Estimated total leaf dry weight at abscission was based on total leaf weight on September 8, because all the leaves had fallen from the trees by December. The estimate was calculated from the product of total tree leaf dry weight on September 8 and the fraction of leaf dry weight retained after nutrient resorption in the fall. This fraction was determined by sampling 15 leaflets from eight shoots per tree from three on- and three off-year trees on September 8 and December 13 (mesh bags were used to catch senescing leaflets in December) and calculating the average dry weight per leaflet from both sampling periods. Abscised leaves were analyzed for N, P, and K, and these concentrations were multiplied by their respective total tree dry weights.
Statistical analysis
The experiment was set up as a completely randomized design and one-way analyses of variance were performed by SAS/GLM procedures (SAS Institute 1988, Cary, NC).
Results
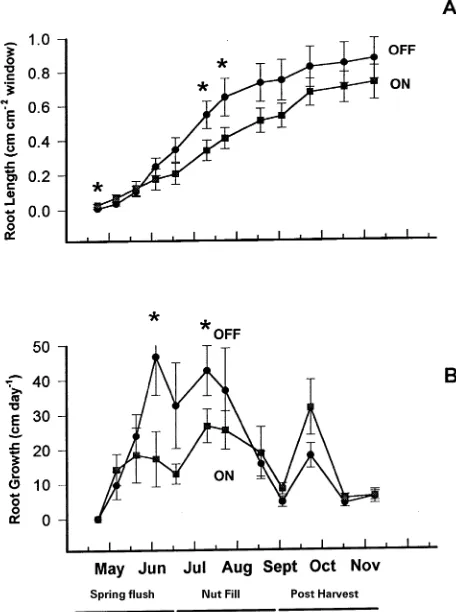
Root growth dynamics over the alternate bearing cycle Root growth varied seasonally and was influenced by alternate bearing. On-year trees initiated root growth one week earlier than off-year trees (data not shown) and, by the third week following anthesis (April 22), on-year trees had three times more white roots growing against the rhizotron windows than
off-year trees (Table 1). Total root length (cm cm−2 of rhizotron window) was 30% greater in on-year trees than in off-year trees in mid-April (Figure 1a). By June, shortly before the initiation of nut fill, a transition occurred and root length, root growth rate, and the number of white roots declined in on-year trees compared with off-year trees (Figures 1a and 1b and Table 1). Root growth rates of off-year trees were nearly twice those of on-year trees during the end of the spring flush and
Table 1. Effects of alternate bearing and season on the number of white roots growing against the rhizotron windows (no. per cm2) during the annual cycle.1
Cropping Spring flush Nut fill Nut maturity Postharvest status April 22 June 16 August 30 October 13
On-year 38.1 a2 15.4 a 26.7 a 14.1 Off-year 11.5 b 52.2 b 6.8 b 14.2
1 Each value is the mean of six replicates on a day representative of
that growth period.
2 Within a column, values followed by different letters are
cantly different at P < 0.05, according to the F test.
Figure 1. Relationship between cumulative root length (A) and root growth rate (B) versus time in mature on- and off-year pistachio trees. Each value is the mean ± SE of six tree replicates. In some cases, the SE is not visible. An asterisk indicates a significant difference at
most of the nut fill periods. Toward the end of the nut fill period as seed reached maturity, root growth rates in both on- and off-year trees decreased (Figure 1b), increased again after harvest and then declined in November. Root growth rates after harvest in September tended to be greater (P < 0.10) in on-year trees than in off-year trees.
Effect of alternate bearing on tree nutrient contents at the end of the spring flush, nut fill, and postharvest--leaf senescence periods
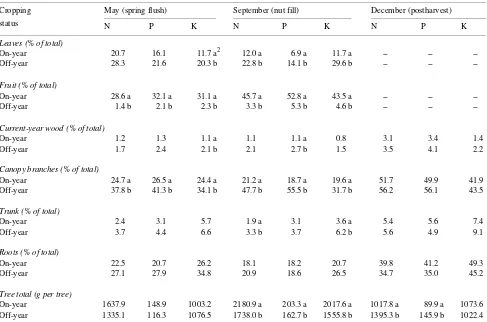
Spring flush No significant differences in tree N, P, and K contents (the product of dry weight and nutrient concentration) were found between on- and off-year trees during the spring flush period (Table 2). The distribution of N, P, and K within the trees, however, varied dramatically. Fruits of on-year trees, for example, comprised approximately 30% of the total tree N, P, and K contents, respectively, whereas fruit in off-year trees represented only 1--2%. In contrast, canopy branches con-tained a much greater proportion of the total tree nutrient content in off-year trees than in on-year trees (Table 2).
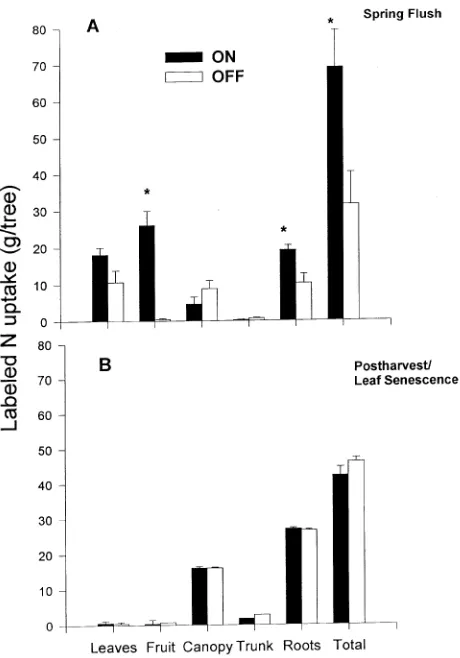
On-year trees took up more than twice as much 15N than off-year trees during the spring flush period (March 17--May 24; Figure 2a). Substantially greater amounts of 15N accumu-lated in the fruit and roots of on-year trees compared with off-year trees. Moreover, leaves from on-year trees contained 70% more 15N than leaves from off-year trees, even though off-year trees had 13% greater leaf area and 11% greater leaf N content (Rosecrance 1996). Fruits and leaves of on-year trees accounted for 67% of the 15N taken up by the tree compared with 38% for fruits and leaves of off-year trees.
Although the proportion of 15N found in fruits was high, the actual amount of label only represented 5.5% of the total N in the fruit (26 g out of a total of 469 g per tree; see Figure 2a and Table 2). Overall recoveries of 15N for off- and on-year trees were 3 and 9%, respectively (data not shown). These relatively low N recovery rates likely reflect: (1) dilution of 15N caused by non-labeled N uptake from the soil (see below); and (2) the short labeling periods used.
Total N uptake between December 13 and May 24 (esti-mated from the difference in total tree N contents) was three to 10 times greater than 15N uptake (cf. Figure 2a and Table 3).
Table 2. Effect of tree cropping status on distribution of N, P, and K and total tree N, P, and K contents in mature ‘‘Kerman’’ pistachio trees.1 Months indicate harvest dates.
Cropping May (spring flush) September (nut fill) December (postharvest)
status N P K N P K N P K
Leaves (% of total)
On-year 20.7 16.1 11.7 a2 12.0 a 6.9 a 11.7 a -- -- --Off-year 28.3 21.6 20.3 b 22.8 b 14.1 b 29.6 b -- --
--Fruit (% of total)
On-year 28.6 a 32.1 a 31.1 a 45.7 a 52.8 a 43.5 a -- -- --Off-year 1.4 b 2.1 b 2.3 b 3.3 b 5.3 b 4.6 b -- --
--Current-year wood (% of total)
On-year 1.2 1.3 1.1 a 1.1 1.1 a 0.8 3.1 3.4 1.4
Off-year 1.7 2.4 2.1 b 2.1 2.7 b 1.5 3.5 4.1 2.2
Canopy branches (% of total)
On-year 24.7 a 26.5 a 24.4 a 21.2 a 18.7 a 19.6 a 51.7 49.9 41.9 Off-year 37.8 b 41.3 b 34.1 b 47.7 b 55.5 b 31.7 b 56.2 56.1 43.5
Trunk (% of total)
On-year 2.4 3.1 5.7 1.9 a 3.1 3.6 a 5.4 5.6 7.4
Off-year 3.7 4.4 6.6 3.3 b 3.7 6.2 b 5.6 4.9 9.1
Roots (% of total)
On-year 22.5 20.7 26.2 18.1 18.2 20.7 39.8 41.2 49.3 Off-year 27.1 27.9 34.8 20.9 18.6 26.5 34.7 35.0 45.2
Tree total (g per tree)
On-year 1637.9 148.9 1003.2 2180.9 a 203.3 a 2017.6 a 1017.8 a 89.9 a 1073.6 Off-year 1335.1 116.3 1076.5 1738.0 b 162.7 b 1555.8 b 1395.3 b 145.9 b 1022.4
1 Each value is the mean of three tree replicates, except from September 10 harvest where only two tree replicates were used.
Moreover, there was a trend for off-year trees to take up more N than on-year trees (P < 0.10) during the spring flush period (Table 3), which is the reverse of the trend noted for uptake of 15N. However, total N uptake and 15N uptake are not directly comparable, because the time periods of N uptake differed (see Discussion).
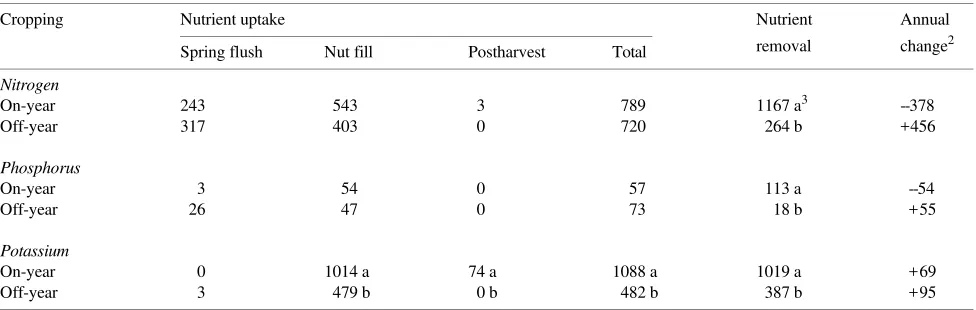
Nut fill On-year trees took up 35% more N (P < 0.10), 112% more K, and similar amounts of P than off-year trees during the nut fill period (Table 3), resulting in 25 to 30% greater whole-tree N, P, and K contents in on-year whole-trees than in off-year whole-trees (Table 2). In on-year trees, nutrients accumulated mainly in the fruit: N, P, and K contents of fruit increased 53, 55, and 65%, respectively, between the end of spring flush and nut maturity (calculated from Table 2). In off-year trees, canopy branches accumulated 80, 89, and 27% of the total N, P, and K absorbed during this period. Moreover, canopy branches of off-year trees contained 78 and 137% more N and P, respectively, than on-year trees at nut maturity in September. Thus, on-year trees accumulated nutrients in fruit, whereas off-year trees stored nutrients in perennial tissues during the nut fill period.
Postharvest--leaf senescence Little or no nutrient uptake oc-curred during the postharvest--leaf senescence period (Table 3). Nutrients did accumulate in perennial tissues during this pe-riod; however, leaf nutrient resorption rather than uptake from the soil could account for this accumulation (Rosecrance 1996). Uptake of 15N was also low during this period and there were no differences between on- and off-year trees (Figure 2b). On-year trees took up 40% less 15N during the postharvest--leaf senescence period than during spring flush (Figures 2a and 2b). On- and off-year trees recovered 4 to 5% of the 15N applied, and of the 15N recovered, > 50% of it remained in the roots in both on- and off-year trees.
Effects of alternate bearing on nutrient distribution, uptake, and removal
Alternate bearing had a marked influence on nutrient distribu-tion, but had little effect on the periodicity of nutrient uptake. Nutrients accumulated in the fruits of on-year trees and in the perennial tree parts of off-year trees over the entire season. However, nutrient uptake occurred primarily during the nut fill period in both on- and off-year trees. Thus, on-year trees took up 69, 95, and 93% of their total annual accumulation of N, P, and K, respectively, during nut fill (Table 3). The correspond-ing values for off-year trees are 56, 64, and 99%. Nutrient uptake was negligible during the postharvest--leaf senescence period irrespective of crop load.
On-year trees removed 4, 6, and 3 times more N, P, and K, respectively, in fruits plus abscised leaves than off-year trees (Table 3). For the orchard (212 trees ha−1), the fruit removed 211 kg ha−1 of N, 23 kg ha−1 of P, and 186 kg ha−1 of K at harvest. At the tree level, fruit removed 46, 52, and 43% of the total N, P, and K present in a 20-year-old heavily cropping pistachio tree (calculated from Table 2).
Crop load influenced the annual accumulation and reduction of N and P in perennial tree parts (Table 3). During an on-year, fruits and abscised leaves removed more N (47%) and P (98%) than was taken up from the soil, indicating that substantial amounts of nutrients were redistributed from storage during the on-year. Conversely, during an off-year, N and P uptake was greater than nutrient removal and pistachio trees accumu-lated nutrients.
The quantities of N, P, and K removed and taken up were similar over the 2-year alternate-bearing cycle (Table 3). That is, the sum of the nutrients removed over a 2-year alternate-bearing cycle equalled the sum of the nutrients taken up by these trees over the same period. These data indicate that off-year trees accumulated nutrients that were used during the following on-year, and that net nutrient accumulation and retention in tree structure were minimal in these mature pista-chio trees.
Discussion
Fruiting effects on root growth
There was a negative relationship between nutrient uptake and root growth. During nut fill in mid-June, for example, roots from on-year trees had 1.5 times less root length, a 50% lower
root growth rate, and 4 times fewer white roots growing against the rhizotron windows than off-year trees. Nevertheless, be-tween May 24 and September 8, N and K uptake were 35 and 112% greater, respectively, in on-year trees than in off-year trees. Moreover, following nut harvest in September, root growth increased 2--3-fold in both on- and off-year trees, although nutrient uptake was negligible during this period. Similar results have been reported in mature prune trees, where heavy-fruiting trees took up 17% more K and had less root growth than trees that were defruited two months earlier (Weinbaum et al. 1994a). In coffee trees, the relationship between root growth and N uptake is also poor (Canell and Kimeu 1971). A negative relationship between root growth and N uptake has been observed in some herbaceous species (Caldwell et al. 1981).
For decoupling between root growth and nutrient uptake to occur, the rate of nutrient uptake per unit of root length in fruiting trees must be higher than that of light-fruiting or defruited trees. Rates of N uptake by roots are reported to be 50% higher in fruiting apple (Hansen 1971a, 1971b), and coffee (Cannell and Kimeu 1971) trees relative to non-fruiting trees. Simulation models have shown that doubling root uptake kinetics is as effective as doubling root growth in increasing N (Barber 1995) and P uptake (Caldwell et al. 1992). Moreover, changes in root uptake rates are more rapid than changes in root proliferation rates (Jackson et al. 1990). Thus, increases in root nutrient uptake rates can compensate for a lack of root growth.
Nitrogen uptake during the spring
During the spring, 15N uptake was twice as high in on-year trees as in off-year trees, whereas total N uptake was 31% higher in off-year trees than in on-year trees (cf. Figure 2a and Table 3). The different time periods over which 15N uptake (between mid-March and late May) and total N uptake
(be-tween mid December to late May) occurred may account for this discrepancy. It is not clear when the 15N was taken up by the pistachio trees, but it is likely that substantial amounts of 15N were absorbed by on-year trees in May when developing fruits became a major sink for nitrogen (Rosecrance 1996). On- and off-year shoots (leaves, fruits, and current-year wood) contained similar amounts of N before May; however, on-year trees contained almost double the amount of N of shoots of off-year trees by late May (Rosecrance 1996). Thus, as fruits became a major nitrogen sink in late spring, 15N was likely taken up from the soil.
The greater total N uptake in off- versus on-year trees between December and May may reflect the potential sink activity of the depleted N storage pools in off-year trees as a result of fruiting the previous year (Rosecrance 1996). Wein-baum et al. (1994b) reported that the percentage of N derived from 15N-labeled fertilizer applied in January to mature pista-chio trees was twice as high in leaves of off-year trees as in leaves of on-year trees when sampled 2 weeks after bud break. We found that off-year pistachio trees had greater N uptake in early spring, whereas on-year trees had greater N uptake in late spring. This is consistent with demand-driven uptake because storage N pools of trees entering an off-year are low (Rose-crance 1996), hence soil N uptake occurs in early spring to support leaf area development. Trees entering an on-year, however, contain significant amounts of storage N that can be utilized for early spring growth and so N uptake from the soil is delayed until late in the spring when internal N pools are exhausted and N is needed for continuous fruit growth and development.
Fruiting effects on N, P, and K uptake
Determination of annual nutrient uptake in mature fruit trees is the first step in developing best management practices for these nutrients to maintain high yields and to minimize fertilizer
Table 3. Uptake of N, P, and K (g per tree) during spring flush, nut fill and postharvest--leaf senescence periods and removal in fruits and leaf litter in on- and off-year trees.1
Cropping Nutrient uptake Nutrient Annual
removal change2 Spring flush Nut fill Postharvest Total
Nitrogen
On-year 243 543 3 789 1167 a3 --378
Off-year 317 403 0 720 264 b +456
Phosphorus
On-year 3 54 0 57 113 a --54
Off-year 26 47 0 73 18 b +55
Potassium
On-year 0 1014 a 74 a 1088 a 1019 a +69
Off-year 3 479 b 0 b 482 b 387 b +95
1
Nutrient uptake determined from differences in tree nutrient contents from sequential tree excavations.
2 Difference between total annual nutrient uptake and removal in fruits and abscised leaves. 3
leaching to groundwater. Nutrients taken up during an on-year were allocated primarily to the fruits, whereas perennial tis-sues were the major nutrient sink in off-year trees. On-year trees took up 167, 12, and 227 kg ha−1 of N, P, and K, respectively, over the season, whereas off-year trees absorbed 136, 15, and 71 kg ha−1 of N, P, and K.
Over the 2-year alternate bearing cycle, there was a balance between nutrient uptake and removal in mature pistachio trees; however, these processes were not balanced in either the off-or on-year. In off-years, nutrient uptake was greater than re-moval, whereas during on-years, more nutrients were removed than were taken up from the soil. Because the net result of these fluctuations is that only small quantities of nutrients accumu-late in perennial tissues over a 2-year alternate-bearing cycle, nutrient removal measured over 2 years can provide a good estimate of nutrient uptake over the same period.
The greater N uptake in on-year trees than in off-year trees during nut fill may reflect a decrease in vascular cycling of amino-N, coincident with the accumulation of N by the devel-oping embryo. The reduction in the cycling of N in the xylem and phloem between roots and shoots may stimulate whole-plant N uptake (Cooper and Clarkson 1989, Lee et al. 1992, Imsande and Touraine 1994) and K uptake (Weinbaum et al. 1994a). It has been observed that pretreating maize roots with amino acids (asparagine and glutamine) inhibited NO3− and NH4+ uptake, whereas decreasing root amino acids with spe-cific N-assimilation inhibitors stimulated N uptake (Lee et al. 1992). In pistachio, it is possible that the removal of amino N by reproductive structures (fruit, seed) stimulated N uptake in on-year trees.
Crop load influenced K uptake more than the uptake of other nutrients, with on-year trees taking up three times more K than off-year trees (Table 3) (cf. Brown et al. 1995). Similarly, heavy-fruiting prune trees took up significantly more K than defruited trees (Weinbaum et al. 1994b). In pistachio, 95% of the K uptake occurred during nut fill (Table 3), and may reflect the roles of K in sugar transport which include: binding to carboxylates and transport (mainly as potassium malate) in the phloem to fruits and roots (Ben-Zioni et al. 1971, Casadesus et al. 1995, Touraine et al. 1988); and acting as an osmoticum to develop pressure gradients in the phloem for the transport and storage of sugars (Giaquinta 1983).
Little nutrient uptake occurred during the postharvest--leaf senescence period (mid-September to December). The sub-stantial quantities of amino acids cycling within the plant during leaf N resorption may have contributed to the low N uptake from the soil during the postharvest--leaf senescence period. Both on- and off-year trees resorbed approximately 50% of their leaf nitrogen during this period (Rosecrance 1996). In soybeans, amino-nitrogen resorbed from leaves in-hibits N uptake (Imsande and Touraine 1994, Touraine et al. 1992).
Another possible cause for the low nutrient uptake during the postharvest--leaf senescence period relates to pistachio’s native range. Pistachio trees have evolved in climates where little or no rainfall occurs during the autumn (Rao et al. 1976),
and, as a consequence, selection pressure for trees that absorb nutrients during this period is likely to be low.
We conclude that the pronounced effect of alternate bearing on tree nutrient demand and uptake has important implications for fertilizer management. The greatest amount of soil nutrient uptake occurred during the nut fill period in both on- and off-year trees. Nutrient uptake in the postharvest--leaf senes-cence period was low in both on- and off-years indicating that fertilizer application many not be effective at this time.
Acknowledgments
The study was supported by a grant from the California Pistachio Industry. We greatly appreciate the help from the staff at S and J Ranch, especially Jerry Allen, where this work was conducted. We also thank Grant Aitken, Bob Beede, Al Bonin, Tony Cristler, Louise Ferguson, Jennifer Katcher, Tom Muraoka, Franz Niederholzer, Oswaldo Rubio, Hilary Sampson, Steve Sibbett, and Max Stevenson for their help in the tree excavations.
References
Barber, S.A. 1995. Soil nutrient bioavailability. A mechanistic ap-proach. John Wiley & Sons, New York, 414 p.
Ben-Zioni, A., Y. Vaadia, and S.H. Lips. 1971. Nitrate uptake by roots as regulated by nitrate reduction products of the shoots. Physiol. Plant. 24:288--290.
Brown, P.H., S.A. Weinbaum and G.A. Picchioni. 1995. Alternate bearing influences annual nutrient consumption and the total nutri-ent contnutri-ent of mature pistachio trees. Trees 9:158--164.
Cabrera, M.L. and D.E. Kissel. 1989. Review and simplification of calculations in 15N tracer studies. Fert. Res. 20:11--15.
Crane, J.C. and B.T. Iwakiri. 1981. Morphology and reproduction in pistachio. Hort. Rev. 3:376--393.
Caldwell, M.M., L.M. Dudlye, and B. Lilieholm. 1992. Soil solution phosphate, root uptake kinetics and nutrient acquisition: implica-tions for a patchy soil environment. Oecologia 89:305--309. Caldwell, M.M., J.H. Richards, D.A. Johnson, R.S. Nowak and R.S.
Dzurec. 1981. Coping with herbivory: photosynthetic capacity and resoruce allocation in two semiarid Agropyron bunchgrasses. Oe-cologia 50:14--24.
Cannell, M.G.R. and B.S. Kimeu. 1971. Uptake and distribution of macro-nutrients in trees of Coffea arabica L. in Kenya as affected by seasonal climatic differences and the presence of fruits. Ann. Appl. Biol. 68:213--230.
Casadesus, J., L. Tapia and H. Lambers. 1995. Regulation of K+ and NO3− fluxes in roots of sunflower (Helianthus annuus) after changes
in light intensity. Physiol. Plant. 93:279--285.
Chalmers, D.H. and B. Van Den Ende 1975. Productivity of peach trees: Factors affecting dry-weight distribution during tree growth. Ann. Bot. 38:423--432.
Conradie, W.J. 1992. Partitioning of nitrogen in grapevines during autumn and utilization of nitrogen reserves during the following growing season. S. Afr. J. Enol. Vitic. 13:45--51.
Cooper, H.D. and D.T. Clarkson. 1989. Cycling of amino-nitrogen and other nutrients between shoots and roots in cereals----a possible mechanism integrating shoot and root in the regulation of nutrient uptake. J. Exp. Bot. 40:753--762.
Giaquinta, R.T. 1983. Phloem loading of sucrose. Annu. Rev. Plant Physiol. 34:347--387.
Golomb, A. and E.E. Goldschmidt. 1987. Mineral nutrient balance and impairment of the nitrate-reducing system in alternate-bearing Wilking mandarin trees. J. Am. Soc. Hort. Sci. 112:397--401. Hansen, P. and J. Grauslund. 1978. Levels of sorbitol in bleeding sap
and in xylem sap in relation to leaf mass and assimilate demand in apple trees. Physiol. Plant. 42:129--133.
Hansen, P. 1971a. The effects of cropping on the distribution of growth in apple trees. Tidsskr. Planteavl 75:119--127.
Hansen, P. 1971b. The effects of cropping on the uptake, contents, and distribution of nutrient in apple trees. Tidsskr. Planteavl 75:615--625.
Hansen, P. 1973. The effect of cropping on the growth and uptake of nutrients by apple trees at different levels of nitrogen, potassium, magnesium, and phosphorus. Acta Agric. Scand. 23:87--92. Hauck, R.D. and J.M. Bremner. 1976. Uses of tracers for soil and
fertilizer nitrogen research. Adv. Agron. 28:219--266.
Head, G.C. 1969. The effects of fruiting and defoliation on seasonal trends in new root production on apple trees. J. Hort. Sci. 44:175--81. Heim, G., J.J. Landsberg, R.L. Watson and P. Brain. 1979. Eco-physi-ology of apple trees: dry matter production and partitioning by young golden delicious trees in France and England. J. Appl. Ecol. 16:179--194.
Imsande, J. and B. Touraine. 1994. N demand and the regulation of nitrate uptake. Plant Physiol. 105:3--7.
Jackson, R.B., J.H. Manwaring and M.M. Caldwell. 1990. Rapid physiological adjustment of roots to localized soil enrichment. Nature 344:58--60.
Johnson, R.S. and S.A. Weinbaum. 1987. Variation in the tree size, yield, cropping efficiency, and alternate bearing among ‘‘Kerman’’ pistachio trees. J. Am. Soc. Hort. Sci. 112:942--945.
Kappel, F. 1991. Partitioning of above-ground dry matter in ‘‘Lambert’’ sweet cherry trees with and without fruit. J. Am. Soc. Hort. Sci. 116:201--205.
Legaz, F. E. Prim-Millo, E. Primo-Yufera, C. Gil, and J.L. Rubio. 1982. Nitrogen fertilization in citrus I. Absorption and distribution of nitrogen in calamondin trees (Citrus mitis Bl.), during flower-ing, fruit set and initial fruit development periods. Plant Soil 66:339--351.
Lee, R.B, J.V. Purves, R.G. Ratcliffe and L.R. Saker. 1992. Nitrogen assimilation and the control of ammonium and nitrate absorption by maize roots. J. Exp. Bot. 43:1385--1396.
Maggs, D.H. 1963. The reduction in growth of apple trees brought about by fruiting. J. Hort. Sci. 38:119--128.
Millard, P. and G.H. Neilsen. 1989. The influence of nitrogen supply on the uptake and remobilization of stored N for the seasonal growth of apple trees. Ann. Bot. 63:301--309.
Miller, H.G. 1995. The influence of stand development on nutrient demand, growth and allocation. Plant Soil 169:225--232. Munoz N, J. Guerri, F. Legaz, and E. Primo-Millo. 1993. Seasonal
uptake of 15N-nitrate and distribution of absorbed nitrogen in peach trees. Plant Soil 150:263--269.
Nii, N. 1993. Fruiting effects on leaf characteristics, photosynthesis, and root growth in peach trees. J. Jpn. Soc. Hort. Sci. 62:519--526. Rao, M.S.V., W.V. Abbott, and J.S. Theon. 1976. Satellite-derived global oceanic rainfall atlas (1973 and 1974). NASA Goddard Space Flight Center. Washington, DC, 410 p.
Rosecrance, R.C. 1996. Effect of alternate bearing on nutrient uptake, storage, and root growth in mature pistachio (Pistachia vera L.) trees. Ph.D. Diss., Univ. California, Davis, 121 p.
Smith, P.F. 1976. Collapse of ‘‘Murcott’’ tangerines trees. J. Am. Soc. Hort. Sci.101:23--35.
Smith, G.S., J.G. Buwalda and C.J. Clark. 1988. Nutrient dynamics of a kiwifruit ecosystem. Sci. Hort. 37:87--109.
Stassen, P.J.C., H.W. Stindt, D.K. Strydom and J. H. Terblanche. 1981. Seasonal changes in nitrogen fractions of young Kakamas peach trees. Agroplantae 13:63--72.
Touraine, B., N. Grignon and C. Grignon. 1988. Charge balance in NO3− fed soybean. Estimation of K+ and carboxylate recirculation.
Plant Physiol. 88:605--612.
Touraine, B. B. Muller, and C. Grignon. 1992. Effect of phloem-translocated malate on NO3− uptake by roots of intact soybean
plants. Plant Physiol 93:1118--1123.
Weinbaum, S.A. and P.M. Neumann. 1977. Uptake and metabolism of
15N-labeled potassium nitrate by French prune (Prunus domes-tica L.) leaves and the effects of two surfactants. J. Am. Soc. Hort. Sci. 102:601--604.
Weinbaum, S.A., F.J.A. Niederholzer, S. Ponchner, R.C. Rosecrance, R.M. Carlson, A.C. Whittlesey and T.T. Muraoka. 1994a. Nutrient uptake by cropping and defruited field-grown ‘‘French’’ prune trees. J. Am. Soc. Hort. Sci. 119:925--930.
Weinbaum, S.A., G.A. Picchioni, T.T. Muraoka, P.H. Brown and L. Ferguson. 1994b. Nitrogen usage, accumulation of carbon and nitrogen reserves, and the capacity for labelled fertilizer nitrogen and boron uptake varies during the alternate-bearing cycle in pistachio. J. Am. Soc. Hort. Sci. 119:24--31.