Manipulating flux through plant metabolic pathways
Anthony J Kinney
The past two years have seen a marked increase in patent applications for novel methods of altering the level and spectrum of commercially important products in plants. Results from these studies have proven surprising, showing that in many cases those enzymes traditionally thought of as flux-controlling have no impact on product formation when they are directly altered by genetic manipulation. In many cases, successful induction of increased flux throughout an entire pathway has been achieved by targeting one of the terminal enzymes in the pathway.
Addresses
DuPont Experimental Station, P.O. Box 80402 Wilmington, DE 19880-0402, USA; e-mail: [email protected]
Current Opinion in Plant Biology1998,1:173–178 http://biomednet.com/elecref/1369526600100173
Current Biology Ltd ISSN 1369-5266
Introduction
The manipulation of metabolic pathways, an art restricted to microbes only a few years ago, is rapidly becoming commonplace in transgenic plants. The recent advent of rapid sequencing of cDNA libraries has led to the discovery of many new plant genes related to primary, secondary and energy metabolism. Coupled with improve-ments in the transformation efficiency of plants, this technology can now be used to produce new traits in agriculturally important crops. These traits include the improvement of human food or animal feed quality [1,2], the production of industrially useful molecules in plants [3,4•], the production of pharmaceuticals in plants [5••,P1] and the manipulation of a plant’s ability to defend itself against pathogens (as reviewed in [6]).
The ability to manipulate plant metabolism at will is, however, dependent upon an understanding of metabolic regulation. Generally, increased or reduced expression of a gene encoding a metabolic enzyme will produce the desired phenotype only if that enzyme has significant control of the flux through the entire pathway in question. This review will demonstrate that enzymes previously regarded as the regulatory step in a given pathway may not prove to be the most efficient point at which to manipulate the pathway to increase product levels. I will illustrate this point by comparing some different strategies used in attempts to increase flux through a variety of important branches of metabolism. I will then go on to describe the flipside of these strategies, where increased product levels can be suppressed by the resultant increase in catabolism, or can be maintained by the direct downregulation of catabolic enzymes. Finally, I will discuss the potential for
producing novel classes of compounds in plants by genetic engineering.
Early targets in flux control
An example of phenotype alteration may be found in fatty acid biosynthesis in developing oilseeds [4•]. Tenfold overexpression of an enzyme involved in the synthesis of palmitoyl-ACP, a beta-ketoacyl-ACP synthetase I, did not result in increased palmitate in the oil of transgenic rape, soy or tobacco. On the other hand, twofold overexpression of a palmitoyl-ACP thioesterase in rape and soy plants resulted in a doubling of oil palmitate content in those plants. Thus, it is apparent that the thioesterase has a relatively high control co-efficient in plant oil biosynthesis. This experiment also illustrates an effective method which improves our understanding of the regulation of a metabolic pathway: manipulating the expression levels of potential control enzymes in the pathway and analyzing the resulting changes in flux or pathway product.
When this kind of analysis is done the results are often surprising. Thus, for example, the action of phosphofruc-tokinase (PFK) is cited in many text books as a regulatory step in glycolysis. Genetically engineered plants, however, that overexpress genes encoding this enzyme do not usually display any change in glycolytic or respiratory flux [7••]. One obvious explanation of this observation is that some other step has become rate-limiting, resulting in an accumulation of substrate at some other point of the pathway. Biochemical analysis of the transformed tissue suggests that this is not the case, however. Rather there appears to be an attenuation of the increased substrate concentration caused by PFK overexpression as the flux passes down the pathway [8••]. Thus, there was little or no increase in substrate concentration of the terminal enzyme of the pathway, and hence no change in pathway product.
The conclusions the authors drew from these experiments [8••] is that over-expression of single enzymes early in metabolic pathways may be of limited effectiveness in increasing overall flux through that pathway. One possible corollary of this hypothesis is that, in some cases, changes in the expression level of genes encoding enzymes close to the terminal reaction of a pathway may have more effect on pathway product than changes in the expression of genes encoding enzymes normally thought of as flux generating or as committing steps for a particular branch of that pathway. Although this seems counter-intuitive, there are some recent examples in plants which may support this postulate.
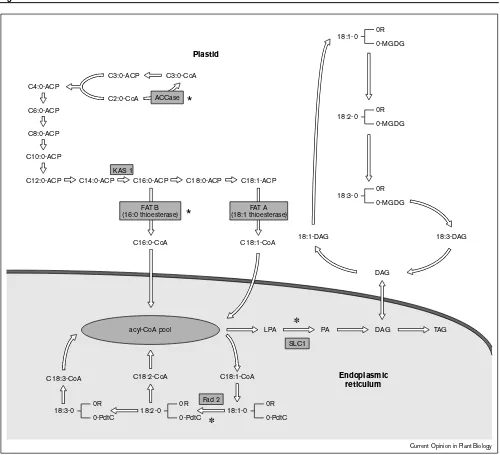
cytoplasm as acyl-CoA moieties which are then assembled onto the glycerol backbone by fatty acyltransferases. There is a unique acyltransferase for placing a fatty acyl chain on each of the three carbons on the glycerol backbone.
The reaction which is often cited as the flux-generating or committing step in the synthesis of fatty acids, and hence of triacylglycerol, is the conversion of acetyl-CoA to malonyl-CoA [4•]. This reaction is catalyzed by the
Figure 1
C4:0-ACP
C6:0-ACP
C3:0-ACP C3:0-CoA
C2:0-CoA
C8:0-ACP
C10:0-ACP
C12:0-ACP C14:0-ACP C16:0-ACP
C16:0-CoA C18:1-CoA 18:1-DAG 18:3-DAG
C18:3-CoA C18:2-CoA C18:1-CoA
18:2-0
✽
✽
18:3-0 0R 0-PdtC
C18:0-ACP C18:1-ACP
LPA PA DAG
DAG
TAG ACCase
FAT B
(16:0 thioesterase) (18:1 thioesterase)FAT A
18:1-0 0R
0-PdtC
0R
0-PdtC
18:1-0 0R
0-MGDG
18:2-0 0R
0-MGDG
18:3-0 0R
0-MGDG
Current Opinion in Plant Biology Fad 2
SLC1 acyl-CoA pool
Plastid
Endoplasmic reticulum
*
*
KAS 1
enzyme acetyl-CoA carboxylase. It has not yet been possible, however, to demonstrate any significant changes in the total oil content of transgenic oilseeds overex-pressing genes for acetyl-CoA carboxylase, the supposedly flux-generating step of fatty acid biosynthesis [3].
Late targets in flux control
The yeast gene SLC 1-1 was isolated as a variant of the yeast geneSLC 1, which itself was isolated from a mutant unable to make sphingolipids [9]. SLC 1-1 was able to complement the mutant and it was suggested that its function was to transfer long-chain acyl groups to the sn-2 position of phosphatidylinositols. The mutant gene,slc 1, had a single base change which caused it to lack this function. Recently it was shown that theSLC 1-1-gene was also able to transfer acyl groups to the sn-2 position of plant triacylglycerols when it was expressed in Arabidopsis and oilseed rape seeds [10••]. Furthermore, transgenic seeds over-expressingSLC 1-1had an increased total oil content. Thus, in this instance, it appears that increased activity of a terminal reaction resulted in increased flux through the entire pathway. It will be interesting to determine if this is an isolated case or if similar results will be obtained for other oilseed plants or for other metabolic pathways.
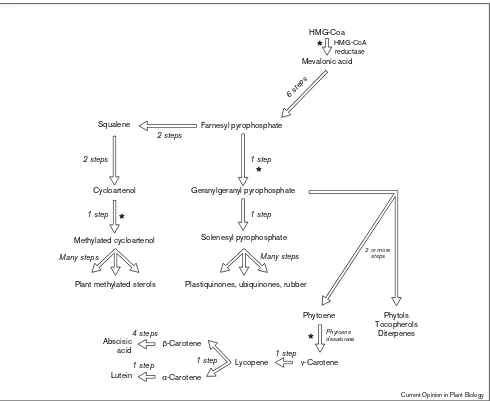
Another example of increasing flux into product by increasing the activity of a near-terminal enzyme comes from the isoprenoid pathway. This is a highly branched pathway which originates with 3-hydroxy-3-methyl glu-taryl coenzyme A (HMG-CoA) [11]. From the essentially linear conversion of HMG-CoA to ubiquinone there are many branches to important metabolites such as cytokinins, squalene, sterols, carotenoids and tocopherols. The activity of the enzyme HMG-CoA reductase was for many years believed to be a rate-limiting step in sterol biosynthesis and possibly that of other isoprenoids. Increasing the activity of this enzyme was then an obvious target for increasing flux through the entire pathway and thus the concentration of useful isoprenoid end-products. A few years ago, however, it was shown that increasing the catalytic activity of HMG-CoA reductase in transgenic tobacco, corn and tomatoes resulted in an accumulation of only cycloartenol rather than sterols or other isoprenoids [P2]. More recently a number of groups [P3••,P4••,P5•] have demonstrated that overexpression of phytoene desaturase, an enzyme close to the terminal reactions of carotenoid biosynthesis, resulted in a large increase in the concentration of the orange pigment lutein in various transgenic plants — thus supporting the hypothesis that increased levels of terminal enzymes in a pathway may indeed have more effect on pathway products than directly manipulating those enzymes that were traditionally thought of as rate-limiting. The results of metabolic flux experiments in transgenic plants must be interpreted with care if they are to be used to devise a strategy for the increased production of products of commercial value.
Catabolic targets in flux control
In some cases the flux through a pathway may be greatly increased as a result of increased expression of one or more enzymes in that pathway. The steady-state concentration of pathway product, on the other hand, may not change because of some other effect, such as an increased rate of catabolism of the product. Attempts to increase the free lysine content of seeds are a good example of this. A key reaction in the biosynthesis of free lysine in plant seeds is the conversion of aspartic semialdehyde to 2,3-dihydrodipicolinate, catalyzed by the enzyme dihydrodipicolinate synthase. In plants, the activity of this enzyme is inhibited by free lysine which regulates the flux through the synthetic pathway. The rate of lysine synthesis has been greatly increased in transgenic tobacco, soy, corn and barley meal by the expression of a gene encoding a feedback-insensitive dihydrodipicolinate synthase from eitherE. coli orCorynibacerium glutamicum.
[12,13]. In a number of these experiments, however, the steady-state level of lysine remained constant even though the flux through the entire pathway had been significantly increased. This was because lysine catabolism had also increased in these transgenic plants [13]. Increases in lysine were only observed when the increase of flux into lysine was so great that the activity of the first lysine catabolic pathway member, lysine ketoreductase, became saturated [12,13].
One of the most effective ways of changing flux through any pathway is by silencing the expression of genes which encode enzymes at or beyond branch points in the pathway. In many cases the resultant phenotype of such experiments is similar to that predicted. For example, again in the developing oilseed, the synthesis of polyunsaturated fatty acids may be regarded as a branch pathway in oil biosynthesis. Oleoyl-CoA may be incorporated into phosphatidylcholine (PC) for desatu-ration to linoleoyl-PC and linolenyl-PC, or it may be incorporated directly into triacylglycerol. Silencing the gene which encodes the omega-6 desaturase responsible for the conversion of oleoyl-CoA to linoleoyl-CoA acid in soybean results in a large increase in the oleic acid content of the triacylglycerol with a corresponding reduction in polyunsaturated fatty acid content [4•]. Indeed, this particular example of pathway engineering has resulted in the commercial production of oxidatively stable vegetable oil from transgenic soybeans [14•].
Production of novel compounds
Figure 2
Current Opinion in Plant Biology
HMG-Coa
Mevalonic acid
1 step 2 steps
1 step 2 steps
1 step
6 steps
Squalene
Geranylgeranyl pyrophosphate
Solenesyl pyrophosphate Cycloartenol
Methylated cycloartenol
Many steps Many steps
Plant methylated sterols Plastiquinones, ubiquinones, rubber
Phytoene Phytols Tocopherols
Diterpenes
Phytoene desaturase
γ-Carotene Lycopene
1 step 1 step
β-Carotene
α-Carotene Abscisic
acid Lutein
4 steps
1 step
2 or more steps
*
*
*
*
Farnesyl pyrophosphate
HMG-CoA reductase
Overview of isoprenoid metabolism in plants. The pathway must be envisioned as two main branches: the sterol and terpenoid pathways. The six reactions leading to squalene (via geranyl pyrophosphate), however, are common to both branches and thus sterol and lutein biosynthesis may also be regarded as branches of a single linear pathway from HMG-CoA to ubiquinones. All plants contain both sterol and terpenoid pathways but the specific products synthesized depend upon the plant species and tissue type. Potential flux control steps are marked with asterisks. The pathway begins with mevalonic acid from acetyl-CoA. The rate limiting step in this 3-step pathway is thought to be HMG-CoA reductase. Increasing HMG-CoA reductase activity in tobacco leaves, however, resulted in an increased cycloartenol content, suggesting that geranylgeranyl pyrophosphate synthesis is rate-limiting but squalene synthesis is not. A near-terminal reaction,δ-24 methylation, must have a high control coefficient for this sterol branch of the pathway. Increasing phytoene desaturase in a number of plants resulted in a large increase in lutein, suggesting that this enzyme has a high control coefficient in the terpenoid branch of the pathway.
source of aliphatic carbon compounds, although current major crop plants produce only a handful of different fatty acids with limited nonfood applications. Triacylglycerol synthesis, however, is an attractive target for modification for a number of reasons. First, for the high level oleic acid soybean example above, it has been shown that large changes in the types of fatty acids found in the triacylglycerol can be made without affecting the viability of the crop plant or seed germination [14•]. Secondly, nature has already provided a precedent for producing unusual fatty acids in seed oil, over 400 different kinds of fatty acids have been observed in the oils of exotic oilseed
plants. And lastly, it is theoretically possible to produce plant oils with entirely new functionality by expressing only one or two heterologous genes [4•].
A recent example of this is the production of acetylenic fatty acids in the oils of transgenic seeds [P6••]. Acetylenic acids, such as crepenynic acid, are industrially useful in the production of paint and other coatings, as well as plastics and lubricants. Crepenynic acid is found in the triacylglycerols of a number of exotic oilseed plants such as
Figure 3
β-aspartyl phosphate
Aspartic
β-semialdehyde
2-3 dihydrodipicolinate Threonine Methionine 5 steps
Lysine
Current Opinion in Plant Biology
Lysine turnover in plants. In common with oil and isoprenoid biosynthesis, lysine synthesis originates with acetyl-CoA via the TCA cycle. Lysine can also be catabolised back to acetyl-CoA. Potential flux control steps are marked with asterisks. The enzyme dihydrodipicolinate synthase (DHDPS) is strongly down-regulated by free lysine. Removing this feedback regulation by expressing a prokaryotic DHDPS gene in plants can result in large increases in free lysine in transgenic plants, confirming that this enzyme has a high control coefficient for product synthesis. Large increases in saccharopine were also seen in transgenic high lysine plants, demonstrating that the catabolic part of the pathway is very active and that lysine ketoglutarate reductase (LKR) is rate-limiting for this catabolism.
still esterified to phosphatidylcholine. The acetylase is very closely related to known plant fatty acyl-phospholipid desaturases. Recently the C. alpina gene encoding this acetylase was expressed in transgenic Arabidopsis plants, which subsequently were shown to produce low amounts of crepenynic acid in their seed oil [P6••]. Thus the addition of a single enzyme created a whole new branch point in fatty acid metabolism and triacylglycerol biosynthesis. Additional genes, perhaps encoding for acyltransferases which are more efficient at attaching novel fatty acyl-CoAs to the glycerol backbone, may be needed before commercially significant concentrations of acetylenic fatty acid end-products will be made in domestic crop plants.
Acetylases are members of the mixed-function mono-oxygenase family, which as well as desaturases includes enzymes that catalyze the formation of epoxy, hydroxy and keto groups [15•]. The catalytic function of these enzymes is similar enough to fatty acid desaturation that it has been possible to re-engineer desaturases so that they will
also catalyze related mono-oxygenase reactions, such as hydroxylation [16••]. Being able to produce fatty acids with a desired functional group at a specific position in the acyl chain opens up, among other things, the possibilities of new polymers and composites, improved PVC plasticizers and better paints.
Conclusions
The future of pathway engineering, therefore, may well be in protein engineering. Pathway enzymes do not always catalyze the desired reaction at the required rate to change flux through the pathway, or they may not catalyze precisely the desired reaction. The ability to engineer enzymes with precisely the right catalytic activity to produce novel pathway products or increased amounts of existing products may well be the key to long term success in plant metabolic pathway engineering.
References and recommended reading
Papers of particular interest published within the annual period of review have been highlighted as:• of special interest
•• of outstanding interest
1. Kinney AJ:Improving soybean seed quality.InInduced Mutations and Molecular Techniques for Crop Improvement.
Vienna: International Atomic Energy Agency: 1995:101-113. 2. Herbers K, Sonnewald U:Manipulating metabolic partitioning in
plants.Trends Biotech1996,14:198-205.
3. John ME, Keller G:Metabolic pathway engineering in cotton: biosynthesis of polyhydroxybutyrate in fiber cells.Proc Natl Acad Sci USA1996,93:1268-1277.
•
4. Kinney AJ:Genetic engineering of oilseeds for desired traits.
InGenetic Engineering. Edited by Setlow JK. New York: Plenum Press 1997,19:149-166.
Comprehensive review of the metabolic engineering of fatty acid biosynthetic pathways in plants.
••
5. Hezari M, Croteau R:Taxol biosynthesis: an update.Planta Medica1997,63:291-295.
An update on the biosynthesis of taxol, a diterpenoid chemotherapeutic agent, with useful discussion on target for pathway engineering to increase production. A good example of the potential for engineering secondary metabolism to produce a pharmaceutically important chemical.
6. Dixon RA, Lamb CJ, Masoud S, Sewalt VJH, Paiva NL:Metabolic engineering: prospects for crop improvement through the genetic manipulation of polypropanoid biosynthesis and defense responses.Gene1996,179:61-71.
••
7. Thomas S, Mooney PJF, Burrell MM, Fell DA:Finite change analysis of glycolytic intermediates in tuber tissue of lines of transgenic potato overexpressing phosphofructokinase.
Biochem J1997,322:111-117.
Using transgenic plant lines for metabolic control analysis. With its com-panion paper (see [8••]) postulates novel view of flux generating steps in metabolic pathways. A different and interesting view of metabolism.
••
8. Thomas S, Mooney PJF, Burrell MM, Fell DA:Metabolic control analysis of glycolysis in tuber tissue of potato (Solanum tuberosum): explanation for the low control coefficient of phosphofructokinase over respiratory flux.Biochem J1997,
322:111-117.
With its companion paper [7••] expands on a novel view of flux-generating steps in metabolic pathways with the postulation that flux-generating steps may not be ideal targets for pathway flux manipulation.
9. Nagiec MM, Wells GB, Lester RL, Dickson RC:A suppressor gene that enables Saccharomyces cerevisiae to grow without making sphingolipids encodes a protein that resembles anE. colifatty acyltransferase.J Biol Chem1993,268:22156-22163.
••
composition in the Brassicaceae by expression of a yeast sn-2 acyltransferase gene.Plant Cell1997,9:909-923.
First demonstration that flux through oil biosynthetic pathways in plants may be manipulated by increasing the catalytic activity of a terminal reaction, in this case a yeast acyl-transferase. Expressing the yeast gene in plants results in an increased seed oil content. If this result turns out to be a general effect then this would be a viable way of increasing the world’s vegetable oil production.
•
11. Scolnik PA, Bartley GE:A table of some cloned genes involved in isoprenoid biosynthesis.Plant Mol Biol Rep1996,14 :305-319.
State of the art of our understanding of plant isoprenoid metabolic pathways. A very good, compact review with a lot of information.
12. Falco SC, Guida T, Locke M, Mauvais J, Sanders C, Ward RT, Weber P:Transgenic canola and soybean seeds with increased lysine.Biotechnology1995,13:577-582.
13. Brinch-Pedersen H, Galili G, Knudsen S, Holm PB:Engineering of the aspartate family biosynthetic pathway in barley.Plant Mol Biol1996,132:611-620.
•
14. Kinney AJ, Knowlton S:Designer oils: the high oleic acid soybean.InGenetic Engineering for the Food Industry. Edited by Harlander S, Roller S. London: Blackie Academic; 1997:193-213. Review of the development of a soybean line with genetic modified metabolic pathway. From gene cloning to product analysis, commercialization and safety issues. The other chapters in this book are also well worth reading, es-pecially the ones concerning the safety and public acceptance of genetically engineered plants.
•
15. Shanklin J, Cahoon EB, Whittle E, Lindqvist Y, Huang W, Schneider G, Schmidt H:Structure-function studies on desaturases and related hydrocarbon hydroxylases.In
Physiology, Biochemistry and Molecular Biology of Plant Lipids.
Edited by Williams JP, Kahn MU, Lem NW. Dordrecht: Kluwer Academic; 1997:6-10.
Good overview of mono-oxygenase type desaturase-related enzymes.
••
16. Broun P, Somerville CR:Accumulation of ricinoleic, lesquerolic, and densipolic acids in seeds of transgenic Arabidopsis plants that express a fatty acyl hydroxylase cDNA from castor bean.
Plant Physiol1997,113:933-942.
This paper reports important ground work for re-engineering of pathway en-zymes to produce novel pathway products. By inserting a gene from castor bean intoArabidopsis, the authors report transgenic plants containing novel
hydroxy fatty acids. This is a good example of manipulating the direction of a pathway flux by expressing a heterologous gene.
Patents
• of special interest
•• of outstanding interest
P1. Maloney M:Expressing recombinant polypeptide as fusion with oil body protein.International patent application December/21st/1995 WO9621029.
P2. Chappel J, Cuellar RE, Saunders CA, Wolf FR:Plant sterol accumulation and pest resistance by increasing copy number of HMG-CoA reductase gene in tobacco, tomato and corn.
European patent application October/10th/1991 EP480730.
••
P3. Brinkhaus FL, Englisah J, Eschenfeldt WH, Hauptman R, Ausich R, Mukharji I, Poroffitt JH, Yarger JG, Yen HB:Accumulating colored native carotenoids in transgenic plants.International patent application October/27th/1995 WO9613149.
This paper demonstrates that the manipulation of terminal enzymes of iso-prenoid metabolism, in this case phytoene desaturase, can lead to an in-creased carotenoid content in plants.
••
P4. Grierson D, John I, Karvouni Z, Taylor J, Turner A, Watson C:
New isolated DNA encoding melon phytoene desaturase.
International patent application July/6th/1995 WO9602650. A similar study to that cited in [P3••]. Manipulation of terminal enzymes of isoprenoid metabolism to increase carotenoid content of plants.
•
P5. Braun CJ, Trulson AJ:Visual identification of transgenic plant material by production of carotenoid pigment encoded by cassette containing Erwinia phytoene desaturase. International patent application March/29th/1997 WO9714807.
Use of technology in [P3••] and [P4••] as a selection for transgenic plant tis-sue with engineered metabolism. Makes good use of a genetic manipulation which results in a colored phenotype, in this case as a selectable marker.
••
P6. Gummeson P, Lee M, Lenman M, Sjoedahl M, Stymne S:New acetylase used for production of crepenynic acid from linoleic acid.International patent application February/14th/1997 WO9737033.
![Figure 3also catalyze related mono-oxygenase reactions, such ashydroxylation [16••]. Being able to produce fatty acids with](https://thumb-ap.123doks.com/thumbv2/123dok/1037162.929450/5.578.42.281.117.385/figure-catalyze-related-oxygenase-reactions-ashydroxylation-produce-fatty.webp)