Characterization of salt-induced changes in gene expression in
tomato (
Lycopersicon esculentum
) roots and the role played by
abscisic acid
Jun-Zhi Wei
1, Ananchanok Tirajoh, Jollanda Effendy, Aine L. Plant *
Department of Biological Sciences,Simon Fraser Uni6ersity,8888Uni6ersity Dri6e,Burnaby,BC,Canada V5A1S6
Received 12 April 2000; received in revised form 10 July 2000; accepted 12 July 2000
Abstract
Examination of tomato (Lycopersicon esculentumMill) root mRNA profiles by differential display-polymerase chain reaction (DD-PCR) revealed that a salt treatment induced, promoted or repressed the expression of a number of genes. The majority of the observed changes were indicative of a rapid and transient salt-induced alteration in gene expression. Twenty partial cDNAs corresponding primarily to salt-induced or up-regulated mRNAs were subsequently cloned and sequenced. The role of abscisic acid (ABA) in regulating salt-responsive gene expression in roots was explored. The DD-PCR data indicate that the majority of the salt-induced changes in the root mRNA profile occurred in an ABA-independent manner. The expression of genes corresponding to six cDNAs was shown unequivocally to be responsive to a salt treatment by RNA blot hybridization. Just two of these were responsive to exogenous ABA and, in salt-treated roots of the ABA-deficient mutantflacca, all were expressed to a level comparable to that in the wild-type. The identity of two of the salt-responsive partial cDNAs is known. The deduced amino acid sequence of one was similar to that of laccases that polymerize a variety of substrates to form resilient structures within the cell wall. One other shared amino acid sequence similarity with the C-terminus of a tobacco pathogen-induced oxygenase (PIOX). It is possible that the PIOX is involved in generating signaling molecules that mediate a general stress response. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Abscisic acid; Differential display; Gene expression;Lycopersicon esculentum; Salt stress
www.elsevier.com/locate/plantsci
1. Introduction
Roots play a number of important roles during plant growth and development and typically are the first and critical part of the plant to encounter soil salinity, a major stress that drastically affects crop productivity. Approximately 10% of the world’s arable land and 23% of the cultivated land is covered with various types of salt-affected soils
and this situation will worsen due to the continu-ous build-up of salt in cultivated soils as a result of irrigation [1]. When growing in saline soils, roots have to cope with two types of stresses. The first of these is an osmotic stress resulting from high salt concentration in the soil that results in lowered water potential and a consequent loss of cell turgor in the roots. The second is an ionic stress induced by changes in the concentrations of specific ions in the root-growing medium and within root tissues. These stresses in turn cause a reduction in the water uptake and the inhibition of root elongation [2].
Physiological and metabolic changes in salt-af-fected roots are accompanied by changes in gene expression. Salt alters the accumulation of several polypeptides in roots [3 – 8] and subsequently a
Abbre6iations: ABA, abscisic acid; AC, Ailsa Craig; DD-PCR, differential display polymerase chain reaction; flc, flacca; MS, Mu-rashige and Skoog; PIOX, pathogen-induced oxygenase; ROS, reac-tive oxygen species; RT, reverse transcription.
* Corresponding author. Tel.: +1-604-2914461; fax: + 1-604-2913496.
E-mail address:[email protected] (A.L. Plant).
1Present address: USDA-ARS, Forage and Range Research Labo-ratory, Utah State University, Logan, UT 84322-6300, USA.
number of cDNA clones corresponding to salt-re-sponsive genes have been identified and isolated from cDNA libraries constructed using RNA from salt-treated plant tissues [9 – 13]. Those that have been isolated from salt-treated roots include ger-min [14], the early salt-stress responsive cDNAs
from Lophopyrum elongatum [9] and several
cD-NAs associated with salt-tolerance in rice [15,16]. The expression of many of these genes in roots is responsive to abscisic acid (ABA) and it has subse-quently been proposed that ABA is the primary regulator of salt-induced changes in gene expres-sion [17]. ABA is known to regulate the expresexpres-sion of many genes in response to other environmental stresses, particularly water-deficit-stress [18]. How-ever, in salt-challenged plants, a role for ABA has largely been based on enhanced gene expression in response to the application of exogenous ABA to unstressed plant tissues. Several studies have ad-dressed the role of endogenous ABA in regulating salt-responsive genes, however they, have exam-ined expression in either leaves or whole seedlings [19 – 22]. Very few studies have examined directly the role of endogenous ABA in eliciting salt-in-duced changes in gene expression in roots.
The recently developed differential display or DD-PCR technique [23] provides a sensitive and flexible approach to the identification of differen-tially expressed genes. This method has been used successfully to identify several cDNAs correspond-ing to genes regulated by gibberellic acid [24,25], ozone [26], salt [27], heat [28], senescence [29], sucrose [30] and those differentially expressed dur-ing development [31 – 34]. Differential display was used in this study to analyze changes in mRNA populations that occur in salt- and ABA-treated tomato roots and to identify novel salt-responsive genes. DD-PCR and RNA blot hybridization data are presented that indicate that a substantial pro-portion of the gene expression that occurs in
salt-treated roots appears to be regulated
independently of ABA.
2. Methods
2.1. Materials
Seeds of tomato (Lycopersicon esculentum Mill. cv. Ailsa Craig) and the near-isogenic ABA-defi-cient mutantflacca(flc) were germinated in
moist-ened vermiculite contained within a plastic grid (1.5×1.5 cm) that was lined with a plastic mesh and housed in a plastic tray. Upon germination, the roots grew down through the mesh to contact the Murashige and Skoog (MS) nutrient solution (2/3-strength, [35]) in the tray below. The MS nutrient solution was changed twice weekly and aerated. Plants were maintained in a growth cham-ber (Conviron Basic Model I25L Incubator) in the light at 25°C, 70% relative humidity for 16 h; in the dark at 21°C and 70% relative humidity for 8 h.
2.2. Experimental treatments
Six-week-old plants were used for all the experi-ments. A salt treatment was imposed by the addi-tion of NaCl to the MS nutrient soluaddi-tion to reach a final concentration of 170 mM. Plants were exposed to NaCl for varying periods of time rang-ing from 0 to 24 h. Exogenous ABA and com-bined NaCl/ABA treatments were imposed by exposing plants to 100 mM ABA (mixed isomers,
+ / − cis/trans ABA; Sigma) and 100 mM ABA
together with 170 mM NaCl, respectively. Plants were exposed to these treatments for 24 h during which time aeration of the media was maintained. Control plants were transferred to and maintained in MS nutrient solution for the duration of the experimental period. Following each treatment the roots were harvested and frozen in liquid N2
be-fore storing at −80°C until needed. All treat-ments were performed at least twice.
2.3. RNA extraction
Frozen roots were ground to a fine powder in liquid nitrogen. Total RNA for DD-PCR was extracted using the Plant RNeasy System (Qiagen, Mississauga, Ontario, Canada) following the man-ufacturer’s instructions. Total RNA for Northern blot hybridization analyses was extracted using the LiCl-phenol method described by Prescott and Martin [36].
2.4. Differential display
Co-lumbia, Vancouver, BC, Canada). Each reverse transcription (RT) reaction contained 5 mM KCl, 10 mM Tris – HCl (pH 8.3), 4 mM MgCl2, 20mM
dNTPs, 1.5 mM anchor primer (T11GG, T11GC,
T11CG, or T11CC), 0.2 mg RNA, 20 units RNase
inhibitor (Perkin Elmer, Foster City, CA, USA) and 50 U MuLV reverse transcriptase (Perkin Elmer, Foster City, CA, USA). The RT reaction was performed at 37°C for 60 min followed by incubation at 95°C for 5 min. The subsequent PCR contained 2 mM MgCl2, 0.25 mM arbitrary
primer (TACAACGAGG, TGGATTGGTC,
CTTTCTACCC, TTTTGGCTCC,
GGAAC-CAATC, AAACTCCGTC, TCGATACAGG, or TGGTAAAGGG), 1.5mM anchor primer, 20 mM
dNTPs, 2ml RT products, 0.5 U DNA polymerase
supplied with its own buffer (Ultratherm, Bio/Can Scientific, Mississauga, Ontario, Canada), and 0.074 mBq 33P-dATP (Amersham, B’aie d’Urfe,
Quebec, Canada). Each PCR cycle consisted of 94°C for 30 s, 40°C for 2 min and 72°C for 30 s, the reaction was subjected to 40 cycles with a 5 min extension at 72°C following the final cycle. One-fifth of the PCR products was loaded on a 6% acrylamide/8.3M urea gel. The gel was run for 3.5 h at 55 W constant power after which it was transferred to Whatman 3MM paper, dried under vacuum at 80°C for 2 h, and exposed to an X-ray film (Kodak X-Omat blue XB-1) for 24 – 48 h. Bands of interest were excised from the gel and the resulting gel slices were boiled in 100ml dH2O for
15 min. After centrifugation, the supernatant was directly used for reamplification, which was per-formed using the same primer set and PCR condi-tions as described above except the dNTP concentration was 200 mM and no isotope was
added. RT reactions were performed at least twice for each RNA sample, and the subsequent PCR step was duplicated at least twice for each primer combination.
2.5. Cloning, sequencing and analyses
Reamplified cDNA products were cloned into plasmid vectors using the TA Cloning System from Invitrogen (San Diego, CA, USA). The cloned partial cDNA sequences were sequenced using an ABI 377 automatic sequencer. The nucle-otide sequences obtained were submitted to the NCBI server for BLASTN and BLASTX searches against nucleotide and protein sequences deposited in various databases [38].
2.6. RNA blot hybridization analyses
Total RNA (20 mg) was size separated on a
formaldehyde denaturing 1.2% agarose gel accord-ing to Sambrook et al. [39]. RNA was capillary transferred to a positively charged nylon mem-brane (Boehringer Mannheim, Laval, Quebec, Canada) using 20×SSC as the transfer medium. RNA was fixed to the membrane by UV-crosslink-ing for two min (UV Stratalinker 2400) followed by baking at 80°C for 30 min. Membranes were prehybridized in 100 mM tetrasodium pyrophos-phate, 50 mM sodium phospyrophos-phate, 7% SDS, 1 mM EDTA at 65°C for 2 h (FSB) [40]. Partial cDNA inserts were labeled with 1.85 MBq a-32P-dCTP
(Amersham, B’aie d’Urfe, Quebec, Canada) using the Random Prime-it kit (Stratagene, La Jolla, CA, USA). Hybridization continued in the same buffer containing 107– 108 cpm 32P-labelled probe
for 16 h at 65°C. Membranes were washed two times in FSB/1% SDS at 65°C for 45 min each time and then once in the same buffer at 68°C for 45 min. The washed membranes were exposed to autoradiography film (Kodak X-Omat blue XB-1) with a single intensifying screen at −80°C. All RNA blots were performed twice.
3. Results
3.1. Effect of a salt treatment on root RNA populations
To determine how rapidly changes in the mRNA population occurred following the onset of a salt treatment, time course experiments were carried out. RNA was isolated from tomato roots 0, 0.5, 2, 8 and 24 h following the onset of a salt treatment and used for DD-PCR. The results
ob-tained confirmed the majority of the previously observed salt-induced, -enhanced and -repressed partial cDNAs. In addition, more novel induced or up-regulated cDNA products generated from RNA isolated between 0.5 and 8 h following the onset of a salt treatment were identified, indicating
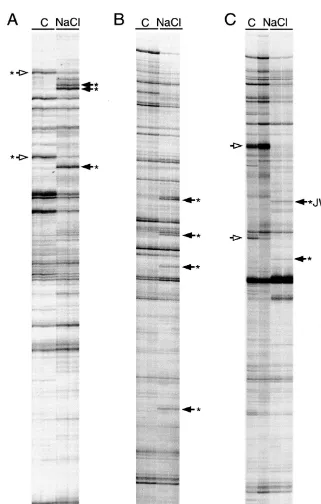
Fig. 1. DD-PCR products generated from mRNA isolated from roots exposed to 170 mM NaCl (NaCl) or MS nutrient solution (C) for 24 h. DD-PCR was performed with primers [T11]GC or [T11]CC and TACAACGAGG, respectively (A and B) and
[T11]CC and GGAACCAATC (C). Duplicate lanes displaying products generated from two independent PCR amplifications are
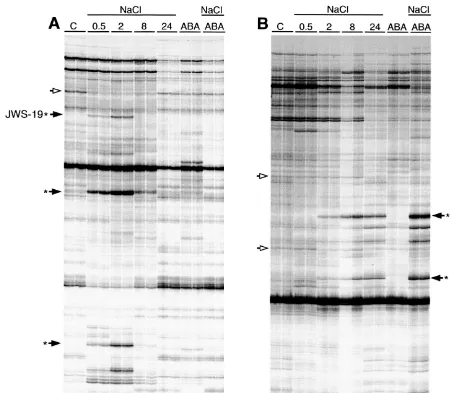
Fig. 2. DD-PCR products generated from mRNA isolated from non-treated roots (C), roots exposed to NaCl for 0.5 h (NaCl, 0.5), 2 h (NaCl, 2), 8 h (NaCl, 8) and 24 h (NaCl, 24), and roots exposed to ABA (ABA) or combined NaCl/ABA (NaCl/ABA) treatments for 24 h. DD-PCR was performed with primers [T11]CC and TGGATTGGTC (A) and [T11]CC and GGAACCAATC
(B). Duplicate lanes displaying products generated from two independent PCR amplifications are shown. DD-PCR products that exhibited consistent differences are indicated. Arrows with a closed and open head indicate cDNAs derived from RNA that was more or less abundant in salt-treated roots, respectively. Arrows marked with an asterisk indicate DD-PCR products that were excised from the gel and cloned. The original JWS-17 and JWS-19 bands are marked.
that several genes were transiently expressed dur-ing a period of salt-stress (see JWS-19 in Fig. 2). The salt-affected DD-PCR products were catego-rized into salt-induced, up-regulated or down-reg-ulated groups (Table 1). Table 1 also indicates the time-dependent accumulation of various members of each group in salt-affected roots.
3.2. Effect of an ABA and a combined ABA/salt treatment on root RNA populations
To investigate the role played by ABA in medi-ating salt-induced changes in root RNA
alone (Fig. 2, Table 1). However, in several in-stances the combined treatment either further en-hanced or further repressed the accumulation of certain cDNAs over that induced by salt alone (see JWS-17 in Fig. 2, Table 1).
3.3. Cloning and sequencing of salt-responsi6e
cDNA fragments
Differential display clearly demonstrated that changes in the mRNA population of roots oc-curred during a period of salt stress. A total of 46 cDNA products of interest were excised and 40 of these were successfully re-amplified and cloned. The majority of these represented DD-PCR prod-ucts with an induced or enhanced accumulation in salt-treated roots. One represented a DD-PCR product with a repressed accumulation and one other accumulated only in response to ABA. The insert of each of these clones was sequenced and this revealed that, among the 40 cDNA clones, 20 contained distinct sequences.
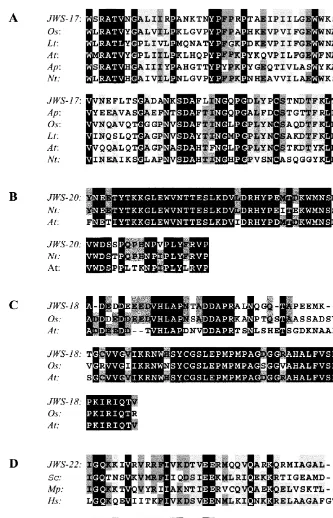
Each of the 20 cDNA sequences (Table 2) was submitted to the NCBI server for comparison to sequences residing in the GenBank nucleotide and dbEST databases using BLAST programs [38]. Four DD-PCR products were similar to known sequences deposited in these databases. The nucle-otide sequence of the partial cDNA JWS-17 was similar to that of laccase genes isolated from
vari-ous plant species. At the amino acid level JWS-17 shared 58% identity with a portion of the N-termi-nal domain of the laccase from Acer pseudopla
-tanus, while the percent identity shared with
laccase proteins from Liriodendron tulipifera, Oryza sati6a, Arabidopsis thaliana and Nicotiana
tabacum ranged from 48 to 56%. An alignment of
the amino acid sequences is shown in Fig. 3A. The nucleotide sequence of the cDNA insert of JWS-20 was similar to the DNA sequence of a tobacco oxygenase (piox) and Arabidopsis feebly-like protein. At the amino acid level there was 91% identity between the amino acid sequence derived from JWS-20 and the C-terminal portion of the tobacco PIOX (Fig. 3B). The partial cDNA, JWS-18, shares 67 and 65% amino acid sequence iden-tity with the central portion of a rice clone and an Arabidopsismitotic control protein DIS3 that both share similarity with Schizosaccharomyces pombe DIS3 (Fig. 3C). The JWS-22 insert shares 46, 50 and 41% amino acid sequence identity with the C-terminus of a yeast DNA repair protein, a hypothetical helicase from Mycoplasma pneumo -niae and a human helicase-like transcription fac-tor, respectively (Fig. 3D). Two other partial cDNAs were similar to deposited sequences. JWS-1 shares 89 – 90% nucleotide sequence identity with a part of five cDNAs derived fromBotrytis cinerea grown under conditions of nitrogen deprivation (Table 2). These cDNAs are all similar to an
Table 1
Accumulation of DD-PCR products corresponding to RNA in salt-, ABA- and ABA/NaCl-treated tomato roots
Typea
aType ‘A’, ‘B’, ‘C’ etc. correspond to groups of DD-PCR products with different accumulation patterns. ‘+’, ‘++’ etc.
Fig. 3. (A) Alignment of the deduced amino acid sequence of JWS-17 with the amino acid sequence of laccases fromO.sati6a
(Os) (AAC04576);Liriodendron tulipifera(Lt) (AAB17191);A.thaliana(At) (AAC33238)A.pseudoplatanus(Ap) (AAB09228) and
N. tabacum (Nt) (JC5229). Residues that are identical in all the sequences are shaded with black, identical in five out of six sequences are shown as black letters on a dark gray background and in four out of six are shown as black letters on a light gray background. (B) Alignment of the deduced amino acid sequence of JWS-20 with the amino acid sequence of PIOX from N.
tabacum (Nt) (CAA07589) and A. thaliana feebly-like protein (At) (AAF24612). Residues that are identical in all the three sequences are shaded in black and those identical in two of the three sequences are shown as black letters on a dark gray background. (C) Alignment of the deduced amino acid sequence of JWS-18 with the deduced amino acid sequence derived from an O. sati6a cDNA (Os) (BAA85401) and an A. thaliana mitotic control protein DIS3 (At) (AAD32908). Residues that are
identical in all the three sequences are shaded in black and those identical in two of the three sequences are shown as black letters on a dark gray background. (D) Alignment of the deduced amino acid sequence of JWS-22 with those of a Saccharomyces cere6isiaeDNA repair protein (Sc) (P32849), aMycoplasma pneumoniaehypothetical helicase (Mp) (P75093), and aHomo sapiens
Table 2
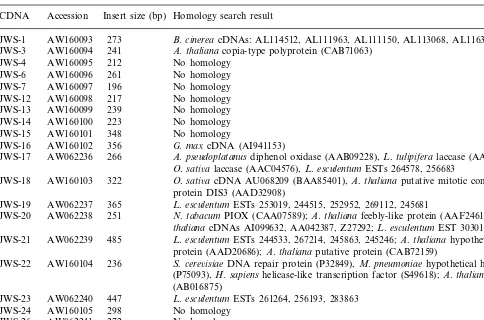
Homology search result for partial cDNAs derived from salt-treated tomato rootsa
Insert size (bp)
CDNA Accession Homology search result
273 B.cinereacDNAs: AL114512, AL111963, AL111150, AL113068, AL116383 JWS-1 AW160093
241
AW160094 A.thaliana copia-type polyprotein (CAB71063) JWS-3
AW160095
JWS-4 212 No homology AW160096
JWS-6 261 No homology 196
AW160097 No homology JWS-7
JWS-12 AW160098 217 No homology 239
AW160099 No homology JWS-13
223
JWS-14 AW160100 No homology 348
AW160101 No homology JWS-15
356 G.maxcDNA (AI941153) JWS-16 AW160102
266
AW062236 A.pseudoplatanus diphenol oxidase (AAB09228),L.tulipifera laccase (AAB17191), JWS-17
O.sati6alaccase (AAC04576),L.esculentumESTs 264578, 256683
322
JWS-18 AW160103 O.sati6acDNA AU068209 (BAA85401),A.thaliana putative mitotic control protein DIS3 (AAD32908)
365
JWS-19 AW062237 L.esculentumESTs 253019, 244515, 252952, 269112, 245681
JWS-20 AW062238 251 N.tabacumPIOX (CAA07589);A.thalianafeebly-like protein (AAF24612); A.
thaliana cDNAs AI099632, AA042387, Z27292;L.esculentumEST 303018 485
JWS-21 AW062239 L.esculentumESTs 244533, 267214, 245863, 245246; A.thalianahypothetical protein (AAD20686);A.thaliana putative protein (CAB72159)
236
JWS-22 AW160104 S.cere6isiaeDNA repair protein (P32849),M.pneumoniae hypothetical helicase (P75093),H.sapiens helicase-like transcription factor (S49618);A.thalianaDNA (AB016875)
JWS-23 AW062240 447 L.esculentumESTs 261264, 256193, 283863 298
JWS-24 AW160105 No homology 272
AW062241 No homology JWS-26
AW062242
JWS-27 429 L.esculentumESTs 275366, 242631; L.penelliiEST 309721
aRNA blot hybridization analyses indicated that JWS-17, -19, -20, -21, -26 and -27 correspond to salt-responsive genes. JWS-7
was down-regulated by salt, JWS-13 and -23 correspond to constitutively expressed genes, and JWS-24 was ABA-responsive. No expression was detected for the remainder.
aconitase gene ofAspergillus terreus. JWS-3 shares 31% amino acid sequence identity with an Ara -bidopsis hypothetical protein that itself is similar to a putative reverse transcriptase of Arabidopsis (Table 2). Five other cDNAs were similar to vari-ous ESTs of tomato or soybean and hypothetical proteins of Arabidopsis (Table 2). No significant similarity to any sequence residing in the data-bases was found for the cDNA inserts of the remaining nine clones. It is possible that the short length of some of the differential display products prevented a higher rate of putative identification. The sequence for each of the JWS clones has been deposited in the Genbank EST database (see Table 2 for accession numbers).
3.4. RNA blot hybridization analyses
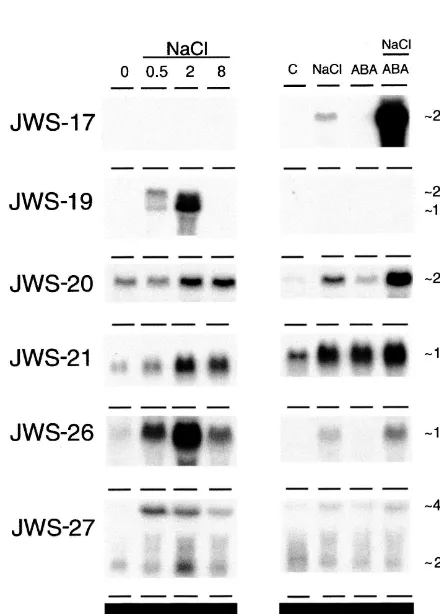
The majority of the isolated DD-PCR cDNAs correspond to mRNAs whose abundance in-creased in salt-treated roots. RNA blot
hybridiza-tion analyses were, therefore, carried out to confirm that the corresponding genes were ex-pressed in a salt-dependent manner. RNA was isolated from tomato roots treated with 170 mM NaCl for 0, 0.5, 2, 8, and 24 h and used for RNA blot hybridization analyses. Of the 20 cDNAs, the expression of the genes corresponding to two was constitutive whereas that of six others was induced or up-regulated in a salt-dependent manner. Sev-eral others gave no signal on RNA blots or corre-spond to genes expressed at a very low level in salt-treated roots. It is possible that these do not represent salt-responsive genes or, that they corre-spond to low abundance mRNAs that are not readily detected by RNA blot hybridization analyses.
following a salt treatment when a low level of transcript was present and at 24 h when the level increased. The estimated size (2.1 kb) of the tran-script corresponding to JWS-17 is consistent with the reported size of laccase mRNA from L. tulip -ifera [41]. A different expression pattern was ob-served for the gene corresponding to JWS-20 for which mRNA was clearly present at the 0 h time point. Transcript accumulation was observed at 2, 8 and 24 h after the onset of a salt treatment. The JWS-20 transcript was approximately 2.3 kb, which corresponds to the reported size of the piox transcript [42]. A low level of JWS-21 expression was apparent at 0 h and the level increased
there-after for the duration of a 24 h salt treatment. Genes corresponding to JWS-19, -26 and -27 were all transiently expressed in salt-treated roots (Fig. 4). Expression of the genes corresponding to each of these cDNAs was induced rapidly, within 0.5 h of a salt stress and, in the case of JWS-26 and – 27, was apparent at 2 and 8 h after which it declined. Expression of the gene corresponding to JWS-19 was not apparent beyond the 2 h time point. Two transcripts hybridized to the JWS-19 and -27 probes, both of which accumulated in a transient manner in salt-treated roots.
The expression pattern of the genes correspond-ing to these partial cDNAs was similar to that observed on the original differential display gel indicating that the overall pattern of displayed cDNAs on these gels was representative of actual changes in the root RNA population (Figs. 2 and 4, compare the expression patterns for JWS-17 and -19).
3.5. Regulation of gene expression by ABA
It has been proposed that ABA regulates changes in gene expression that occur in salt-stressed roots [17]. A comparison of DD-PCR products generated from salt or ABA-treated roots revealed that distinct RNAs accumulated in response to each of these treatments (Fig. 2, Table 1). This finding suggested that there might be an ABA-independent component that is involved in the regulation, at least in part, of many of the salt-induced changes in root RNA population. Therefore, the expression of genes corresponding to the six salt-responsive cDNAs was examined in the roots of ABA-treated plants as well as those exposed to a combined ABA/salt treatment. In addition, expression was examined in salt-treated roots of flacca (flc), an ABA-deficient mutant of tomato.
The expression of the genes corresponding to JWS-20 and -21 was responsive to exogenous ABA (Fig. 4). The transcript level induced by ABA was equivalent to that present in salt-treated roots for 21 but significantly lower for JWS-20. No expression of genes corresponding to any of the other partial cDNAs was apparent in ABA-treated roots. The imposition of a salt treatment together with exogenous ABA elicited an en-hanced transcript level over that present when either treatment was applied alone for JWS-17,
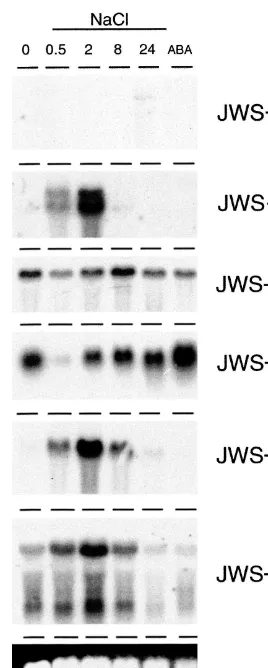
Fig. 5. Expression of genes corresponding to DD-PCR prod-ucts in salt-treatedflaccaroots. RNA was isolated from roots exposed to 170 mM NaCl for 0 h (0), 0.5 h (NaCl, 0.5), 2 h (NaCl, 2), 8 h (NaCl, 8) and 24 h (NaCl, 24), and roots exposed to ABA (ABA) for 24 h. Blots were hybridized with JWS-17, -19, -20, -21, -26 and -27. Exposure times were the same as those listed in the legend for Fig. 4. A representative ethidium-stained agarose gel is shown to indicate approximate equal loading of RNA samples.
sion was apparent inflcroots at the 0 h time-point than in AC roots. In addition, a lower transcript level corresponding to JWS-21 was apparent at the 0.5 h time point in flc roots relative to AC roots. In the roots offlc, only the genes corresponding to JWS-20 and JWS-21 were expressed in response to exogenous ABA.
4. Discussion
Differential display was used to visually exam-ine and compare the patterns of expressed mR-NAs present in salt- and ABA-treated tomato roots to that of non-treated roots. Subsequently, a number of salt-responsive partial cDNAs were isolated and the expression of the corresponding genes was examined in roots exposed to salt.
In previous studies [8,43] it was observed that a salt treatment resulted in the altered synthesis and accumulation of a number of prominent polypep-tides in tomato roots. In the present study, salt-in-duced changes in the accumulation of a number of different RNAs was demonstrated (Figs. 1 and 2). Both are indicative of a salt-induced activation and repression of gene expression. A substantial proportion of the changes in RNA accumulation that occurred in salt-treated roots were transient in nature and many occurred rapidly, within 30 min of a salt treatment. The rapid induction of gene expression is consistent with the salt-enhanced ac-cumulation of specific polypeptides in tomato roots in which the earliest changes were observed within 30 min of a salt treatment [8]. It is also consistent with the rapid induction of gene expres-sion previously observed in salt-treated roots [13,16,17]. In addition, Gulick and Dvorak [9] isolated a suite of early salt-induced (ESI) cDNA clones corresponding to genes that are expressed within 6 h of a salt treatment in L. elongatum roots. In these roots, the expression of many of the esi genes in response to salt was transient.
Genes corresponding to six of the 20 partial cDNAs were expressed in roots in a salt-depen-dent manner. This low percentage of salt-regulated genes may result, in part, from the insensitivity of RNA blot hybridization analyses with regard to detecting changes in RNA levels that can be read-ily demonstrated by PCR. In general, gene expres-sion was induced rapidly following the application of salt and several of the genes were expressed in a -20, and -26. The transcript level for JWS-21 was
approximately equivalent to that present in ABA or salt-treated roots. No transcript corresponding to JWS-19 was present in roots exposed to a combined treatment while the level of JWS-27 mRNA was approximately equivalent to that present in salt-treated roots.
expres-transient manner. The time-dependent accumula-tion of RNA coincided with the pattern observed for the original partial cDNA on differential dis-play gels and thus provided validity to the visual information derived from these gels. It was not possible to determine whether the altered RNA levels observed in salt-treated roots were a result of the osmotic or ion toxicity effects of the salt treatment. Nevertheless, it is likely that many of the changes were a consequence of the osmotic component of the stress for the following reasons. Firstly, previous studies have demonstrated a rapid induction of salt-responsive gene expression that was, in part, an osmotic response [17]. Sec-ondly, salt and water-deficit-stress elicit the accu-mulation of the same set of polypeptides in tomato seedling roots [43], and thirdly, differential display products recently identified in water-deficit-stressed tomato roots included two that were iden-tical to the partial cDNAs described here (Jin, Morem and Plant, unpublished data).
Exogenous ABA elicited the expression of just two of the partial cDNAs. This confirmed earlier observations that an ABA treatment induced changes in protein profiles and RNA abundance that were often distinct from those effected by salt (Fig. 2) [8]. Those genes that were responsive to ABA correspond to two of the three that were expressed 24 h after the imposition of a salt treat-ment. None of the genes expressed in a rapid and transient manner in salt-treated roots were ABA-responsive and it is possible that their expression is not regulated by ABA since endogenous ABA accumulation does not occur until 2 hours follow-ing a salt treatment [8].
Many previously characterized salt-responsive genes are also expressed in non-stressed roots that have been exposed to exogenous ABA and these have subsequently been described as ABA-respon-sive [16,17,44,45]. However, induction of gene ex-pression by exogenous ABA, while indicating that ABA may mediate expression in vivo, does not by itself demonstrate that endogenous ABA is actu-ally involved. Indeed, there are several examples that demonstrate that exogenous ABA can elicit the expression of salt-responsive genes that do not require ABA for expression [21]. In the roots of flacca, each of the genes corresponding to the salt-responsive partial cDNAs was expressed to a level comparable to that in roots of wild-type plants indicating that their expression may be
independent of ABA. However, since flc roots have the capacity to accumulate a limited amount of ABA in response to salt [8], expression in the roots of this mutant does not demonstrate that gene regulation was completely independent of endogenous ABA. Nevertheless, expression in flc roots is consistent with the apparent ABA-inde-pendent accumulation of several polypeptides in salt-treated tomato roots [8,43]. An ABA-indepen-dent component that regulates salt-responsive gene expression in roots would also be consistent with the salt-regulated expression of genes encoding osmotin and the 70-kDa subunit of the tonoplast H+-ATPase, which is independent of an elevated level of endogenous ABA in leaves of salt-treated tomato plants [19,20].
The enhanced expression of several genes in response to a combined ABA/salt treatment pro-vided further evidence indicating that ABA is not the only component that regulates gene expression in salt-treated roots. In particular, the level of JWS-17 mRNA present following a combined treatment was dramatically greater than that ob-served when either treatment was applied alone. This is indicative of a synergistic interaction be-tween salt and ABA possibly due to an overlap or shared component(s) between salt- and ABA-in-duced signal transduction pathways. Bostock and Quatrano [46] observed an additive induction of Em expression in rice suspension cells when ABA and salt were applied together suggesting that they activate separate transduction pathways. However, at sub-optimal concentrations, a synergistic rela-tionship was revealed, which they proposed was due to NaCl altering a component of the ABA response pathway such that the cells were more ‘sensitive’ to ABA in the presence of salt. A simi-lar phenomenon may be occurring in salt-treated roots exposed to exogenous ABA.
salt-dependent manner. JWS-17 is very similar to lac-cases (p-diphenol:O2 oxidoreductase) that have
been identified in a range of organisms including bacteria, fungi, insects, and higher plants [47]. The physiological role of these enzymes is for the most part poorly understood. Laccase oxidizes diphenol or dinaphthol compounds with the subsequent reduction of O2 to H2O. The oxidized aromatic
products of these reactions often polymerize with each other or with molecules in the extracellular matrix to form chemically resilient structures that serve to protect the organism from various envi-ronmental stresses [48]. During salt stress, laccase may play a similar role and thereby contribute to a strengthening of the cell wall.
The gene corresponding to JWS-20 was regu-lated in a salt-dependent manner and shared sig-nificant similarity to tobacco PIOX [42]. PIOX is expressed in tobacco cells following attack by Erwinia amylo6ora, wounding and treatment with
salicylic acid, jasmonic acid, and chemical agents that initiate the production of reactive oxygen species (ROS). Interestingly, PIOX shares 33% similarity at the amino acid level with cyclooxyge-nases involved in the formation of prostaglandins, and it has been proposed that it may be involved in the synthesis of lipid-derived signal molecules responsible for mediating the plant response to pathogen attack. More recently, it was demon-strated that the recombinant tobacco PIOX en-zyme is an a-dioxygenase that catalyses the first
step of a-oxidation of fatty acids [49]. The
func-tional significance of this pathway in plants is not known. However, since piox expression is patho-gen and salt stress-inducible, it is possible that the products serve as signaling intermediates that me-diate a general stress response. Alternatively, the PIOX may mediate the degradation of fatty acids released as a result of membrane damage, which itself may be caused by ROS. Further work is necessary to understand the role played by the products of the genes corresponding to the salt-re-sponsive cDNAs, as well as the factors that influ-ence their expression in salt-treated roots.
Acknowledgements
We would like to thank Bin Han for her help with PCR probe preparation and RNA blot hy-bridization, Greg Ehler for assistance with the
production of figures and Dr E.A. Bray for critical reading of the manuscript. J.-Z. Wei was sup-ported by a postdoctoral fellowship from the Nat-ural Sciences and Engineering Research Council of Canada (NSERC). This research was funded by a research grant from NSERC awarded to A.L. Plant.
References
[1] I. Szabolcs, Soils and salinization, in: M. Pessarakli (Ed.), Handbook of Plant and Crop Stress, Marcel Dekker, New York, 1994, pp. 3 – 11.
[2] U. Kafkafi, N. Bernstein, Root growth under salinity stress, in: Y. Waisel, A. Eiskel, U. Kafkafi (Eds.), Plant Roots: the Hidden Half, Marcel Dekker, New York, 1995, pp. 435 – 451.
[3] S. Ramagopal, Salinity stress induced tissue-specific proteins in barley seedlings, Plant Physiol. 84 (1987) 324 – 331.
[4] S. Ramagopal, Differential mRNA transcription during salinity stress in barley, Proc. Natl. Acad. Sci. USA 84 (1987) 94 – 98.
[5] P.J. Gulick, J. Dvorak, Gene induction and repression by salt treatment in roots of the salinity-sensitive Chi-nese Spring wheat and the salinity-tolerant ChiChi-nese Spring X Elytrigia elongata amphiploid, Proc. Natl. Acad. Sci. USA 84 (1987) 99 – 103.
[6] W.J. Hurkman, C.K. Tanaka, The effects of salt on the pattern of protein synthesis in barley roots, Plant Phys-iol. 83 (1987) 517 – 521.
[7] R.D. Chen, Z. Tabaeizadeh, Alteration of gene expres-sion in tomato plants (Lycopersicon esculentum) by drought and salt stress, Genome 35 (1991) 385 – 391. [8] C.S. Chen, A.L. Plant, Salt-induced protein synthesis in
tomato roots: the role of ABA, J. Exp. Bot. 50 (1999) 677 – 687.
[9] P.J. Gulick, J. Dvorak, Coordinate gene response to salt stress in Lophopyrum elongatum, Plant Physiol. 100 (1992) 1384 – 1388.
[10] N.R. Forsthoefel, D.W. Vernon, J.C. Cushman, A salin-ity-induced gene from the halophyte M. Crystallinum
encodes a glycolytic enzyme, cofactor-independent phos-phoglyceromutase, Plant Mol. Biol. 29 (1995) 213 – 226. [11] J.T. Jones, J.E. Mullet, A salt- and dehydration-in-ducible pea gene, Cyp15a, encodes a cell-wall protein with sequence similarity to cysteine proteases, Plant Mol. Biol. 28 (1995) 1055 – 1065.
[12] G.J. King, V.A. Turner, C.E.Jr. Hussey, E.S. Wurtele, S.M. Lee, Isolation and characterization of a tomato cDNA clone which codes for a salt-induced protein, Plant Mol. Biol. 10 (1988) 401 – 402.
[14] W.J. Hurkman, B.G. Lane, C.K. Tanaka, Nucleotide sequence of a transcript encoding a germin-like protein that is present in salt-stressed barley (Hordeum 6ulgare
L.) roots, Plant Physiol. 104 (1994) 803 – 804.
[15] A. Moons, J. Gielen, J. Vandekerckhove, D. van-der Straeten, G. Gheysen, M. van Montagu, An abscisic-acid- and salt-stress-responsive rice cDNA from a novel plant gene family, Planta 202 (1997) 443 – 454.
[16] A. Moons, A. De Keyser, M. van Montagu, A group 3 LEA cDNA of rice, responsive to abscisic acid, but not jasmonic acid, shows variety-specific differences in salt stress response, Gene 191 (1997) 197 – 204.
[17] A.F. Galvez, P.J. Gulick, J. Dvorak, Characterization of the early stages of genetic salt-stress response in salt-tol-erant Lophopyrum elongatum, salt-sensitive wheat and their amphiploid, Plant Physiol. 103 (1993) 257 – 265. [18] A.L. Plant, E.A. Bray, Regulation of gene expression by
abscisic acid during environmental stress, in: H.R. Lerner (Ed.), Plant Responses to Environmental Stresses, Marcel Dekker, New York, 1999, pp. 303 – 331. [19] M.L. Binzel, J.R. Dunlap, Abscisic acid does not medi-ate NaCl-induced accumulation of 70-kDa subunit of tonoplast H+-ATPase message in tomato, Planta 197
(1995) 563 – 568.
[20] S. Grillo, A. Leone, Y. Xu, M. Tucci, R. Francione, P.M. Hasegawa, L. Monti, R.A. Bressan, Control of osmotin gene expression by ABA and osmotic stress in vegetative tissues of wild-type and ABA-deficient mu-tants of tomato, Physiol. Plant 93 (1995) 498 – 504. [21] A. Savoure, X.-J. Hua, N. Bertauche, M. van Montagu,
N. Verbruggen, Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis follow-ing cold and osmotic stresses in Arabidopsis thaliana, Mol. Gen. Genet. 254 (1997) 104 – 109.
[22] N. Strizhov, E. Abraham, L. Okresz, S. Blickling, A. Zilberstein, J. Schell, C. Koncz, L. Szabados, Differen-tial expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis, Plant J. 12 (1997) 557 – 569.
[23] P. Liang, A.B. Pardee, Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction, Science 257 (1992) 967 – 971.
[24] E. Knaap, H. Kende, Identification of a gibberellin-in-duced gene in deep water rice using differential display of mRNA, Plant Mol. Biol. 28 (1995) 589 – 592. [25] X. Chen, B. Wang, R. Wu, A gibberellin-stimulated
ubiquitin-conjugating enzyme gene is involved ina
-amy-lase gene expression in rice aleurone, Plant Mol. Biol. 29 (1995) 787 – 795.
[26] Y.K. Sharma, K.R. Davis, Isolation of a novel ozone induced cDNA by differential display, Plant Mol. Biol. 29 (1995) 91 – 98.
[27] Y. Nemoto, N. Kawakami, T. Sasakuma, Isolation of early salt-responding genes from wheat (Triticum aes
-ti6umL.) by differential display, Theor. Appl. Genet. 98 (1999) 673 – 678.
[28] C.P. Joshi, S. Kumar, H.T. Nguyen, Application of modified differential display technique for cloning the 3%
region from three putative members of wheat HSP70 gene family, Plant Mol. Biol. 30 (1996) 641 – 646.
[29] T. Kleber-Janke, K. Krupinska, Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions, Planta 203 (1997) 332 – 340.
[30] T.C. Tseng, T.H. Tsai, M.Y. Lue, H. Lee, Identification of sucrose-regulated genes in cultured rice cells using mRNA differential display, Gene 161 (1995) 179 – 182.
[31] L.A. Brigham, H.-H. Woo, S.M. Nicoll, M.C. Hawes, Differential expression of proteins and mRNAs from border cells and root tips of pea, Plant Physiol. 109 (1995) 457 – 463.
[32] R.R. Johnson, H.J. Cranston, M.E. Chaverra, W.E. Dyer, Characterization of cDNA clones for differentially expressed genes in embryos of dormant and nondormant
A6ena fatua L. caryopses, Plant Mol. Biol. 28 (1995) 113 – 122.
[33] J.Q. Wilkinson, M.B. Lanahan, T.W. Conner, H.J. Klee, Identification of mRNAs with enhanced expression in ripening strawberry fruit using polymerase chain reac-tion differential display, Plant Mol. Biol. 27 (1995) 1097 – 1108.
[34] D.M. Tieman, A.K. Handa, Molecular cloning and characterization of genes expressed during early tomato (Lycopersicon esculentum Mill) fruit development by mRNA differential display, J. Am. Soc. Hortic. Sci. 121 (1996) 52 – 56.
[35] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Plant Physiol. 15 (1962) 473 – 497.
[36] A. Prescott, C. Martin, A rapid method for the quantita-tive assessment of levels of specific mRNAs in plants, Plant Mol. Biol. Rep. 4 (1987) 219 – 224.
[37] D. Bauer, H. Muller, J. Reich, H. Riedel, V. Ahrenkiewl, P. Warthoe, M. Strauss, Identification of differentially expressed mRNA species by an improved display technique (DDRT-PCR), Nucl. Acids Res. 21 (1993) 4272 – 4280.
[38] S.F. Altschul, T.L. Madden, A.A. Schaffer, J. Zhang, Z. Zhang, W. Miller, D.J. Lipman, Gapped BLAST and PSI-BLAST: a new generation of protein database search programs, Nucl. Acids Res. 25 (1997) 3389 – 3402.
[39] J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular Cloning: A Laboratory Manual, second ed, Cold Spring Harbour Laboratory Press, New York, Cold Spring Harbour, 1989.
[40] N.H. Brown, F.C. Kafatos, Functional cDNA libraries from Drosophila embryos, J. Mol. Biol. 203 (1988) 425 – 437.
[41] P.R. LaFayette, K.L. Eriksson, J.F.D. Dean, Character-ization and heterologous expression of laccase cDNAs from xylem tissues of yellow-poplar (Liriodendron tulip
-ifera), Plant Mol. Biol. 40 (1999) 23 – 35.
[42] A. Sanz, J.I. Moreno, C. Castresana, PIOX, a new pathogen-induced oxygenase with homology to animal cyclooxygenase, Plant Cell 10 (1998) 1523 – 1537. [43] S. Jin, C.S. Chen, A.L. Plant, Regulation by ABA of
[44] H. Amitai-Zeigerson, P.A. Scolnik, D. Bar-Zvi, Tomato
Asr1 mRNA and protein are transiently expressed fol-lowing salt stress, osmotic stress and treatment with abscisic acid, Plant Sci. 110 (1995) 205 – 213.
[45] J. Espartero, J.A. Pintor-Toro, J.M. Pardo, Differential accumulation of S-adenosylmethionine synthetase tran-scripts in response to salt stress, Plant Mol. Biol. 25 (1994) 217 – 227.
[46] R.M. Bostock, R.S. Quatrano, Regulation of Em gene expression in rice, Plant Physiol. 98 (1992) 1356 – 1363.
[47] J.F.D. Dean, K.L. Eriksson, Laccase and the deposition of lignin in vascular plants, Holsforschung 48 (1994) 21 – 33.
[48] P.R. LaFayette, K.L. Eriksson, J.F.D. Dean, Nucleotide sequence of a cDNA clone encoding an acidic laccase from sycamore maple (Acer pseudoplatanus L.), Plant Physiol. 107 (1995) 667 – 668.
[49] M. Hamberg, A. Sanz, C. Castresana, a-oxidation of
fatty acids in higher plants, J. Biol. Chem. 274 (1999) 24503 – 24513.