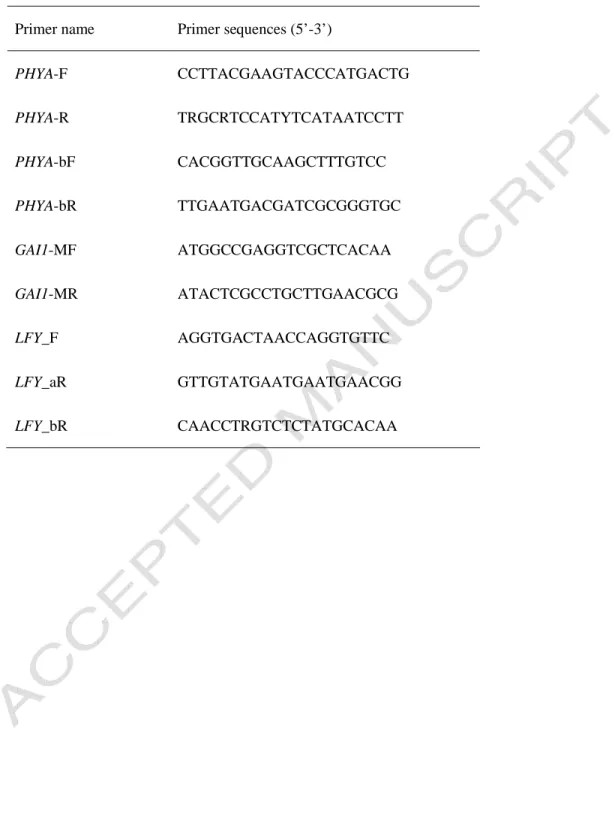
Phylogenetic and biogeographical complexity of Northern Hemisphere Magnoliaceae inferred from three nuclear datasets. Some of the ancient relict mesic forests that once covered much of the temperate regions of the Northern Hemisphere are today found in the southeastern US, as well as east to central China and central to southern Japan. About two-thirds of the species are currently distributed in temperate and tropical regions from East to Southeast Asia (Fig. 1).
One of the first important findings from molecular evidence was the polyphyly of the Magnolia section Rhytidospermum, with the North American Magnolia tripetala closely related to the Asian counterparts, rather than to M. The studies also showed that Manglietia and Michelia were nested within Magnolia. The ArabidopsisGA INSENSITIVE (GAI) locus and related genes of the DELLA subfamily encode growth regulators and have been implicated in quantitative variation for developmental traits (Peng et al. Subsequently, the divergence time of the lineage was specifically estimated to be mya based on plastid trnK, psbA_trnH and atpB_rbcL sequences using strict molecular clocks calibrated with fossil evidence (Azuma et al., 2001).
To update our biogeographic understanding of the family, we performed age estimation using “relaxed clock” analyzes and multiple fossil calibrations ( Renner, 2005 ). Previous classifications of the family by Dandy (1978b), Law (1984) and Nooteboom (1985) were also considered in our discussion.
ACCEPTED MANUSCRIPT
Results
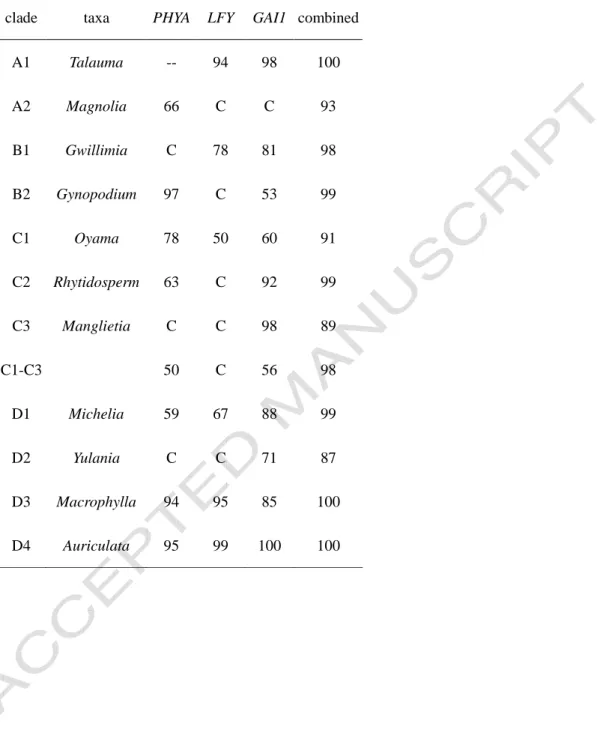
Clade B2 (subgenus Gynopodium in Figlar and Nooteboom, 2004) included the traditionally recognized Parakmeria and Manglietiastrum (Nooteboom, 1985), and was well supported by the nuclear data (PB = 99%, PP = 1.00 and MB = 100%) . The nuclear and chloroplast data sets for the remaining 31 taxa were thus combined for a phylogenetic analysis to evaluate the relationships among the deep clades of the family. However, the species was nested in a clade that included both subsection Yulania and section Michelia in the low-support nuclear data set (PB = 54%).
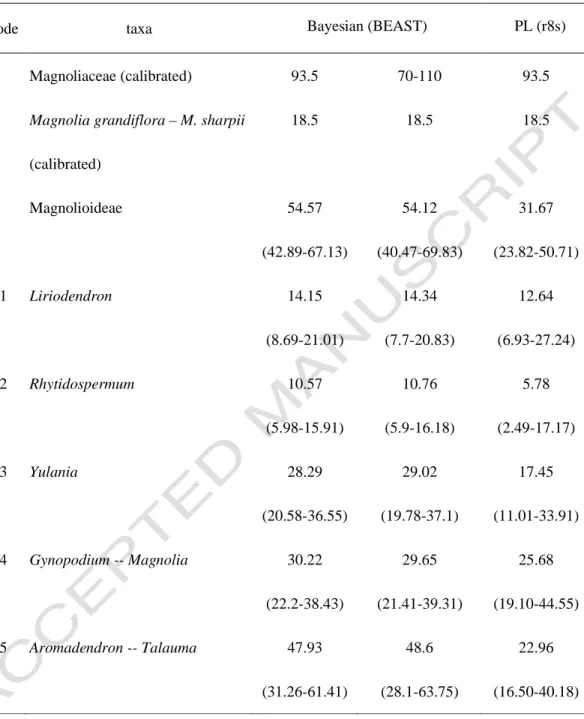
Magnolia elegans (previously known as Aromandron elegans) was sister to the Michelia group with high support in the chloroplast data (PB=100%), while it was quite isolated in the nuclear data (Fig. 4). The other was from subsection Rhytidospermum, between Magnolia tripetala and its Asian counterparts (node 2 in Fig. 6 and Table 4), which were calculated to have diverged about 10.57 mya. The split in the Yulania group (between the only North American Magnolia acuminata and all other Asian members, node 3 in Fig. 6 and Table 4) appeared to be older (28.29 billion years), although with support from low.
The tropical-subtropical segregated group of the subgenus Gynopodium – section Magnolia (node 4 in Figure 6 and Table 4) produced a divergence time of 30.22 mya. Finally, the disjunction for subsections of Aromadendron and Talauma (node 5 in Figure 6 and Table 4) was estimated at 47.93 mya.
Discussion
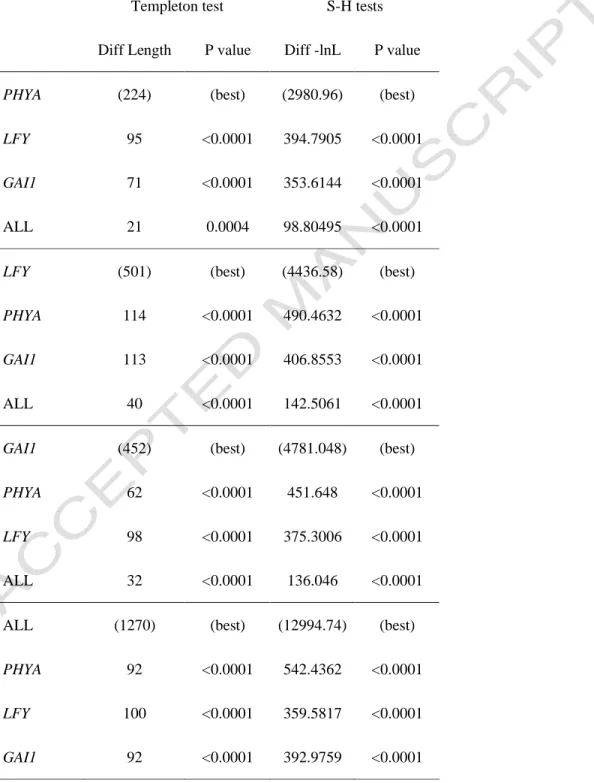
Our sampling included only a few representatives of the section, and additional sampling from the other two subsections will be necessary to determine their phylogenetic relationships. Chloroplast DNA data indicated that these two groups constitute distinct lineages separated from the Asian-North American Rhytidospermum clade (including one North American species of M. tripetala). It is difficult to compare the fit of the model used in Bayesian analysis with PL (Roger and Hug, 2006).
Seeds of the Yulania group are usually flat, symmetrical, and cordiform to bean-like in shape (Tiffney, 1977; Azuma et al., 2001). Fossil seeds agreed with the divergence of the Yulania group in the middle Oligocene to early Miocene (Dorofeev et al., 1974; Figlar, 1993; Tiffney, 1977;). The crown group of the subfamily Magnolioideae was estimated to be 54.57 mya old, inferring an early diversification of the extant magnolias at the Paleocene-Eocene boundary in the early Tertiary.
This correlates with the "Paleocene-Eocene Thermal Maximum" event, which marked the start of the Eocene, the fastest and most extreme global warming recorded in geologic history (Lourens et al., 2005). Since the age of the tribe Magnolioideae was determined to be 93.5 mya based on fossil evidence (Frumin and Friis), the crown age was estimated to be about half as old as its stem age (Table 4). Fossils suggest that Magnolioideae arose before the Tertiary, and that the divergence of the extant magnolias (except Liriodendron) occurred at the earliest in the Eocene.
The great Eocene diversification of the family is also supported by the marked increase in fossils of the magnolias during the same period. Phylogenetic utility of the LEAFY/FLORICAULA gene in the Caesalpinioideae (Leguminosae), gene duplication and a new insertion. Molecular phylogeny of Magnolia based on chloroplast DNA sequence data (trnK intron, psbA-trnH and atpB-rbcL intergenic spacer regions) and floral fragrance chemistry. Eds.), Proceedings of the International Symposium on the Family Magnoliaceae.
Divergence of the phytochrome gene family dates angiosperm evolution and suggests that Selaginella and Equisetum arose before Psilotum. The structure and function of phytochrome A, the roles of the whole molecule and of its different parts. The phytochrome gene family in grasses (Poaceae), a phylogeny and evidence that grasses have a subset of the loci found in dicotyledonous angiosperms.
Molecular phylogenetics of the Magnoliidae, cladistic analyzes of nucleotide sequences of the plastid gene rbcL. A phylogenetic study of chloroplast DNA from the disjunct section Rytidospermum of Magnolia (Magnoliaceae) in eastern Asia and eastern North America. Some aspects of the plant geography of the Northern Hemisphere during the Late Cretaceous and Tertiary.
Bootstrap values for each clade inform maximum parsimony analyzes of each of the three nuclear sequences and of the combined data (C: clade collapsed; --: single sample).