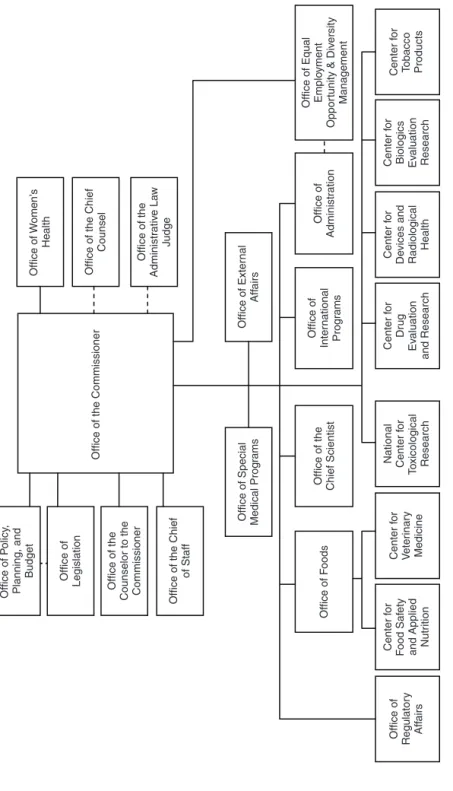
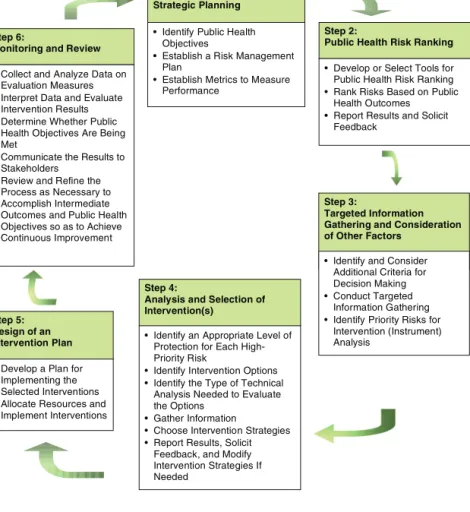
See Table 2-2 for details on the responsibilities of the FDA centers and offices involved in food safety. The FDA has six program centers: (1) the Center for Biologics Evaluation and Research, (2) the Center for Drug Evaluation and Research, (3) the Center for Devices and Radiological Health, (4) the Center for Food Safety. . Further description of the proposed approach to risk-based food safety management Step: Strategic Planning.
The committee therefore views the risk-based approach as the underlying structure for all FDA food safety decisions. The provision of these resources is critical to the success of FDA's future food safety risk management activities.
Recommendation 3-1: The type of risk-based food safety approach outlined by the committee in Box 3-2 should become the operational
Furthermore, although the FDA states in many of its documents that it operates under a risk-based framework, many of the characteristics of a risk-based system that the committee considers necessary (notably, strategic planning, comprehensiveness, transparency, external review of risk assessment and intervention analysis programs, and risk communication) are not sufficient in the agency's current approach. The required resources (personnel, data, models) to design and support a risk-based food safety management system are extensive, and FDA does not have the human capacity, data infrastructure, or organization to support such a function at this time. The committee offers the following recommendations to improve the management of food safety at FDA.
The following recommendations include essential steps that require special attention in implementing a risk-based approach.
The FDA, in collaboration with partners, should identify metrics with which to measure the effectiveness of the food
Food Safety: FDA's imported seafood safety program shows some progress, but further improvements are needed. Federal Review of Food Safety: High-Risk Designation Can Bring Needed Attention to Fragmented System. Letter Report on the Reiew of the Food Safety and Inspection Series Proposed risk-based approach to and application of recognition for public health.
As part of the strategic planning process (Step 1 in the risk-based system described in Chapter 3), the responsibilities of all parties in achieving the desired level of food security should be articulated. The FPP makes several statements regarding the responsibilities of the various parties in the food safety system. In addition, the FPP supports the exploration of new roles for third-party certification as part of the overall food safety assurance system.
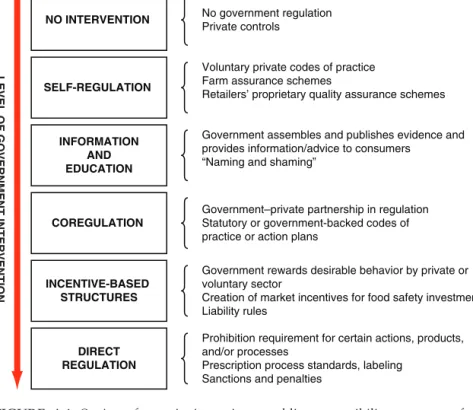
The above statements indicate that FDA is focusing on the need for shared responsibility in the design of its food safety program. In several exchanges with FDA staff, however, the committee did not find that FDA has a well-thought-out approach to defining food safety responsibilities beyond these general statements. At one end of the spectrum, food security is entirely an individual, private responsibility and there is no intervention by public agencies.
Whatever governance models are chosen or policy interventions used to achieve them, food security will always be the responsibility of many partners. Different intervention choices incorporate different responsibilities to ensure that the desired level of food security is achieved. Overall, however, third party action is clearly an important part of a risk-based system of shared responsibility for food safety.
The Committee found that the FPP does not express a clear approach to the roles of private parties and other governments in ensuring the food safety of products imported into the United States. The structure of private standards for food safety management has been developing particularly rapidly in the last decade (Henson, 2008).
To ensure food safety, the FDA should develop a plan for defining the extent of and form for sharing responsibilities with
The committee did not receive any results from this pilot program and could not evaluate it as an approach to shared responsibility for food safety. Food safety in the United States is the responsibility of suppliers, farmers, food handlers, processors, wholesalers and retailers, food service companies, consumers, third-party organizations, and government (federal and state) agencies both in the United States and abroad. It is therefore unrealistic to expect the FDA, or any government agency, to have sufficient resources to manage food safety without the help of others who share this responsibility.
A risk-based approach to the choice and design of interventions (steps 4 and 5 in a risk-based system) requires a comprehensive understanding of the policy intervention toolkit and a roadmap for choosing and designing interventions. Furthermore, developing an approach for defining the roles of other responsible parties is part of strategic planning in a risk-based food safety system. In essence, this roadmap should also serve to assign shared responsibility among the federal government, the private sector, third parties, other governments, states, and consumers.
Designing new management approaches to achieve food security is currently the subject of experimentation by other governments and debate among scientists. The committee found that FDA has made ad hoc efforts in this direction, but lacks a clear regulatory philosophy for assigning responsibility for food safety or a comprehensive strategy for selecting the level and intensity of interventions as part of the country's risk-based strategic planning. approach.
The FDA should develop a comprehensive strat- egy for choosing the level and intensity of policy interventions needed
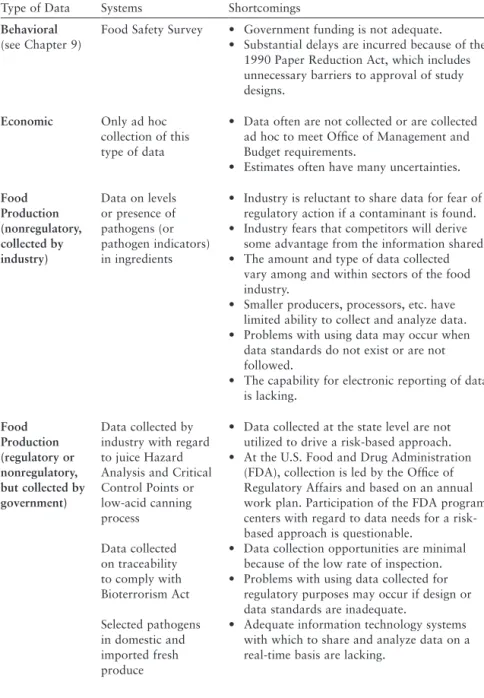
To support the achievement of this goal, the FDA's information infrastructure must provide a foundation for risk-based decision making in all aspects of food safety management. To support risk-based decision-making, the FDA must have information related to the production, processing, and storage of food, including the size of the regulated industry and the distribution channels. With coverage in every state and territory, the FDA's field staff and delegates are well positioned to generate and provide data that can be used in the agency's risk-based decision making.
The participation of FDA program centers regarding data needs for a risk-based approach is controversial. This redaction of information delays data sharing; at worst, it prevents or delays FDA's use of the information to protect public health. The response states that the CDC withholds food safety data from the FDA until it "analyzes" and "interprets" the data.
There is also evidence of significant limitations and delays in the flow of food safety information from the FDA to state and local governments. The FDA's responses to the committee's questions about data sharing identify several federal laws that limit what food safety information it can share with state and local authorities. These federal laws clearly pose real barriers to information sharing between the FDA and state and local governments.
The Committee does not know whether this regulation has prevented or delayed the sharing of essential food safety information by State and local governments with the FDA. Many different groups collect food safety data for various purposes that can be valuable to the FDA's regulatory mission. The FDA should also assist state and local food safety agencies in providing such training to state and local employees.
During the spinach-related outbreak in 2006, e.g. failure in the FDA's email system for delays in responding to the outbreak (FDA Science Board, 2007). As noted in Chapter 3, one way to meet FDA's analytical staffing needs would be to create functional teams to support the risk-based approach.
Data collection by the FDA should be driven by the recommended risk-based approach and should support risk-based
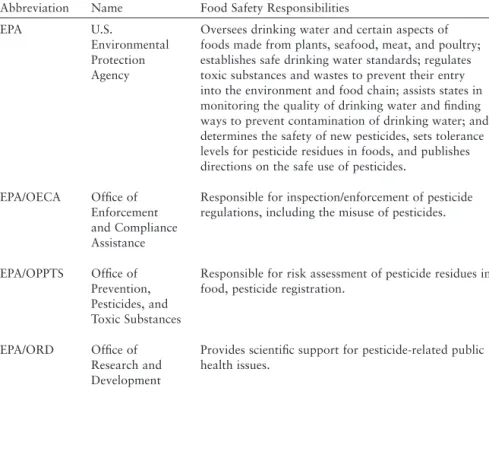
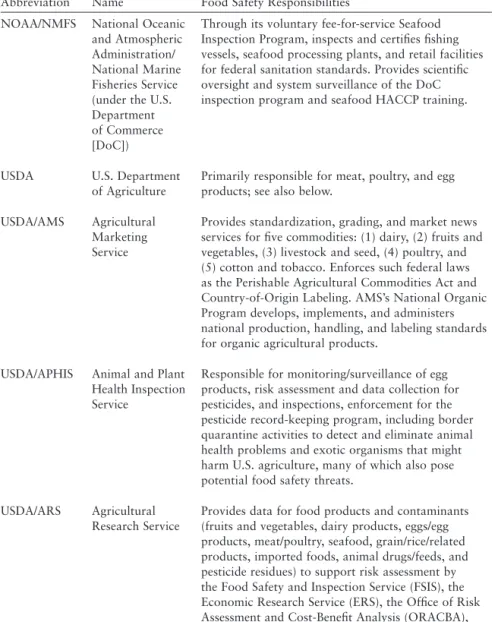
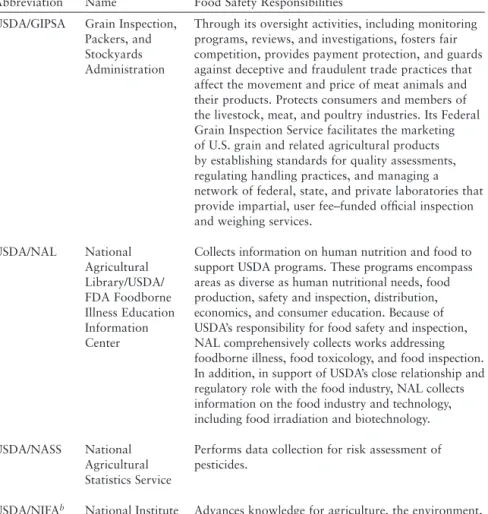
With respect to sharing data with other relevant partners (eg, CDC, U.S. Department of Agriculture, food industry), it appears that the legal regime now in place would allow for a significant increase in such data sharing. Non-legal barriers, both technological (e.g. inadequate IT) and cultural (e.g. unnecessary delays in sharing data or lack of trust), continue to limit the sharing of data between partners. In support of a risk-based approach driven by data, the committee makes the following recommendations.
Recommendation 5-2: The FDA should evaluate its personnel needs to carry out its roles in collecting, analyzing, managing, and commu-
The FDA should evaluate statutes and policies governing data sharing and develop plans to improve the collection and
To protect public health, federal, state, and local agencies and industry must share more information about food safety and share it more quickly than is the case now. Identifying specific beliefs to target to improve restaurant employees' intentions to perform three important food safety behaviors. Food and Drug Administration's (FDA's) food safety research functions are performed primarily by three intramural funded centers—the Center for Food Safety and Applied Nutrition (CFSAN), the Center for Veterinary Medicine (CVM), and the National Center for Toxicology Research (NCTR) )—with ' some involvement of the Office of Regulatory Affairs (ORA).
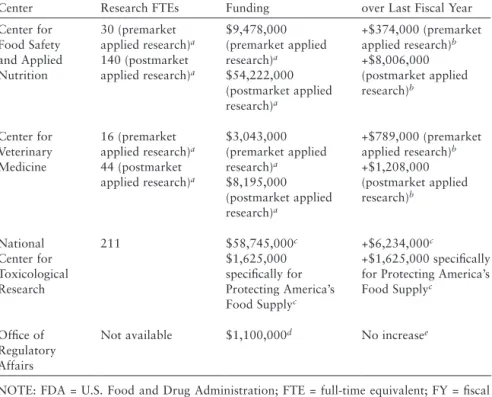
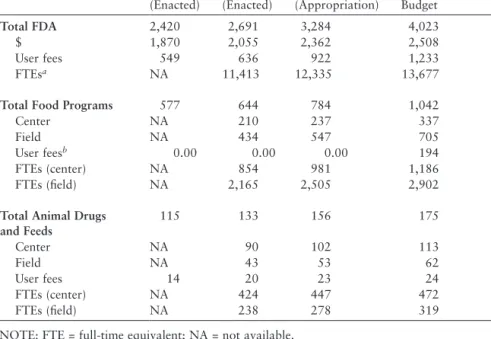
The agency's food safety research mission is also supported by external research centers in formal collaboration with academic institutions, as well as several other activities. Food security research at these intramural and extramural centers is summarized by topic in Appendix F. For FY 2010, center-specific resource allocations for research are summarized in Table 6-1. In general, the agency's food safety research initiatives can be categorized as follows:
Information on the proportion of FTEs dedicated to food safety compared to nutrition was not available, but the vast majority of the research focus is food safety, with an emphasis on chemical and microbiological public health hazards and, more recently, , in food protection. As with research FTEs, the committee was unable to obtain information on funds allocated solely to CFSAN's food security mission. As mentioned, however, most of the research in CFSAN has been devoted to food security.
In support of the center's mission, KKRT has identified seven Centers of Excellence (see Box 6-2). The committee was not provided with information with which to determine the percentage of KKRT's research budget devoted to food safety. The committee reviewed information received from FDA related to NCTR, including NCTR Strategic Plan 00-0, Accepted Publications FY00, Food Publications NCTR 00-00, and a summary of food safety expenditures and food safety research FTE for NCTR, 2009a,b,c).
Of the five strategic goals, Goal 3 deals directly with food safety, while Goals 4 and 5 include broad support for FDA's mission, which clearly includes food safety. It should be noted that many of NCTR's food safety publications are on items not regulated by the FDA, such as processed eggs and poultry (e.g., Kiess et al., 2007; Khan et al., 2009 ).