The concentration of Ir in the oceans is fairly uniform with depth and location, ranging from 2.9 to 5.7 x 108 atoms kg-1. The amount of Ir in Ktr boundary sediments is ::::: 103 times the total amount in the oceans. Part H: The biogeochemistry of methyl bromide (CH3Br) in the oceans was investigated using a steady-state mass balance model.
Part II, Methyl bromide: Ocean sources, ocean sinks, and climate sensitivity, establishes the importance of the marine biosphere in the production of methyl bromide. Finally, Part III, C02 Stability and Heterogeneous Chemistry in the Martian Atmosphere, is an exploration of the chemistry of a clearly abiotic atmosphere. A satisfactory explanation for the C02:CO ratio in the Martian atmosphere has long been elusive (the "C02 stability problem").
Not So Noble: An Introduction to the Low-Temperature Geochemistry of Rhenium and Iridium
Overview
Current dating of the Chicxulub structure demonstrates that a massive collision occurred 65 million years ago (Swisher et al., 1992). A critical entry point for these questions is the chemistry of PGEs in natural waters. The main obstacles to a better understanding of PGE in natural waters have been analytical.
Excellent, comprehensive reviews of the PGE include the historical survey of McDonald and Hunt (1982), the geochemical review edited by Cabri (1981), and the chemical reviews of Hartley (1991).
The Noble Metals
- Iridium and the Other PGE
- Rhenium
A thorough recent review of the literature on PGE and Re in the solid Earth and meteorites by Blum (1990). By the mid-19th century, their inertness made these metals useful in early electrochemical research by M. Around this time, the commercial use of PGE was aggressively promoted by P.
Modern non-catalytic applications include the use of Pt in jewelry and in optical glass processing. One of the more recent applications of Pt is the use of the compound cisplatin (PtC12(NH3 )2) as an antitumor drug. It is also a component of the Paris standard meter stick, which is a 90% · Ptl10% Ir alloy.
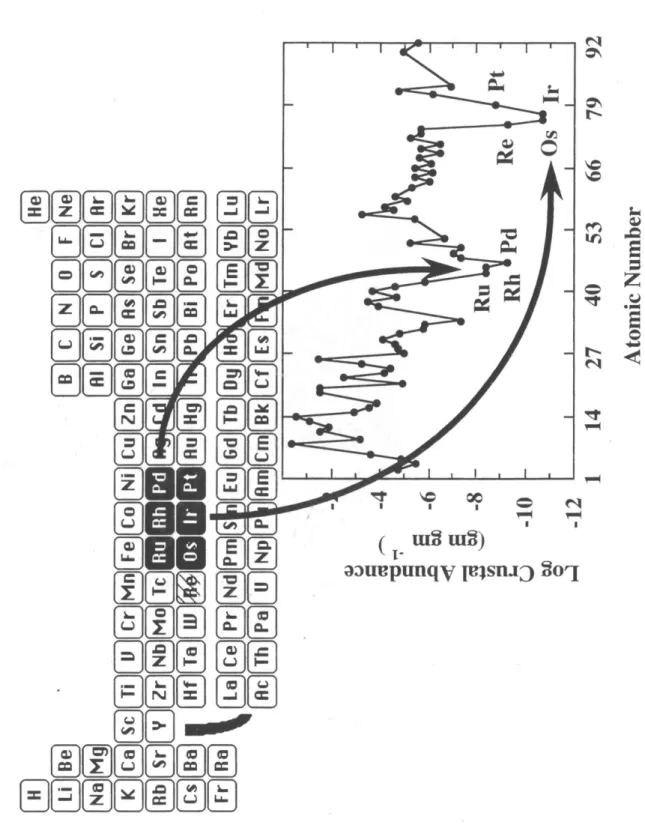
In the case of the Bushveld complex, chromium and sulphide orebodies contain 3 to 20 ppm PGE. PGEs have a very siderophilic character on the solid Earth; these elements prefer to be bonded to each other, either to Fe or Ni, rather than to O or S. Therefore, during the accretion and differentiation of the Earth, PGE and Re separated into the metallic phases Fe~Ni, which eventually formed the Earth's core , leaving the crust and mantle heavily depleted in siderophiles.
Thermodynamic models of mantle PGE content cannot easily reproduce chondritic relative abundances of. The partitioning of PGE and Re between the mantle and crust is better understood, although the mineralogical controls on this behavior are not well understood.
The Ignoble Elements: PGE Solution Chemistry
Various models have been used to explain PGE abundances in the mantle, including the impact of a Mars-sized object after nucleation. Ir, Os and Ru are very compatible in the mantle and therefore do not distribute easily in the melts that form the crust. Recent findings suggest that PGE solution chemistry may redistribute these elements in the environment (Bowles, 1986; Fuchs and Rose, 1974; Wallace et al., 1990).
Because Pt metal was discovered in such deposits, it's ironic that the discovery of the noble metals might have been delayed if it weren't for the chemistry of the solution. As noted by Cousins and Kinloch (1976), the idea that the character of precious metals determines PGE distributions in the environment "appears to be somewhat overstated". Conversion between the two species is easily achieved in the laboratory by acid or base titration, and is completely reversible.
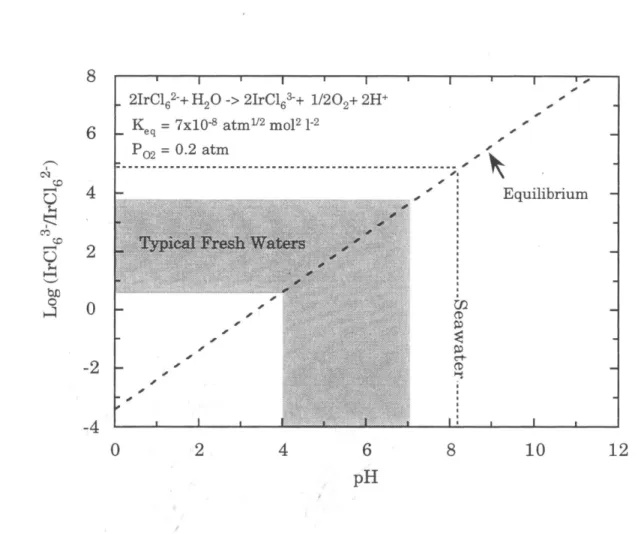
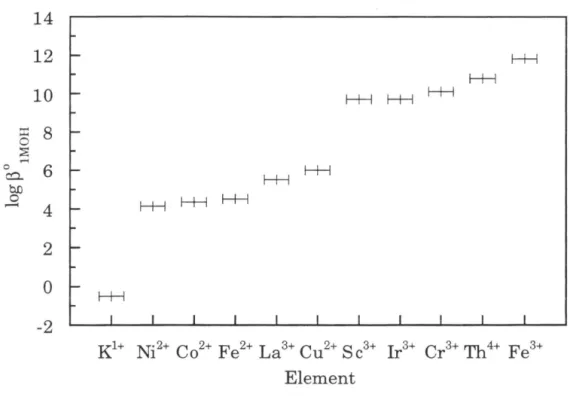
Thus, Ir is likely to be highly reactive toward phases commonly found in natural waters, such as Fe-Mn oxyhydroxides and clay mineral surfaces. In the oceans, the slow scavenging of Ir by settling particles or autogenous phases can be an important removal mechanism. The affinity of the PGE for organic ligands in natural waters has been considered theoretically and is probably important for Pt and Pd (Wood, 1990; Wood et al., 1994).
However, Ir has a rich organometallic chemistry in the laboratory in the Jrl+ valence state (Cotton and Wilkinson, 1988). In oxidizing environments, Re is present in the heptavalent oxidation state, which forms strong bonds with oxygen.
Cabri, Analytical methods for the platinum group elements, in Platinum group elements: mineralogy, geology, recovery, edited by L. Hoffman, Platinum group metals in common plants of boreal forests: developments in analytical methods and the application of biogeochemistry to exploration strategies, J. Rose, Het geochemical behavior of platinum and palladium in the weathering cycle in the Stillwater Complex, Montana, Econ.
Bertine, Comparative marine chemistries of platinum-group metals and their periodic table neighbors, in Metal Speciation: Theory, Analysis, and Application, edited by J. Boynton, Chicxulub Crater: A Possible Cretaceous/Tertiary Boundary Impact Crater in the Yucatan Peninsula, Mexico, Geology. Rubi, High-pressure and high-temperature experiments on mantle-core fission in the accreting Earth, Sci.
Lopezoliva, Age, deposition and biotic effects of the Cretaceous-Tertiary boundary event at Mimbral, NE Mexico, Palaios. Wasson, Siderophilic inter-elemental variations in Cretaceous-Tertiary boundary sediments from Caravaca, Spain, Earth Planet. Hay, Geochemistry of rhenium and osmium in recent sediments from the Black Sea, Geochim.
Vlassopoulos, Solubility and spectroscopic studies of the interaction of palladium with simple carboxylic acids and fulvic acid at low temperature, Geochim. The Platinum Group Elements (PGE) in the Periodic Table, and their abundances in average continental crust.
Rhenium in Seawater I
Confirmation of Generally Conservative Behavior
Abstract
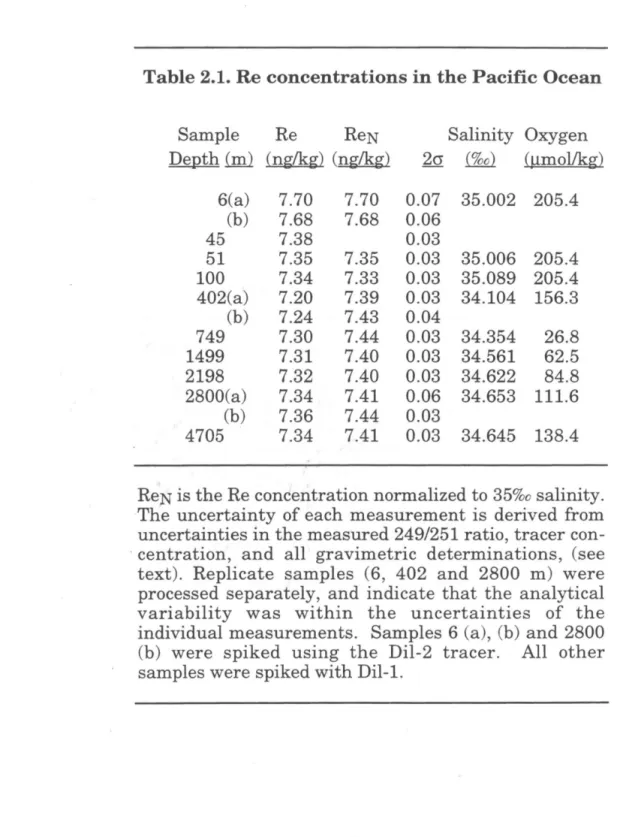
For example, studies of the marine distribution of Pt have each yielded a different trend with depth. To solve the problem of seawater Re concentration and its distribution with depth, we have applied ID-NTIMS to the study of Re in a profile of the Pacific Ocean. Since approximately 1.5 ng Re was typically separated from seawater quantities, the resulting blank correction was less than 3%o of the total sample Re present.
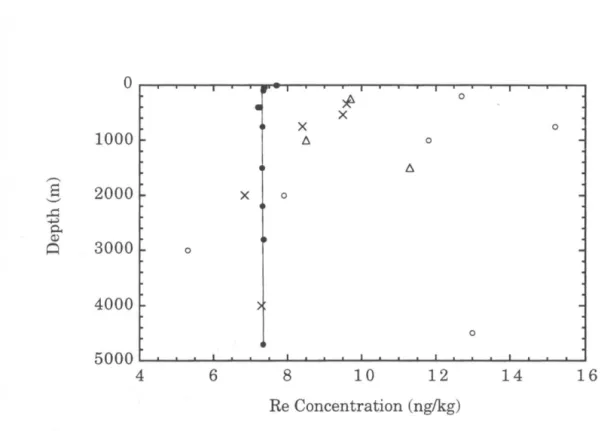
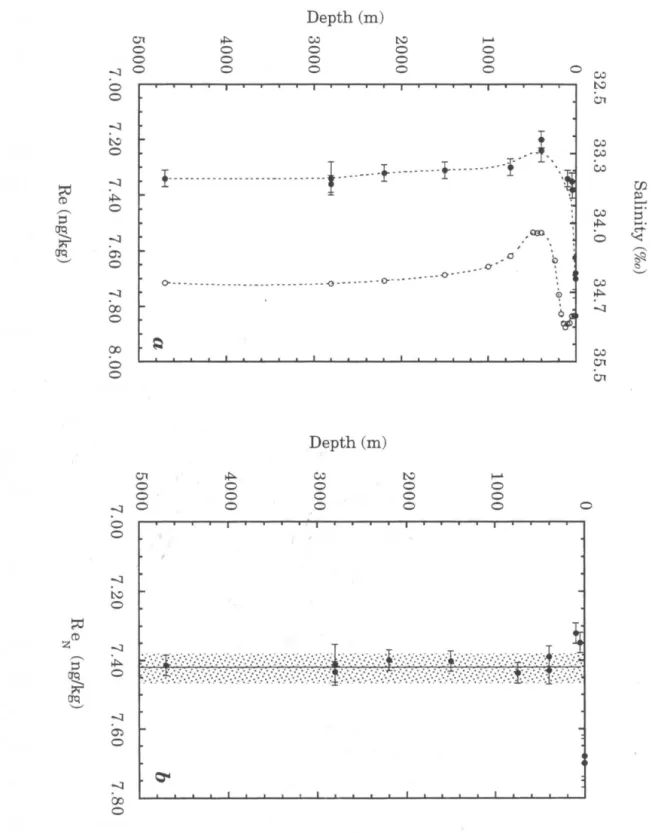
A comparison of the depth profile determined in this study with the Pacific profiles of Koide et al. Our determination is within the broad range of the analyzes of Scadden (1969), Matthews and Riley (1970), and Koide et al. 1987), but is significantly higher than the value from the Atlantic study by Olafsson and Riley (1972). As suggested by Koide et al. 1987), we suspect that the uncertainty in these earlier studies is largely due to variation in the yield of the chemical separation of Re from seawater.
It should be noted that some of the previous studies included data from several locations (see Table 2.2). This discrepancy has since been traced to an error in the calibration of the 185Re spike used for the ICP-MS study (Colodner et al., 1995). The salinity-normalized data suggest that the 51 m sample may also be somewhat depleted in Re relative to deep water, although this difference is within measurement uncertainties.
The measured Re concentration at the surface is :::: 4% higher than the average of the other samples, which is well above the analytical uncertainty bands. This interpretation would require a nearly constant amount of Re to be lost from most of the samples, to account for the uniformity of the salinity-normalized values below 402 m.
Goldberg, Determination of rhenium in seawater and sediments by graphite furnace atomic absorption spectrometry, Anal. Turner, The role of particles in regulating seawater composition, in Aquatic Surface Chemistry, edited by W. The uncertainty of any measurement derives from uncertainties in the measured 249/251 ratio, con- tracer.
Replicate samples (6, 402 and 2800 m) were processed separately, indicating that the analytical variability was within the uncertainties of the individual measurements. Uncertainties in (b), (c) and (f) were reported by the authors as ± lcr of the measurements, but were converted to ± 2cr for comparison with this study. No uncertainty estimates are included in (d) and (e); by analogy with (b) and (c), we calculated their uncertainties as ± 2ct.
Our average, (a), includes all measurements below 400 m, and the uncertainty is reported as ± 2cr of these values. Mean values from studies (a) and (f) are reported on a salinity-normalized basis. ;, Rhenium and Iridium in Natural Waters 51. The solid circles are data from this study; other points are from Koide et al.
Rhenium and Iridium in Natural Waters.. a) Depth profiles of salinity (open circles) and rhenium (solid circles) measured in this study. The vertical line represents the mean Re concentration of the samples below 400 m (7.42 ng kg-1), and the shaded area indicates the 95% confidence limits of this value (± 0.04 ng kg-1).
The Filament Loading Blank
A priori, it was not clear whether this variation was due to peak depletion over time, an increase in the blank filament contribution over time, or a combination of these effects. The accurate determination of Re concentrations by isotope dilution requires strict control of the blank contribution of the filament or activator. If this contribution varies during the analysis of a sample, the accuracy of the ratio measurement is reduced.
Such a change may result from the accumulation of Re on the surface of the filament as atoms diffuse from within the metal. Although the gaps calculated from the fixed ratios (Fig. 2.A.l) are small compared to typical sample sizes (z 1 ng), the possibility of a variable gap that could grow to much larger values was of concern. . The effects of sample depletion and gap growth can be decomposed by applying a simple model which assumes that the only contributions to the measured ratios come from the charged material and the filament gap and that these two reservoirs do not mix prior to emission.