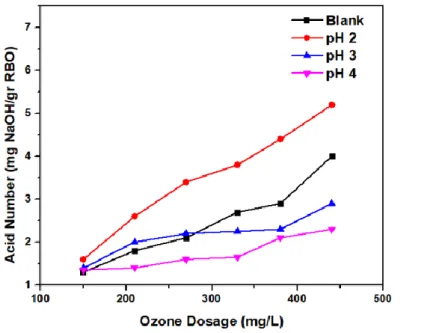
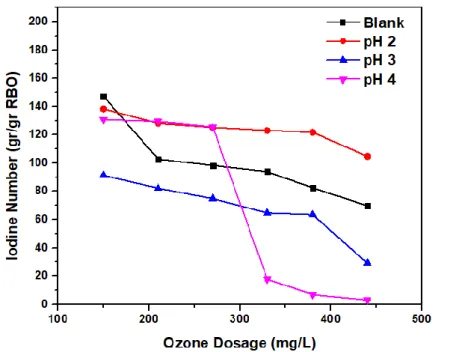
According to this study, pH 4 and an ozone dose of 440 mg O3/L are the optimal parameters used in the RBO ozonation process. Also include hypotheses in the form of a chemical reaction after the addition of ascorbic acid or the presence of tocopherol. These findings indicate that the higher the ozone dose used in oil ozonation, the lower the iodine number.
A reduction in the iodine number indicates the breaking of the double bond due to the breakdown by ozone that generates single bonds in unsaturated fatty acids that create saturated compounds due to the Criegee mechanism. The higher the ozone dose in the RBO ozonation process, the lower the RBO iodine number value because more ozone breaks the double bond (de Almeida Kogawa et al., 2004). This indicates that the lower the concentration of ascorbic acid in RBO, the lower the iodine number.
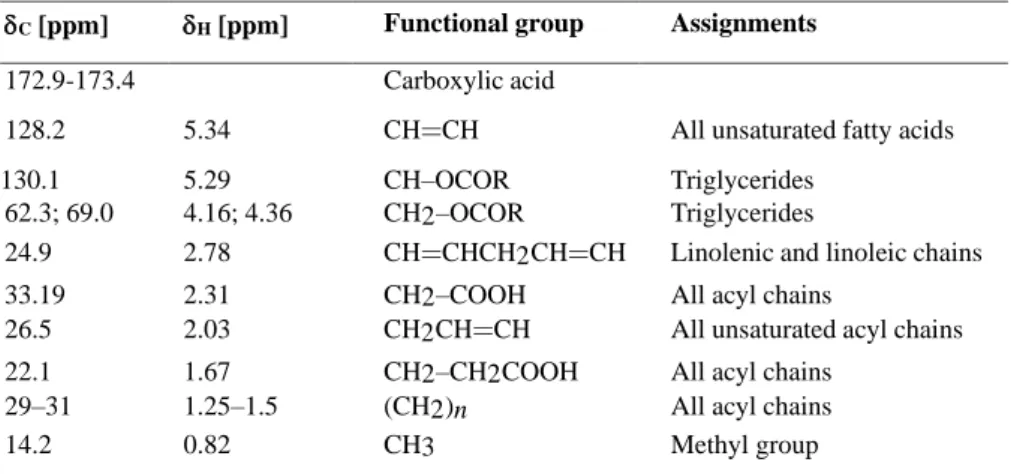
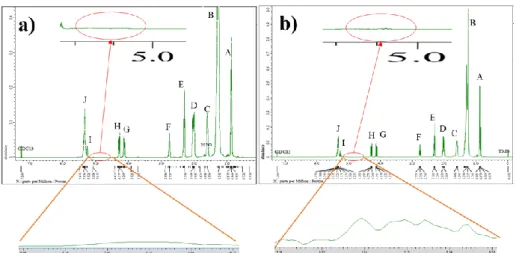
The RBO sample without ozonation forms 8 signals in the 13C NMR spectrum data shown in Figure 7a. The H signal (10 – 35 ppm) is the same as the A,B,C,D,E 1H NMR signal showing methyl compounds and compounds with CH2 bonds, carbonyl bonded or not. 13C NMR spectra for RBO a) before and b) after the ozonation process. Cleavage of the C double bond in linolenic acid (C18:3) and linoleic acid (C18:2) results in an increase in C18:1 and saturated fat.
The purpose of adding ascorbic acid is to lower the pH so that ozone is more stable and reacts more easily with the C double bonds in the fatty acids in RBO;.
However, related to tocopherol, in the analysis and discussion section part, the author does not show the data more clearly, the discussion appears not so deep
In general, this topic is the same as an article published with a link above. The preceding article concentrates on the influence of ozonation time and ozone dosage on the properties of ozonated RBO. In this work, the effects of pH (with the addition of ascorbic acid) and ozone dosage on the properties of ozonated RBO are investigated.
Although the dependent variables evaluated are the same as in the previous study, the modifying factors that are the focus of this study are different.
Can Tocopherol be analyzed using GC-MS?
Comment 5: Need to identify by GC-MS for RBO, before and after ozonization? How to preparation sample? Or Direct Analysis?
What the meaning this sentences?
How to ascorbic acid can stabilize the ozone? Please explain more clearly and put references
How?
Need to be equipped with experimental data related to the content of tocopherol in RBO before and after ozonization
How much amount RBO? ascorbic acid? Ratio?
How much amount RBO? How much mL water?
Need to be equipped with experimental data related to the content of tocopherol in RBO
400 Mhz?
Please write down the condition analysis using GC-MS?
It should be clarified in this article, there is no need to wait for further analysis
Pease make in table
The H-NMR spectra of 6a and 6b looked the same, there was no significant difference in both peak profiles and chemical shifts
The 13C-NMR spectra of 7a and 7b looked the same, there was no significant difference in both
All free fatty acids have such characteristics
Is there any experimental data in this study, that there is Tocopherol in RBO as well as in Ozonized RBO?
Why? Please make some explanation
Can Tocopherol be analyzed using GC-MS?
We have already added the data as shown in Table 2. Note 18: The H-NMR spectra of 6a and 6b looked the same, there was no significant difference in either peak profiles or chemical shifts.
Please to add and describe the structure of compounds that produce during the RBO ozonated in the body of your Results and discussion part
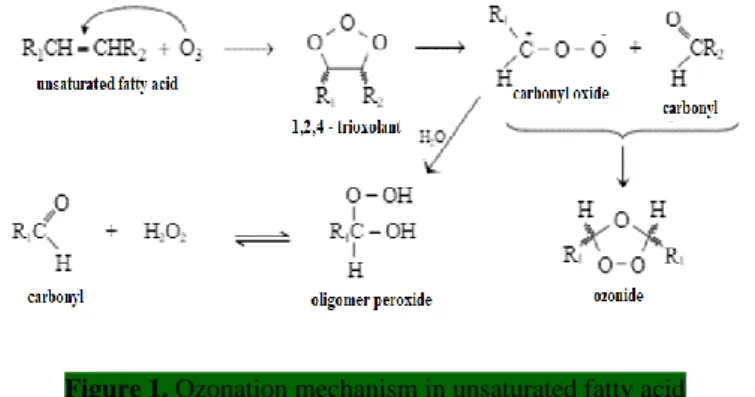
Due to the high concentration of unsaturated fatty acids in RBO, such as oleic acid and linoleic acid, it can be used as a raw material for the production of trioxolane and peroxide as an active medicinal component through the ozonation process with the Criegee mechanism (Criegee, 1975), which is beneficial in the body's fight against free radicals, as shown in Figure 1. According to previous research, trioxolane was not detected in RBO's NMR test, although peroxide was. Since ozone is stable at acidic pH, addition of ascorbic acid attempts to reduce the pH of the RBO and ascorbate emulsion combination until it achieves a low pH.
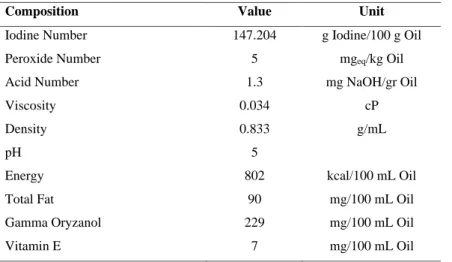
Furthermore, the acid value indicates how much the triglycerides in the oil sample have broken down to create free fatty acids. The density was determined by weighing a pycnometer in the absence and presence of RBO samples. Gas chromatography spectrometry (GCMS) GC Agilent 7890B tandem MSD 5977 A (California, USA) with n-hexane as a solvent was used to evaluate the fatty acid. The sample to be examined was taken ± 10 µL, then mixed solvent in n-hexane to 1000 mL, then vortexed and sonicated for 15 minutes until completely dissolved.
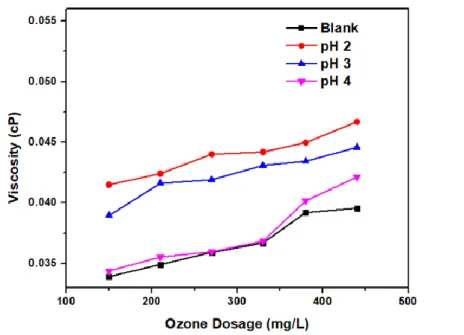
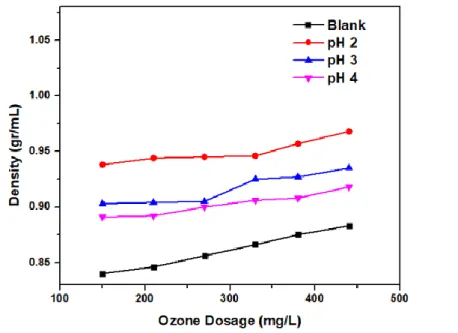
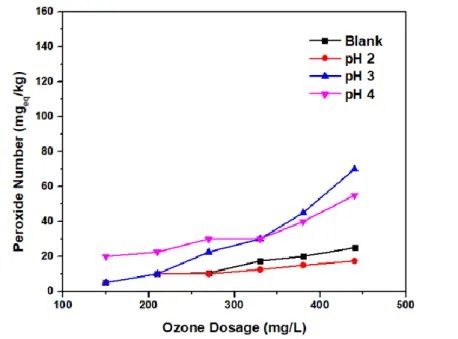
The effect of the pH of the ozonation process on the viscosity is shown in Figure 2, where the ozone dose is 440 mg O3/L, and with a lower pH, the viscosity and density values rise. As more ozone doses were used in the RBO ozonation process, newer chemical compounds were formed, especially peroxide and aldehyde compounds (Figure 3), causing the oil density to increase. Another study on the ozonation of vegetable oils found that a drop in the level of ester chains was caused by a decrease in unsaturated fatty acids due to ozonation, which caused.
The increase in acid number is due to the following factors: (1) Ozone is stable at acidic pH (pH 4), resulting in direct ozonation by O3 (Pera-Titus, Garcı́a-Molina, Baños, Giménez, & Esplugas, 2004; Langlais, Reckhow, & Brink .The amount of ozone reacting with the unsaturated fatty acids produces more ozonide/trioxolane because trioxolane is unstable and easily converted to carboxylic acid and other products; and (3) the addition of ascorbic acid solution to RBO to lower the pH automatically increases the volume of water in RBO. According to the above explanation, the increase in the maximum peroxide value shows that the ozonation process at high doses of ozone causes the entry of many double bonds in the oil, which are oxidized by ozone, with larger ones. and Bocci, 2008).
The presence of high amounts of polyunsaturated fatty acids present in RBO is indicated by (F) = 5.34 ppm with the triplet characterized by the olein proton signal, with the top peak indicating oleic acid and the second peak indicating linoleic acid. Comparison of the 13C NMR spectra between RBO and RBO ozonated at pH 4 with the ozone dose of 440 gr O3/L shows that some of the chemical shifts of the main components involved in the ozonation reaction can be detected in each signal. The RBO sample without ozonation forms 8 signals in the 13C NMR spectrum data shown in Figure 8a.
The D,E,F signal (60-70 ppm) was the same as the G&H1H NMR signal in the 4 ppm range, indicating glycerol and triglycerides. The same trend is also observed in the ozonation of olive oil and sunflower oil, where the ozonation takes place gradually according to the suitability of the ozone gas reactant, and after ozonation, the content of linoleic acid (C18:2) is greater than that of oleic acid (C18:1) (Diaz et al., 2005).