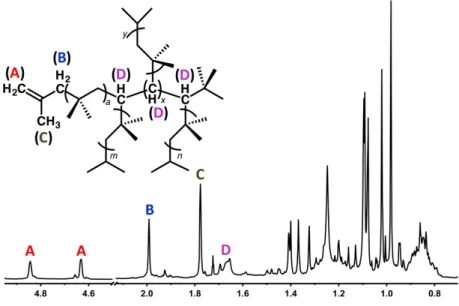
Time-dependent density functional theory (TD-DFT) was used to explain the reactivity by comparing the LUMO energy of hypercloso-B12(OBz)12 and F600 with the HOMO energy of the styrene monomers. Label A indicates protons from the olefinic chain end; B/C, allylic protons of the chain tip; D, methine protons. The original aim of the work presented in this chapter was to improve on the results reported in the initial B12(OR)12 photooxidation paper (Messina et al.
Reproducible spectra could not be collected under the given conditions and we were unable to obtain excitation spectra that matched the absorption spectra of the neutral clusters. We wonder what the nature of the purple species was and whether it could be responsible for the observed fluorescence spectra. Comparison of the UV-visible absorption spectrum of the purple F60 samples showed an uncanny resemblance to the published spectrum of [F60]1-.
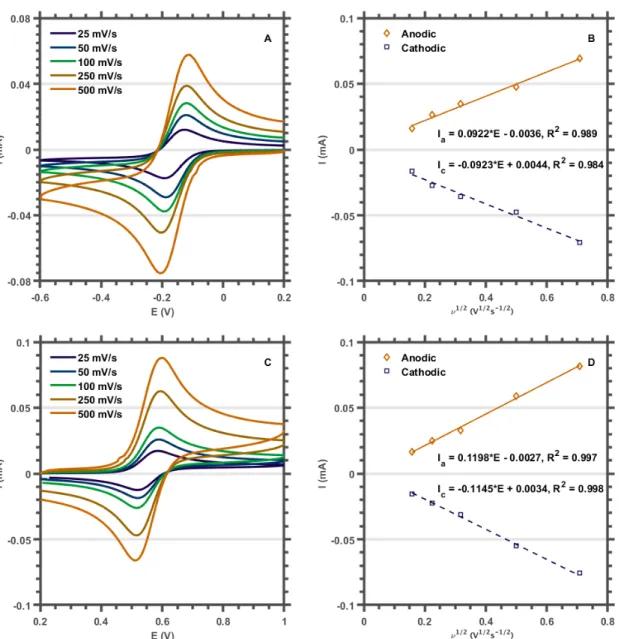
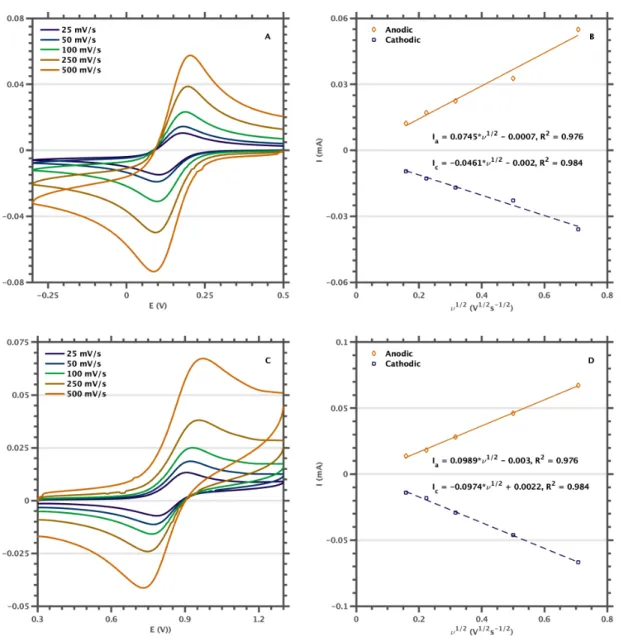
We assumed that the purple species resulted from B120 photoreduction to [B12]1- and that the reported extinction coefficient for [F60]1- was incorrect. The influence of scan rate (n) on the peak potential (ip) was investigated to assess the reversibility of the redox events. After electrochemical characterization, we used spectroelectrochemistry to show that the published extinction coefficient for the [F60]1-525 nm band had been significantly underestimated and that the true spectrum was in good agreement with the purple species we had observed during our initial work .
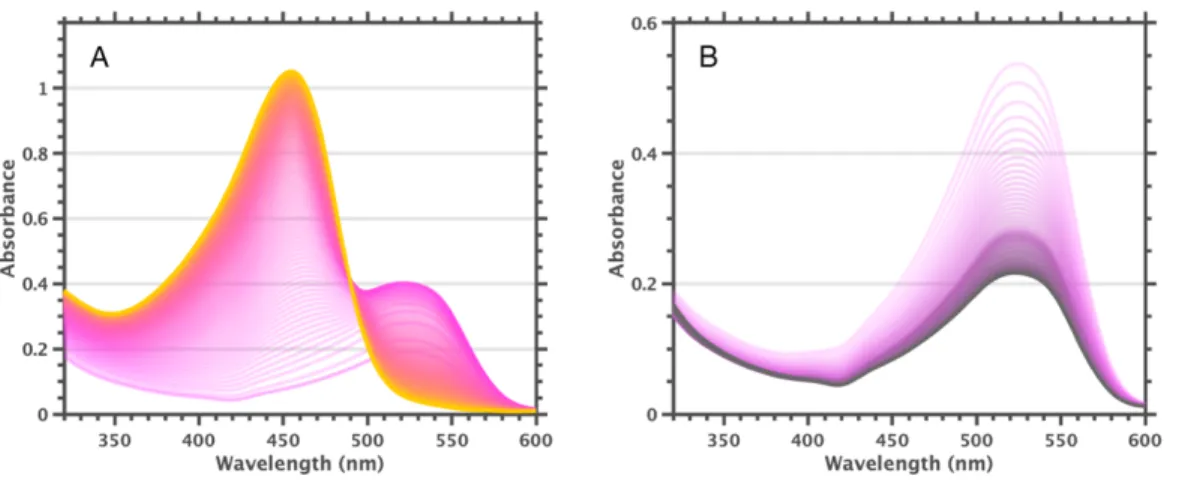
These data led us to conclude that [F60]1- has a visible absorption band at 525 nm with approximately half the extinction coefficient of the 455 nm band of F600.
Interactions with Haloarenes
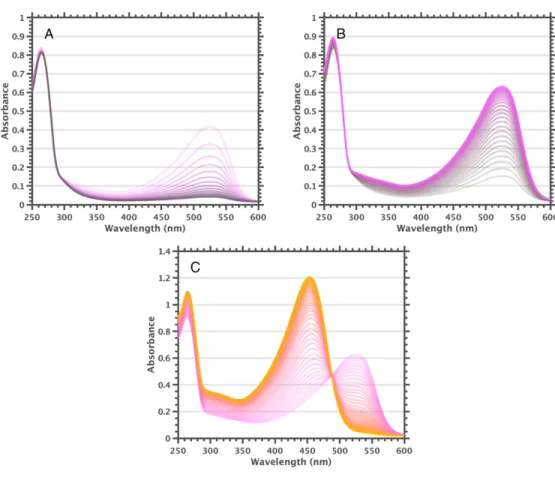
The F840 samples show a prominent 525 nm band even in pure 1,2-difluorobenzene, and addition of a 1,2-diiodo-tetrafluorobenzene species leads to complete conversion to [F84]1-. More halogenated haloarenes result in a higher conversion rate to the [B12]1- state (hexafluorobenzene » 1,2-diiodo-tetrafluorobenzene > . 1,2-difluorobenzene > 1,2-dichlorobenzene > chlorobenzene); no 525 nm shoulder is observed under low light conditions for F600 in chlorobenzene, while the solutions contain little vol%. These observations indicate that interactions between clusters and haloarenes result in a new species exhibiting the 525 nm band, apparently [B12]1-.
Addition of triethylamine to solutions of F600 results in rapid conversion to [F60]1- (high triethylamine concentration leads to reduction to colorless [F60]2-). In fact, reversible bleaching and ingrowth of the 455 nm and 525 nm bands can be achieved by addition of base followed by addition of acid, or vice versa. It is noteworthy that the size of the 455 nm band at 26 mM trifluoroacetic acid is approximately the same as the size of the 525 nm band of the starting solution.
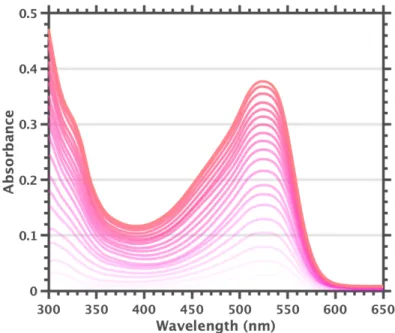
The UV-visible spectrum of equilibrated solutions of 2-MeTHF F600 at room temperature indicates complete conversion to [F60]1-. However, we cannot currently achieve temperatures low enough to clearly separate the 455 nm band. It is worth nothing to observe vibrio progression in the 525 nm band at cryogenic temperatures, with a spacing of .
Based on these observations, we hypothesize that F600 in 2-MeTHF is completely converted to [F60]1- by (photo)oxidation of the solvent and that cooling to cryogenic temperatures causes disproportionation, giving a spectrum dominated by the F600 455 nm band. After these procedures, no 525 nm shoulder was observed in the UV-visible spectra of F600 samples prepared under low light conditions in dichloromethane, benzene, and toluene. A smaller 525 nm shoulder could be observed for benzene and toluene with prolonged and/or intense light exposure.
Further purification of 1,2-difluorobenzene, or use of solvents from different manufacturers, did not remove the 525 nm shoulder. Solutions of F600 in 2-MeTHF, which appears to undergo complete conversion to [F60]1-, provide further support for assigning the 600 nm fluorescence band to [F60]1-. We now believe that the samples used for the reported extinction coefficient measurements dominated the colorless dianionic species, creating the illusion of a relatively weak band 525 nm band.
Under low light conditions, no 525 nm band is observed in the UV-visible spectrum of F600 in dichloromethane, benzene, and rigorously purified toluene, suggesting that. However, after sufficient illumination, the 525 nm band increases even in samples prepared with rigorously purified solvents.
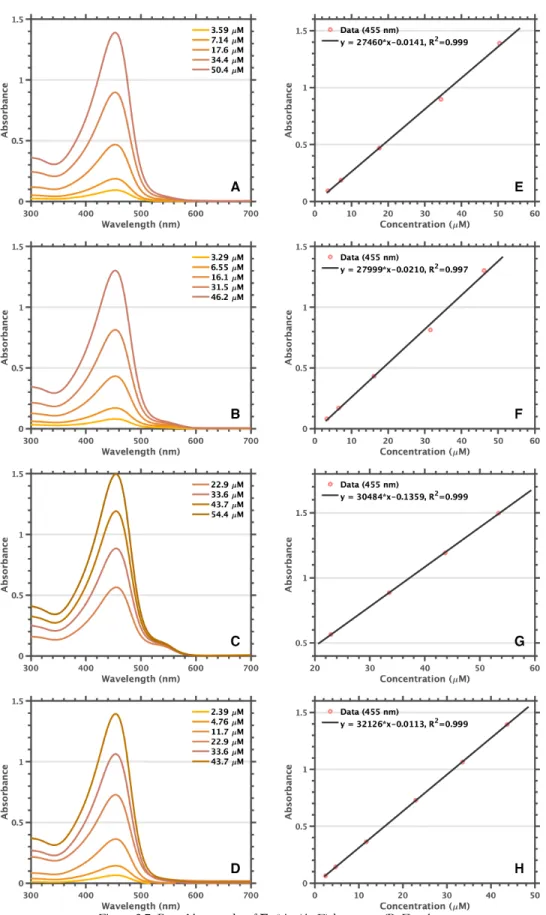
Experimental Details
Spectroelectrochemical data were collected on a Pine WaveNow potentiostat, using a 0.5 mm quartz spectroelectrochemical cell (BASi) and gold grid working electrode (BASi). Hexafluorobenzene (Sigma-Aldrich, NMR grade) was added in 1 µl increments until a total of 18 µl had been added. A 1 vol% solution of trifluoroacetic acid was added to the sample in 25 µl increments until a total of 500 µl had been added.
After completion of the acid titration, the sample was titrated with a 1 vol% solution of triethylamine in 1,2-dichlorobenzene, added in 25 µl increments until a total of 225 µl had been added. A small piece of F600 was added to the tube and vacuum dried for >30 minutes, during three argon/vacuum cycles. UV-visible samples were prepared in screw-cap cuvettes with a path length of 1 cm in a nitrogen-filled glove box.
Variable temperature NMR spectra were collected on a Varian 400 MHz spectrometer with OneProbe broadband autotuning. UV-visible spectra were corrected by subtracting the solvent background spectra collected at each temperature point. After preparation, the sample was illuminated with a 450 nm laser pointer to achieve complete conversion to [F60]1-.
The samples were immersed in liquid nitrogen to obtain a 2-MeTHF glass, which resulted in an instantaneous color shift from deep purple to bright yellow. Solid compounds were handled in the open air, but all steps after adding solids to the sample compartment were performed using standard air-free techniques. Preliminary fluorescence spectra were collected from all samples, whether [B12]1- was present or not.
All steps after addition of compound to the sample preparation vessel were performed under argon/vacuum using standard airless techniques. Solvent was added by cannula, the samples mixed and finally transferred to the EPR tube under argon reflux. An Unpaired Electron Confined in an Icosahedral Borane Cage: Synthesis and Crystal Structure of the Blue, Air-Stable {[Closo-B12(CH3)12]·}– Radical.