Proceedings of the United States
National Museum
SMITHSONIAN INSTITUTION
•WASHINGTON, D.C.
Volume123 1967 Number3607
The Role
oftheDepressor Mandibulae Muscle
in Kinesis ofthe
Avian
SkullBy
Richard Zusi AssociateCurator, Division ofBirdsIntroduction
Basic structuresof the facial
and
palatal portions of thebird's skull areunderstandableonlyin termsofthekineticpropertyoftheskull—
the ability to
move
the upperjaw
with respect to the braincase.Although
avian kinesiswas known
to 18th-century anatomists and has been analyzed variously in terms of muscles, ligaments,and
adaptive modifications, the importantcomplex
of structures consti- tuting the jaw-quadrate linkage has not been studied sufficiently inany
species to permita detailed evaluationof the actionsof themajor jaw
muscles.My
purposehereisto examineasinglemuscle,M.
depressormandib- ulae, to determine the effectsupon jaw
action of variations in muscle configurationand
variations in thejaw
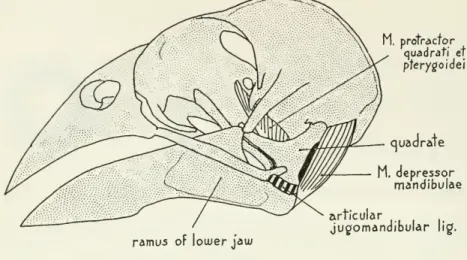
articulation through which the muscle acts. (See fig. 1 for structures under discussion in this paper.)My methods
include experiments based on stimulation of muscles in live birds, construction of models of the muscle-ligament sj'stem,and
manipulation of freshand
preserved specimens.The
1
M. protractor quadrati et pterygoidei
ramus of lower jaw
M. depressor mandibulae articular
juc/omandibular lig.
Hesperfphona vespertine
ramus of lower jaw
postorbital ligament
M. protractor quadrati et pfery^oidei
drate
M. depressor mandibulae retroarticular process articular
jugomandibular lig.
Gallus domestfcus
Figure1.
—
Structural features of theheadintheevening grosbeak (Hesperiphonavespertina)and domestic chicken {Gallus domesticus). (Adductor and retractor muscle groups removed.)
no. 3607 KINESIS
OF AVIAN SKULL —
ZUSI3 most commonly
stated action ofM.
depressor mandibulae is to open the lower jaw, but emphasis herein will be placed on the interaction between the postorbital ligamentand
the depressor mandibulae in protraction (raising) of the upper jaw. Several authorshave
postu- lated such a protraction effect through the postorbital ligament (Kripp, 1933,pp. 556-559;Starck, 1940, pp. 618-620; Barnikol, 1952, pp. 382-384; Zusi, 1962, p. 47; Bock, 1964, pp. 19-22), explainingits action, withsome
variations, as follows: the ligament is a virtually unstretchableband
runningfrom
the cranium to the mandibleand
attaching anterior to the quadrate articulation,where
the ligament provides a fulcrum aroundwhich
the lowerjaw
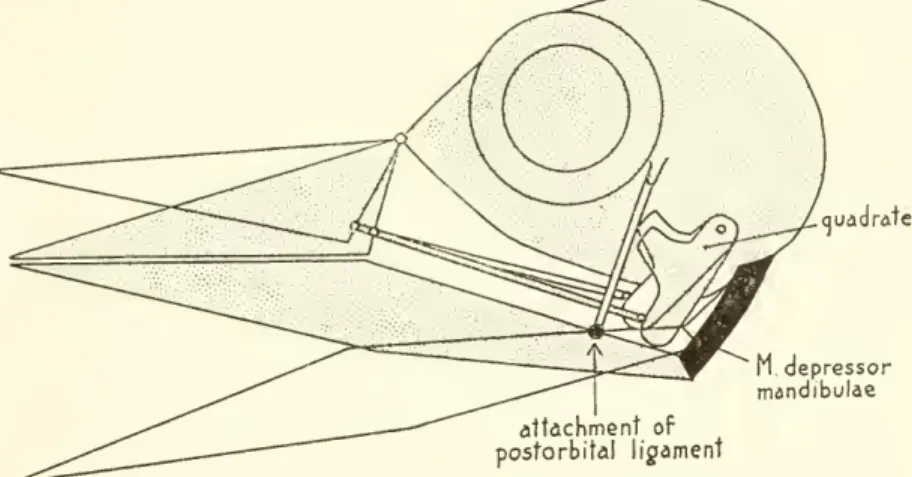
pivots (see fig. 2).The
depressormandibulaerotates that portion of the lowerjaw
lyingM depressor mandibulae attachment of
postorbital ligament
Figure 2.
—
Diagram of protraction of the upper jaw through the depressor mandibulae and postorbitalligament. Thelowerjawpivots abouttheattachmentofthe ligament.As thelowerjawopens, the quadrateis pushed forwardand theupperjaw israised.
behind the attachment
upward and
forward, rocking the quadrates forwardand
therebyprotracting theupperjaw.The
importantpointis that thelower
jaw
pivots about the ligamentary attachmentrather than about the quadrate articulation as iscommonly
stated.Bock
(1964) has explored the significance of the postorbital ligament
and
has presented anumber
of hypotheses about avian kinesis that willbe discussedbelow.
The
group of structures that support the lowerjaw form
a closely interactingfunctionalcomplex thatispartofthe largercomplexofthe entire kineticmechanism.The
supportsystemincludes the quadrate, the rami of the lower jaw, the jaw-quadrate articulation,and
the musclesand
ligaments associatedwith these structures.Most
of thejaw
muscleshavethe potentialformoving
thequadrateeitherthroughdibulararticulations.
Motion
of the quadrateshas a effecton
motion of the upperjaw and on
the lowerjaw
as well;motion
of the lowerjaw may
in turn induce shifting of the quadrates through features of thejaw
articulationand
ligaments. Understanding the functional properties of the complex kineticmechanism
is thus verydifficult, but it is essential for the interpretation of variation of these structures in birds.
The
depressormandibulae
has considerable potential for evolu- tionary development of functional-anatomical variation. Because of its superficial position, its originmay expand
posteriorly over the neck muscles or dorsallyand
anteriorly over the skulland
adductor musclesproviding variationin bothsizeand
angleof pull of themuscle in different birds.The
musclemay
play a rolein protraction of the upperjaw
as well as in depression of the lower jaw,and
therefore, itmight be expected to
show
modifications for feedingmethods
that requireforceful opening of both jaws or the upperjaw
alone, or for resisting forces on thebill.During
the spring of 1963, Ulrich Kalkofen, then a senior honors student undermy
supervision at the Universityof Maine, undertook a series of pilot experiments to test hypotheses about the role of the postorbital ligament in kinesis (Kalkofen, 1963).The
data obtained formthebasis forthe followingsectiononjaw
action. Iwishtothank
Paul C. Harris for providing the chickens used for this studyand Jon Greenlaw
for his help in performing the experiments. Financial assistancewas
providedby
theCoe
ResearchFund (R
625-49) of the University of Maine.The
final organization of the paper bene-fited greatly
from
the constructive criticisms of George E.Watson and
Paul Slud. Iam
indebted to Walter J.Bock
for providingme
with a translation of the 1958 paper
by
Yudin.Experiments on
Jaw
ActionMethods. —
Live birds used for experiment were the eveninggrosbeak (Hesperiphonavespertina)
and
the domestic chicken (Gallus domesticus)—
hens of parentage female barredPlymouth Rock X
male Rhode
Island red. These species were especially suitable because they were readily obtainableand
easily kept in captivity,and
because the postorbital ligament is poorly developed in the grosbeakand
stronglydevelopedin the chicken.For
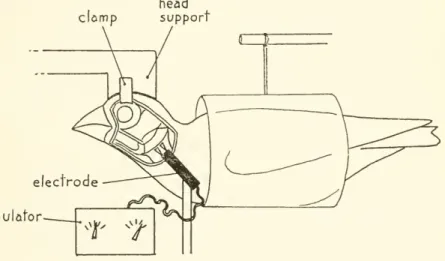
eachspeciesawooden head
supportwas
constructed toconform with the contour of the mid-dorsal surface of the cranium; each supportwas
held rigidly in placeby
metal clamps that penetrated under the supraorbital rims of the skull. Birds were first weighedno. 3607 KINESIS OF AVIAN
SKULL —
ZUSI5 and
then anaesthetizedby
intramuscular injection of equitheesin (0.20 ml. per 100grams body
weight, ormore
if necessary). Great carewas
taken to ensure that thebirdswere anaesthetized completely beforeand
during the experimentation. Feathers of thehead and
neck weretrimmed and
the skin of both sides of the head reflected as soon as the bird lost consciousness. After the head supportwas
fastened firmly to the bird, the supportwas
held motionlessby
a viseand
thebody
of the bird supportedby
the table or horizontally suspended in a plastic tube (fig. 3).The
exposedjaw
muscles were kept moistand
clean throughout the experimentsby
periodic flushing with avian Ringer's solution at 38° C.The
depressor mandibulae muscles were caused to contract simultaneouslyby
a tetanizingclomp
head support
electrode stimulator
Figure 3.
—
Diagram of experimental setup for stimulating the depressor mandibulae muscles. Thebillwasphotographedasviewedinthediagrambeforeandduringmuscle stimulation.stimulus of 20 volts (using
two Harvard
apparatus stimulators) applied through electrodes.The
tips of both copper wires of each electrode were fitted with platinum wire bent as a triangle with one point of the triangle soldered to the wireand
the opposite side of the triangle placed against the surface ofthe muscle.The
bases of the two triangles of each electrode were parallel, about threemm
apart,
and
were placed perpendicular to the long axis of the muscle.This type of contact gave consistent contractions,
was
easily appliedand
adjusted,and
caused nodamage
to the muscles. Before stimu- lating the muscles simultaneously, eachwas
individually stimulated to see that both were performing in a similar manner.Only
at voltages exceeding 50 (and especially approaching 100) were theretions of other muscles.
Although
20 volts probably did not pro- ducemaximum
contraction of the depressor mandibulae, it produced consistentjaw
motions that could be safely attributed to con- traction of the muscle understudy.The maximum number
of consecutive stimulations, each ofwhich
lasted about
two
seconds,was
18 for agrosbeakand
17 for achicken, duringwhich
the depressor musclesshowed
no signs of fatigue.Despite extensive severing of other
jaw
muscles the birds continued to respond well. All birds were killed while still under anaesthesia.The
natureand
extent of the operative procedures were then checked under a dissecting microscope immediately following each experi- mentalseries.Just before stimulation of the muscles
and
again during stimulationand
steady tetanic contraction, side view photographs of the jaws were taken.The two
photographsfrom
each experimental set were projectedand
traced, one superimposed on the other. Imeasured
the motion of eachjaw
as degrees of arcbetween
the resting positionand
the stabilized position during muscle contraction.The
lowerjaw
has a longer radius ofmotion
than the upperjaw
because itsfulcrum of rotation lies well behind that of the upper jaw.
For
this reason, thetip of the lowerjaw
travels farther than that of the upperjaw
forthesame number
of degreesofmovement. One
thereforecan- not obtain a meaningfulmeasure
of total gape simplyby
adding the degreemotion
of thetwo
jaws, but differences within eachjaw
can becompared
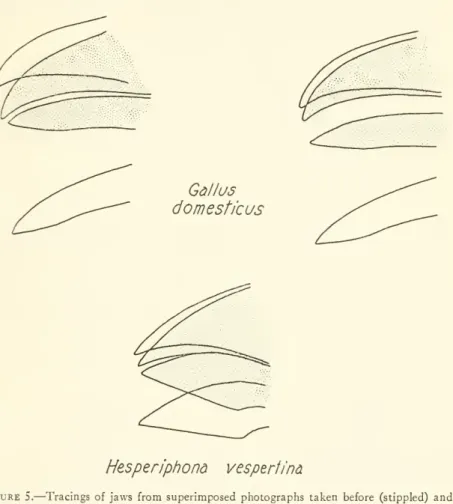
directly.Results. —
Figure 4 presents the degree ofmotion
of eachjaw
in bargraphs, whereasfigure 5shows
several tracings ofthejawsto give the reader a better impression of the actualamount
ofjaw
motion.I
must
emphasize that throughout the experiments the depressors probably never were contractedmaximally and
themajor
protractor muscle of the upperjaw
(protractor quadrati et pterygoidei) did not contributeto theobservedjaw
motion.As
statedbefore, the evening grosbeak lacks a well-defined postorbital ligament, whereas the do- mesticchicken has a stout one.It is clearly indicated in figure 4 that muscle contractions in the chickenproducedrepeatableresults during experimentationwith each bird (see
A
1and
2,4and
7;C
1and
2;D
4and
6, 7and
9)and com-
parableresultsbetween
different birds treatedon
differentdays (A 1,B
1,C
1,D
5).The
results of the experimentsmay
besummarized
as follows:(1) the depressor
mandibulae
causedprotraction (raising) of theupperjaw and
depression of the lowerjaw
in the domestic chickenand
evening grosbeak; (2) the muscle caused opening of both jaws in theNO. 3607
8 PROCEEDINGS OF THE NATIONAL MUSEUM
vol. 123chicken after removal of the postorbital ligament; (3) protraction of the upper
jaw by
the depressormandibulaewas
reduced afterremoval of the postorbital ligamentsin the chicken; (4) depressionof thelowerjaw
occurredwhen
the upperjaw was
held closed in both species;(5) protraction of the upper
jaw
occurredwhen
the lowerjaw was
held closedin bothspecies;and
(6) the postorbital ligament played a role in holding the lowerjaw
partially closed at rest. In addition, protraction in the chicken following removal of the postorbital liga-ment was
about half that of thenormal
condition.A
weight of tengrams
suspendedfrom midway
along the exposedculmen
of the chicken reduced protraction in thenormal
birdand
prevented pro- traction in birdsfrom which
the postorbital ligamentshad
been re-moved.
Limiting or preventingmotion
of onejaw
reduced but did not preventmotion
of the other jaw.The
postorbitalligament alone did not hold the lowerjaw
completely closed at rest (see fig. 4:A
1, 2, 3), but removalof the ligamentshifted thejaw
to amore
depressed resting position.That
the adductor muscles also playedsome
role in support of the restingjaw
is suggestedby B
3, inwhich
thejaw
at restwas most
strongly depressed followingremoval
of both the post- orbitalligamentsand
adductor muscles.Discussion.
— The
fact that the postorbital ligamentand
the depressormandibulae
can together produce protraction of the upperjaw
is demonstratedby
the experiments on domestic chickens described above.The
ligament thus serves to coordinate motions of both jaws during depression of the lower jaw. Coordination ofboth jaws through the depressor mandibulae, however, does not require the presence of the postorbital ligament,
and
the ligamentmust
be regarded as only one of severalmeans
(excluding otherjaw
muscles) of producing coordination. Furthermore, coordination of the jaws in the presence of a postorbital ligament is not obligatory;either
jaw may
also bemoved
independently.Bock
(1964) discussed the role of the postorbital ligament in avian kinesis, basing his conclusions on the results of manipulationof jaws in fresh birds
and
on inferencefrom
the anatomicalstructure of thejaw mechanism.
Hismajor
hypotheses were as follows: (1)Two
basically different kineticmechanisms
exist in birds coupledand
uncoupled.The
jaws are coupled in those species with a post- orbital ligament (or a functionally equivalent ligament), and/or withan
interlocking arrangementof the jaw-quadrate articulation. Birds lacking both of these featureshave
uncoupled jaws. (2)When
the upperjaw
of a coupled bird is held firmly in place, the lowerjaw
cannot be depressed. (3) In uncoupled birds the depressormandib-
ulaedoesnotcontribute to raising theupperjaw
exceptperhapswhen
the mandible is depressed against resistance. (4) In coupled kinesis
KINESIS
OF AVIAN SKULL —
ZUSI 9it is impossible to depress the upper
jaw beyond
the closed position.(5)
The
elevated upperjaw
cannot be lowered without raising the mandible. (6)Only
those few groups ofmodern
birds having un- coupled kinesis couldhave
given rise to an akinetic form.Although the experiments confirm the existence of coordinated jaw motion through the postorbitalligament, they neverthelesscontradict
Gallus domesticus
Hesperiphona
vesperfinaFigure 5.
—
Tracingsofjaws from superimposed photographs taken before (stippled) and during contraction of the depressor mandibulae muscles. (Left: normalbird; middle:normalbird; right:postorbitalligamentscut. Drawings donot presentmaximumopening of either jaw.)
Bock's assumptions 1,2,
and
3 directlyand
4, 5,and
6by
implication.The
avian kineticmechanism
should not beregarded as either strictly coupled or uncoupled; rather, it is likely thatmany
birds arecoupled weakly, but probably few are coupled so strongly that other muscles cannot counteract thejaw
linkage. Approaches toward complete coupling or complete independence ofjaw
actionwould
represent246-573—67 2
10 PROCEEDINGS OF THE NATIONAL MUSEUM
™l. 123special adaptions rather than typical conditions.
To
better under- stand these complex kinetic relationships, threemain
questionsmust
be answered: (1)How
doesthe postorbitalligamentproducecoordina- tion ofthetwo
jaws? (2)How
can thejawsbe alternately "coupled"and
"uncoupled"? (3)What
factors producecoordinationin addition to or in the absence of the postorbital ligament?The
first questionis dealtwith inthe following section,
and
thesecondand
third, under"Coordination
and Independence
ofJaw
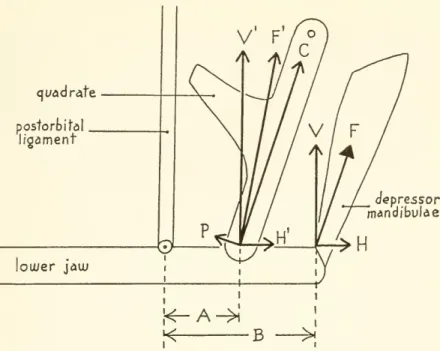
Action."Angle of the Depressor Mandibulae
Zusi (1959) postulated that the depressor
mandibulae
could cause protraction of the upperjaw
if part or allof the muscle pulled at an angle (forwardand upward)
with respect to the long axis of the quadrate.He
stated that the forwardcomponent
of force with respecttothequadratewould
be transmitted to thequadrate through the lower jaw.Bock
(1964, pp. 16, 17) disagreed, saying that the only point of relevancewas
whether or not the pull of the depressorhad
acomponent
directed forward along the axis of the lower jaw,and he
pointed out that such a forwardcomponent would
diminish ordisappearasthelowerjaw was
depressed. Inaddition,heindicated thatany
analysis of muscle angle should include the postorbital ligamentwhen
it is present.Here
I shall present such an analysis in detail because it is of considerable importance for understanding adaptations of the kineticmechanism,
whereas the effect of muscle angle on a simplified system of weightlessand
frictionless levers as presentedby
Zusi (1959)and Bock
(1964, pp. 16, 17) is probably irrelevant to the situation in the avian jaw, even in birds lacking a postorbitalligament.To
explain the relationshipbetween
the postorbital ligamentand motion
of both jaws through force analysis, it is necessary to discusstwo
important variablesdiagrammed
in figure 6: first, the relative lengths of the segmentsfrom
the mandibular attachment of the post- orbital ligament to the center of rotation of thejaw
articulation (A)and from
the ligament's attachment to the insertion of the depressormandibulae
(B);and
second, the angle of the depressormandibulae
in relation to itsneutral axis.
The
force of the depressormandibulae
(F) can be replaced
by two
components,H and
V, running in line withand
vertical to the forcearm
B.To
determine the effect of thesecomponents
on the quadrate, onemust
transfer bothcompo-
nents,
H' and V,
to the jaw-quadrate articulation.The
clockwiseVXB
rotational
component V
is increasedby
theamount V= —
whereas the counterclockwise
component H'
remains the same, andKINESIS
OF AVIAN SKULL —
ZUSI 11quadrate postorbital
ligament
mdepressor mandibulae
Figure6.
—
Diagram offorces produced bythedepressor mandibulaein a simplifiedsup- portingsystem ofthe lower jaw. (F=resultant forceof depressor mandibulae;V
andH =
rectangular components of F;H'=H
transferred to quadratearticulation;V'=V
transferred to quadrate articulationandincreased in relation
V'=
;F'=resultantA
ofcomponents
V
andH';Pand C=rectangu!ar componentsof F';A=work
armfrom fulcrum at attachment of postorbital ligament to quadrate; B=
force arm from post- orbitalligamentto insertionofdepressormandibulae.)a b
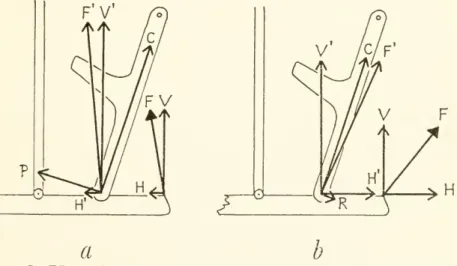
Figure7.
—
Effect of the angle of the depressormandibulaeupon motionof theupperjaw throughthe postorbital ligaments: a, protraction force P increased bycounterclockwise shiftofF(compare withfig.6);b,retractioncomponentR
producedbyclockwiseshiftofF(compare withfig.6). (Symbols definedinfig.6.)
to the skull as viewed
from
its left side.)The
rotational effect of forceF'on
the quadrateis P. It is clear that variationin the angle of the depressormandibulae canincreaseorreduceP
(fig. 7a)and
that the muscle-ligament combination could even produce retraction of the upperjaw
(fig. 76).The
neutral angle of the muscleis the angleat
which
contractionof the depressor mandibulae producesno
motion of either jaw. Bock'sforce analysis (1964, fig. 9) isincorrectbecause he neglected to transfer thebackward component
of force of the depressormandibulae(H
infig. 6ofthispaper) to thejaw
articulation along with theupward component
(V). It is not true that the pres- ence of the ligamentwould
cause protractionupon
contraction of the depressormandibulae under allconditions, asimpliedby
his analysis;rather, theforce
and amount
ofprotraction obtainedand
theamount
of depression of the lower
jaw
as welldepend upon
thetwo
variablesmentioned
above,and
theseforces could reachzero (theneutralstate) under conditions that are not very differentfrom
those existing in birds.The
conditions producing a neutral angle of the depressormandibulae
are not found in birdswhen
the jaws are closed because such conditionswould
prevent opening of bothjaws. Birds inwhich
thegeometry
of the jaw-support system departsmost
strongly from a neutral arrangement probably represent special adaptations for coordination of thejaws through the depressor mandibulae.Just as
components V' and H'
are alteredby
variationsin the angle of F, their effectupon
protraction varies with changes in the relative lengths of theforceand work arms
(Band
A)when
the muscle angle remains the same. IfB
is increased relativelymore
than A,V
increases but
H'
remains the same, with the result thatP
increases.By
contrast,P may
be reduced to zero ifB becomes
relativelyshortened.
Were
the fine of force of the depressormandibulae
to parallel the long axis of the quadrate (regardless of its angle to the long axis of the mandible), its protraction force (P)would
be zero onlywhen B
equalled A. In birds,
B
is always greater thanA and
the depressorcommonly
is nearly parallel to the long axis of the quadrate with the result that a protraction force exists.The
force of protraction can be increasedby
relative lengthening of the retroarticular process of the mandibleorby
shifting the angle of pull of the depressor musclesin a counterclockwise direction, orby
both.The amount
of protraction possible dependsupon
the relative lengths ofA and
of B, of the quadrate,and
of the muscle fibers in the depressor mandibulae, as well as thegeometry
of thepalateand
upperjaw.A
relative increase inA
with respect toB would
increase the degree of protraction with a given contraction of the depressor muscles, at thesame
time re-no. 3607 KINESIS
OF AVIAN SKULL —
ZUSI 13 ducing the force of protraction.The
attachment point of the post- orbitalligament, however,is never far infront of thejaw
articulation as thiswould
reduce theamount by
which the lowerjaw
could be depressed.During
depression of the lowerjaw by
the depressor mandibulae muscles, the resultantforce of each depressormuscle shifts in aclock- wise direction relative to thebones. It is possible that thegeometry
of the entire ligarnentary
mechanism
is such that, insome
birds, the system reaches a neutral condition before the mandible is depressed to the limits allowedby
thejaw
articulation alone. Fullcontraction of the depressor mandibulae thuswould
stabilize both jaws inan
opened position without permitting excessive strain on thejaw
articulation or kinetic articulations that
might
occur in the absence of theligament.Zusi (1959) postulated that birds thatforce their jawsopen against environmental resistance (probers, gapers)
may
derive a protraction effectthrough the depressormandibulaein that the pointofresistance to depression of the lowerjaw
creates anew
fulcrum, replacing that of the postorbital ligament. (In figure 6,A and B would
then extendfrom
the point of resistance near the tip of thejaw
to the quadrateand
to the depressor mandibulae, respectively.) In the light of the above analysis, it is clear that themechanism would work
effec- tively only ifF
were shifted in a counterclockwise direction to offset the loss of protraction force incurredby
the relative increase in the ratio ofA
to B, or if the ratio were reducedby
elongation of the retroarticular process of the mandible. Ihave
not carried the analysis forany
particular species farenough
to beable to saywhether or not the depressor mandibulae alone could produce protraction of the upperjaw
in the presence of environmentalresistance on both jaws.Bock
(1964, p. 17) pointed out that the analysis is difficultand must
include both jaws. It seems likely, however, that species with a strongforwardcomponent
to the pullofthe depressormandib-
ulaewould
be capable of stronger gaping (forceful opening of both jaws), not onlyby
increasingthe forceof depression of the mandible, but alsoby
favoring conditions for protraction of the upperjaw
as well.The
validity of the difference between the adaptations found in the depressormandibulae
muscles of Sturnus vulgarisand
gapers of the Icteridae postulatedby
Zusi (1959) is thus strengthenedby
the above analysis although the explanation originally proposed
was
incorrect.
In species lacking a functional postorbital ligament,
any
structure that resists depression of the lowerjaw
will cause protraction of the upperjaw
through forces similar to thosediagrammed
in figure 6 but onlyinproportionto theamount
ofresistance offeredby
thestructure.temporalis superficialis,
which
inmany
species lies in a position comparable to that of the postorbital ligament but medial to it,may
serve this function in a passive manner.The
pseudotemporalissuperficialis, often highly tendinous,
might
functionally replace the ligamentby
active contraction as well. There is clearly a great need for experimental approaches to the problems of avian kinesis to test the important specific questions thatnow
can be asked.Although
the effect ofmuscle angleon
protraction of the upperjaw
in the absenceof
any
restraining forces anterior to thejaw
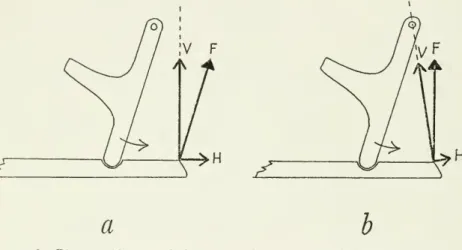
articulationa b
Figure8.
—
Diagramofforcesonthelowerjaw andquadrate fromthe depressormandibulae intheabsenceofthe postorbitalligament:a,withunrestricted rotation of the lower jaw,H
serves to retract thequadrateandV
servesprimarilytorotatethelowerjaw(Hreaches zerowhenFisperpendicular to thelowerjaw);b,withreduction orlossofrotation atthe jaw articulation,H
retracts the jaw-quadrate unit andV
has no rotational effect (H reaches zerowhenFpassesthroughtheoticarticulation of thequadrate). (F=resultant forceofdepressormandibulae;H
andV=
rectangularcomponentsof F;curvedarrows=motionofquadrates underconditionsillustrated.)
probably seldom has a bearing on
jaw motion
in birds, I shall discuss it briefly because it has been mentioned in the literature. If the strutsand
hinges of themodel shown
in figure 8a were weightlessand
frictionless, the effect of muscle anglewould
probably be that describedby Bock
(1964, p. 17)—
that is, protraction or retractionwould depend
only on the muscle's pull forward orbackward from
a 90-degree angle (the neutral angle) to the mandible.Were
there friction orany
other resistance to rotation at the jaw-quadrate hinge, protractionwould
occur if the line of pull of the muscle passed ahead of the cranial articulation of the quadrateand
retractionwould
occurifitpassed behind thearticulation (fig. 86).
The
neutralanglewould
>o. 3607 KINESIS
OF AVIAN SKULL —
ZUSI15
pass through the cranial articulation. In themost common
circum- stances thereissome form
ofresistance to depression of the mandible anterior to its articulation,and
the line of pull of the depressor then has relevanceforprotractiononly ifitanglesforwardfrom
the neutral angle, which is determinedby
thegeometry
of the whole system as already explained. Inany
case, anevolutionaryshiftin thedirection of pull of the depressor mandibulae in a counterclockwise direction represents a functional change along thecontinuum
"retraction—
neutral
—
-protraction" with respect to the muscle's influence on the upperjaw.Coordination and Independence of
Jaw
ActionPostorbital
ligament.—
Coordination of the jaws through the postorbital ligament (or its functional equivalent, as in the lacrymo- mandibular ligament in certain ducks) requires that the ligament attach on the mandible anterior to thejaw
articulation. Should the ligamentary attachment approach thejaw
articulation (or shouldA
infigure6 bereduced) the
amount
ofprotractionobtainedthrough the ligamentwould
be correspondingly reduced. Coordinationwould
reach zerowhen
the ligamentary attachment lay opposite thejaw
articulation.
By
manipulation of the jaws of fresh specimens of the sootytern(Sterna fuscata),Ifoundthat apullonthe depressormuscles caused very little forward orbackward
displacement, relative to the braincase, of thepostorbitalligamentand
lowerjaw
during depression ofthejaw;instead, thequadratewas
displacedforwardand
the upperjaw
raised.On
the other hand,when
motion of the upperjaw was
restricted
and
the quadrates thereby held stationary, depression of the lowerjaw was accompanied by
abackward
shift of the lowerjaw and
postorbital ligament relative to the braincaseand
quadrates.(Strict limits to this
backward
shift were setby
the articular jugo- mandibular ligament.)Whether
thejaw
shiftsbackward
or the quadrate forward, the shift isenough
to bring the attachment point of the postorbital ligament closer to the rotation center of thejaw
articulation
and
to reduce the coordinating action of the ligament.(Manipulation of the jaws
was
accomplished through pulling on the depressor mandibulae muscles orby
applying pressure on thejaw
at theirpoints of insertion. Manipulation of thejawsby
their tipsmay
givedifferent resultsthatdonotcorrespond toactions of the depressor mandibulae muscles.)
The
ability of the lowerjaw
to shiftbackward
with respect to the quadratemay
be deducedfrom
thejaw
motionsofan evening grosbeak that is hulling sunflower seeds.The bud moves
its lowerjaw
to the right or left of the upperjaw
to facilitate manipulation of the seeds.jugal
and
palatal strutsand
therefore cannotmove
independently, one of the mandibular ramimust
slidebackward
along the quadrate while the other remains in place during displacement of thejaw
to the side.The
possibility ofbackward
displacement of the lowerjaw
in the domestic chicken, although less obvious during
normal
feeding of the bird, can be demonstratedby
manipulation of fresh heads.I conclude that the shift of the ligamentary fulcrum toward the
jaw
articulation is one
means
of overcoming strict "coupling"by
the postorbitalligament.In addition to theexperiments already described, three instancesof independent
jaw
action in birds possessing a well-developed post- orbitalligamenthave come
tomy
attention.Two
of theserepresentjaw
motions duringyawning —
one in the night heron (Calherodias leuconotus) observedby me
(see p. 24)and
the other describedby Yudin
(1958, p. 168),who
gave the sequence ofjaw
motions in a gull as follows: (1) lowerjaw maximally
depressed, (2) upperjaw
raised, (3) upperjaw
lowered,and
(4) lowerjaw
raised.The
third instance appears in a series of photographs of the great snipe (Capellamedia) takenby
P. O.Swanberg
(1956). In these birds, protraction of the upperjaw
is restricted to the tipof thebill,where
itwould
bereadily evidentby
comparison with theimmovable
base-line providedby
the rest of the upper jaw. Several photographs
show
the closed billand
itsslightdownward
curve. Plate 74shows
the lowerjaw
slightly openand
in plate 73 quite widely open, but in neither case is thereany
change in thedownward
curve of the tip of the upperjaw and
thus no protraction or coupling.Jaw
ArticulationOne
important question remains to be answered:how
did the de- pressormandibulae
effect protraction in the evening grosbeakand
in the domestic chicken afterremoval
of the postorbital ligaments? In addition to coupling of thetwo
jawsby
the postorbital ligament,Bock
(1964, p. 18) briefly referred to another typeofcoupling through an interlocking of thecondyles of the quadrateand
articularwith the result that the mandible cannot bedepressed without forward motion of the quadrates.He
listed certain families of birds inwhich
this interlocking is well or moderately developed, citing Balaeniceps as a prime example, but heofferednoexplanationofthis action.Although
neither of the species used for experiments in the present study iscoupled through the
jaw
articulation to a high degree, a discussion of themechanism
in Balaeniceps will serve to introduce the general problems of coordination of thejaws through thejaw
articulation.KINESIS
OF AVIAN SKULL —
ZUSI17
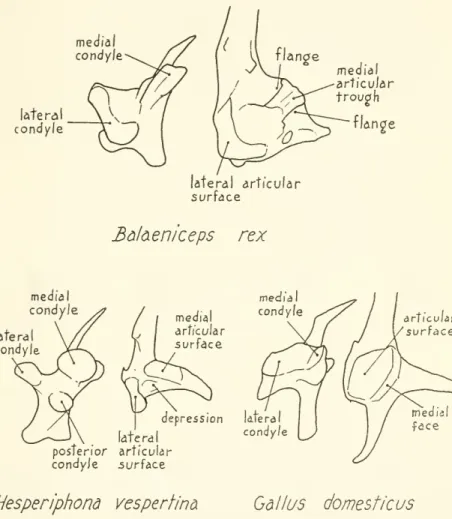
In Balaeniceps (fig. 9), the quadrate hastwo main
condylar sur- faces, the inner ofwhich
(medial condyle) forms a double crest lying parallel to the plane ofmovement
of the quadrate.The
lateral condyle issomewhat
curved, lying approximately perpendicular to the crestand
to the plane of quadrate motion (its posteromedialmedial condyle
latera condyle
lateral articular surface
medial articular trough
flan2e
lateral condyle
Bdl&eniceps rex
medial articular surface
medial condyle
depression Utera condyle
articular surface
late ra posterior articular condyJe surface
Hesperiphona vespertine Gallus domesticus
Figure9.
—
Structure of jawarticulationin Balaeniceps rex,Hesperiphona vespertina,and Gallus domesticus. (Ventral view of right quadrate and dorsal view of left articular shownforeachspecies.)portion is
expanded
into aprominent condyle that isoften referred to as the posterior condyle of the quadrate). These condyles fit into corresponding depressionsorsurfaces ofthe articularbone,and
flanges on the upper edges of the medial articular trough grip the crests of the medial condyle of the quadrate, holding the articulated lower(1930)
and
others. Partly because of the gripping flanges but pri-marily because of the structure of the crests, trough,
and
lateral articular surfaces, the lowerjaw
can be depressed or raised only ifthe quadrate
and
articular surfaces slide along each other in the direction dictatedby
the crestsand
troughs.During
depression of the jaw, this could be accomplished eitherby
a passive spreading of the rami of thejaw
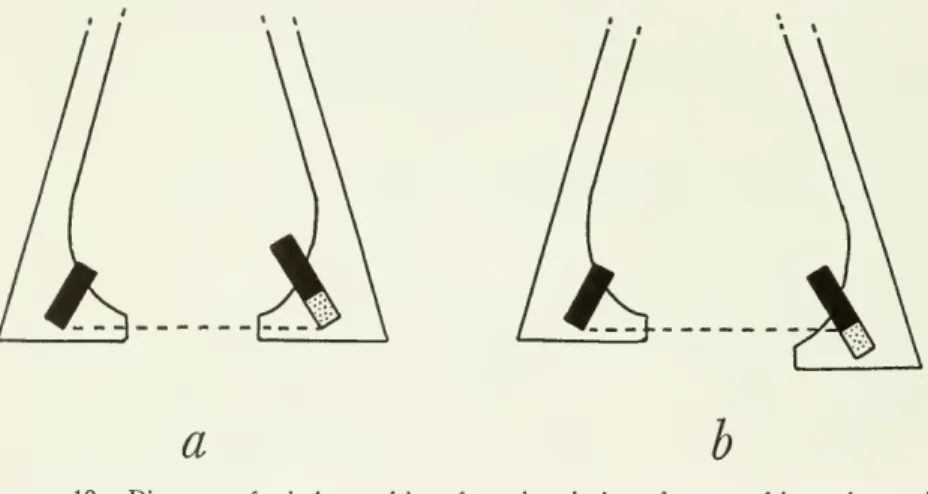
as they slidebackward and upward
along the condyles of fixed quadrates; or the quadrates couldmove
forwardand
inward while thejaw was
depressed without producing a lateral spreading of the rami (fig. 10).Whether
or not thereis aflange gripand
a deepand
well-defined trough of the articular as in Balaeniceps, the condyles ofmany
diverse orders of birds are arranged in such aa b
Figure 10.
—
Diagramsof relative positions from dorsal view oframus oflowerjaw and quadrate(black) whenthejawisclosed(leftramus),andwhendepressed (rightramus):a, quadratemovesanteromedially;b,ramusmovesposterolateral!/.
way
that the planes ofmotion
within thetwo
jaw-quadrate articula- tions converge anteriorly like those of Balaenicepsand downward
rotation of the mandible therefore requires either posterolateral spreading of the rami or anteromedial
motion
of the quadrates.Protraction of the upper
jaw
thus willaccompany
depression of the lowerjaw
if resistance to spreading of the rami isgreater thanresist-ance toforward
and
inwardrotationof thequadrates (whichof course dependsupon
thecombined
resistance of all flexible portions of the upperjaw and
palate). Protractionwould
be preventedif the quad- rates were held in place, but depression of the lowerjaw
could stilloccur through spreading of the rami. Birds vary greatly in the ease of
motion
of the palateand
upperjaw and
in the lateralflexibility of the rami; the extent towhich
the ramimay
contribute to protractionno. 3607 KINESIS
OF AVIAN SKULL —
ZUSI 19 through thejaw
articulation unfortunately has not been established forany
species tomy
knowledge.Manger Cats-Kuenen
(1961, pp. 18, 19) explained the coupling action of thejaw
articulation in detailand
concluded that independentmotion of thetwo
jaws in the hornbill Rhinoplax vigilwas
impossible because the ramihad
no lateral flexibility. I believe, however, that the flexibility necessary for independent depression of the mandible exists in Rhinoplax be- cause evenin a driedskull itis easyto depress thelowerjaw by
press- ing on the retroarticular processes.When
the kineticmechanism
is immobilizedin the driedskull, the raminevertheless readily spread apart during depression of the mandible.
Itisprobablethat theconformationofthejaw-quadratearticulation played a role in protraction through the depressor
mandibulae
in the evening grosbeak (lacking a postorbital ligament)and
in the chicken after removal of the postorbital ligament (fig. 4) although themecha-
nism appears to be different in thetwo
species.The
condyles of thejaw
articulation of thedomestic chicken are quite differentfrom
those ofBalaeniceps (fig. 9), but bothspecieshave
lateralsurfacesproviding broad supportand
medial surfaces providing guidance. In Gallus domesticus the medial condyle of the quadrate slides along a well- defined medial face (corresponding to the trough of Balaeniceps) of the broad, flattened articular surface.The
medial faces of thetwo
rami convergeanteriorly. Depressionofthelowerjaw
in the chicken, therefore, requires spreading of the rami or anteromedial motion of the quadrates just as in Balaeniceps.The
possibility that resistance to lateral spreading of the rami in the chicken isenough
to raise the upperjaw
issuggestedby
the occurrence ofprotraction afterremoval of the postorbital ligaments.The
structureof thejaw
articulation in the evening grosbeak differs sharplyfrom
that of the domestic chicken in that the quadrate possesses three knoblike condylesand
in that the articularhasa deep depression between itstwo
articular surfaces (fig. 9).There
is no well-defined trough of the articular thatwould
serve to guide the motion of the rounded medial condyle of the quadrate; rather, the medial condyle rests on the top of a narrow medial articular surface that slopesdownward and
terminates just in front of the condylewhen
the jaws are closed.As
the lowerjaw
is depressed, the medialand
posterior condyles of the quadrate slide forward on the inclined surfaces of the articular to occupy the space or depression in front.With
contraction of the depressor mandibulae, protraction appears to be causedby
pressure of the articulation surfaces of the lowerjaw
on the quadrate condyles.The
quadrate slides forward easily because of the slope of the articularsurfaces.I
have
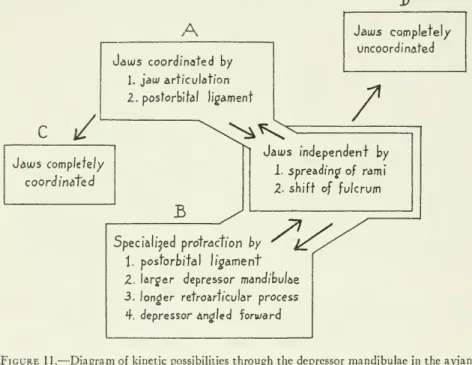
been concerned primarily with the functional properties of onlyonepaired muscle asitacts within thekineticsystems of various species of birds (seefig. 11).The
depressormandibulae musclesserve principally to open the upperand
lower jaws simultaneously. Inmost
species themost
powerful agents for protracting the upperjaw
areundoubtedlytheprotractor quadratietpterygoideimuscles, whichD
A
C /
Jauus coordinated by
1. jawarticulation 2. postorbital ligament
Jaais complete!/
uncoordinated
^*C^ /
Jatus complete//
coordinated
B
Jaius independent by
1. spreadingof rami 2- shift of fulcrum
Specialised protraction by
1. postorbifal ligament Z. larger depressor mandibulae 3. longer retroarticular process 4. depressor angled forward
Figure11.
—
Diagramof kineticpossibilitiesthroughthe depressormandibulaeintheavian jaw: SpeciesA
hasweaklycoordinatedjawsthatmaybemovedindependently. SpeciesBhasdevelopedstronger protraction oftheupperjawthroughthemechanismsof coordi- nationbut can alsoovercome coordination. Species
C
hasno capacityforindependent jaw action, whereas speciesD
has no coordinationthrough the jaw-quadrate complex.(C and
D
arehypothetical.)almost certainly
come
into play duringmaximum
or forceful gaping.The
depressor mandibulae, however,may
be the sole or principal muscles openingboth jawswhen
extensive orpowerful opening of the jaws is not required, as in taking small food itemsfrom
a substrate.The mechanisms by
which the depressor mandibulae can protract the upperjaw
involve the angle of the muscle, the angle of sliding of the jaw-quadrate surfaces, the rigidity of the rami of the lowerjaw
and
of the kinetic mechanism, the presence of a postorbital ligament or a comparable ligament, the length of the retroarticular process ofN0- 36 °7 KINESIS
OF AVIAN SKULL —
ZUSI21
the mandible, the presence ofligamentous adductor muscles,and
the resistance to depression of the lowerjaw by
environmental forces.Other features
may
be involved as welland
a variety of factors probably contribute tojaw
motion in each species.The mecha-
nisms are developed to varying degrees in different groups of birds.Only by
understanding their functional properties canwe
recognize convergence, independent divergent solutions to similar problems,and
adaptive radiation in the kineticmechanism. A
survey of such trends in birds or inany
group of birds has not yet beenmade
in detail, but a few cases of variation in the depressormandibulae complex
should be mentioned here.Certain birds that habitually probe into flowers or fruit
show
a combination of features that suggest a special role of the depressor muscles for protracting the upper jaw. Representatives of the"Coerebidae" (Coereba flaveola), Zosteropidae (Zosterops annulosa),
and
Drepanididae (Vestiaria coccinea) displaylargedepressor muscles, well-developed retroarticular processes of the mandible,and
strong postorbital ligaments (Beecher, 1951a, pp. 277, 283; Moller, 1931, p. 126). Beecher(1951a,p.2S5) statesthat"The
Neotropical nectar- adapted tanagers are non-gapers. Innone
ofthem
isM.
depressor mandibulaemore
highly developed than in Cyanerpes . . .and
it isapparent that these species simplyinsert the billinto flowers
and
sip nectar." Moller (1931, p. 110), however, illustrates Dacnis cayana as having a large depressor mandibulae, which,combined
with itsstrongpostorbitalligament, longretroarticular process,
and
relatively straightcidmen and
gonys strongly suggests that gapingisamong
itsfeeding patterns. Various
members
of the Icteridae display thesesame
features and, in addition,have
the depressor muscles angled well forward (Beecher, 1950, 1951b). Associated with this highly developed gaping adaptation is the straightculmen and
gonys so characteristic ofmany
icterids, essential for effective parting of the grass, earth, or flesh of a fruit with the outer edges of the bill.The
starling (Sturnus vulgaris) is convergent with the icterids, especially Sturnella, in
some
features related to gaping but divergent in others (Zusi, 1959). Certain parrotshave
the depressormandibulae muscles enlargedand
strongly angled forward (Nestor notabilisand
Cyano-rhamphvs
novae-selandiae, illustrated in Hofer, 1950, pp. 457, 463).The
retroarticular process is well developed but the postorbital liga-ment
is lacking. Parrots are wellknown
for their extraordinary mobility of the upper jaw, but no comparative information on thispropertyin the Psittacidaeisavailable.
Among
the probingScolopa- cidae, thewoodcock
(Scolopax)shows
special development of the depressor mandibulaeand
retroarticular process of the mandible(see Marinelli, 1928, fig. 8), possibly in relation to the need for
display a long retroarticular process of the mandible but neither group has an obvious need for powerful depression of the lower jaw.
Rather, the process
may
be related to kinetic action through the depressor mandibulaemuscles.The
examples just given represent birds in which there is a need forstrong, extensive, orrepeated protractionand
in whichacombina- tion of features associated with the depressor mandibulae is adapted to accomplish or to aid protraction. Other examples can be foundamong
the passerinebirds (seeillustrations in Beecher, 1953, pp. 302, 318),but the elucidationof theadaptiveradiation within thesegroups remains a challengefor the anatomist.Evolution
ofthe interlocking jaw articulation.— Bock
(1964, p. 37) postulated an obligatory coupling of the jaws through the postorbital ligamentand
thus foundno
explanation for the presence oftwo
strict couplingmechanisms
(postorbital ligamentand jaw
articulation) in the
same
species or for the evolution of jaw-articula- tion coupling. Explanations for thesephenomena
are possible,how-
ever, withthe
knowledge
that couplingmay
bebypassedand
that the postorbitalligament serves functions other thaja coupling.The
ful-crum
providedby
the postorbitalligamentcauses the posterior portion of the depressed mandible to be rotatedupward and
forward.One
result is that the mandible pushes the quadrates forward at least during the initial phases of depression, but another consequence is
that a firm contactofthe
jaw and
quadratesurfacesisassuredthrough- out motions of the lower jaw.Without
the postorbital ligament (orsome
functional equivalent) the anterior surfaces of thejaw
articula- tionwould
tend to separate during depression of the mandible.As
the condyles of thejaw
articulationmay
serve to coordinatejaw
motions, the ligamentwould
enhance coordinationby
keeping the articulation surfaces in close apposition.The
postorbital ligament could thereby play an important part in the evolution of the inter- locking groovesand
ridges ofthejaw
articulation. Support for the lowerjaw
through interlocking in a speciessuch as Balaenicepsis lost quickly if the rami are spread slightly or the quadrates displaced anteromedially atany
given position of the lower jaw,and
itcannot beassumed
that ligamentary supports are unnecessaryin specialized"articulation-coupled" forms.
The
postorbitalligamentisastructure that probably developed early in the evolution ofmodern
birdsand
has played a role in the evolution of increased articulation-coupling in various groups while being reduced orlost in others. Itslossmay
be associated with the development of
maximum
independence ofjaw
motion.no. 3607 KINESIS OF
AVIAN SKULL —
ZUSI23 Functions
of kinesis.— Bock
(1964, pp. 25-31) discussed six possible functions of kinesis: (1) maintaining the mandible in the closed position, (2) gaping, (3) maintenance of the primary axis of orientation of the bill, (4) faster closing of the jaws, (5)more
wide- spreadand
even distribution ofjaw
muscle attachment,and
(6)shockabsorbing.
Of
these, I believethat thefirstis doubtful becauseit requires the hypothesis ofstrictcoupling ofthejaws foritsexplana- tion
and
because the ligament does not hold the lowerjaw
closed (at least in Gallus domesticus).The
thirdand
sixth are probably of widespread importance—
the third becausemany
species rely onrapid grasping of tiny food items or ofmoving
preyand
the last because of the light construction of thejawsand
palate in birds.I believe that a primary advantage of kinesis is that it provides increased possibilities for diversity of manipulation
by
the jaws.Although birds lack teeth, they nevertheless
must
performmany
manipulativetasks withthebill suchas the capture, holding, orienta- tion, manipulation,
and
swallowing of food, nest-building, preening, defence,and
other activities.Two
fundamental features of skull construction in birds serve, in combination with kinesis, to enhance thediversity of manipulation.First, the tomial edges of the upper
jaw
aredrawn
forwardwhen
raised
and backward when
lowered (fig. 126) because the flexible point of attachment of the upperjaw and cranium
lies above the plane of the tomia. Inmost
birds the articulation of the lowerjaw
lies below the tomial planeand
there is a similar forwardand backward
motion of thetomium
during depressionand
adduction of the lower jaw.A
food item being constrictedby
the jaws, there- fore, iswedged backward
toward the throat rather than pushed forward as itwould
beby
astraight pair of tongs (fig. 12a).Immo-
bility of the upper
jaw would
reduce considerably this effect.Un-
fortunately, I can say nothing about its significance for the living bird although its existence can scarcely be doubted.
The
effect is greatest in relatively shortand
deep bills,where
itmay
providemore
effective seed-cracking forces or help to keep seedsfrom
slipping forward in the bill during nibbling.
A
second type of manipulation ismade
possibleby
the anterior placement of the flexiblecranial attachmentof theupperjaw
relative tothe articulation ofthe lowerjaw
(fig. 13).The
upperjaw
thereby rotates about a shorter radius than the lower,and
the tomial edges can be opposed in a greater variety ofways
thanwould
be possible otherwise, especially if the upperjaw
can be retracted below the resting position as discussedby
Beecher (1951b, p. 413)and Yudin
(1965, p. 68).
Bock
(1964, pp. 23, 24) argued against suchretraction in a coupled skull, butI haveshown
here that coupling can be over-tions to retraction of the upper
jaw
below the resting position thus areeliminated.While
watchinga nightheron (Calherodiasleueonotus) at close range in theNew York
Zoological Park, I observed retrac- tion of the upperjaw when
the birdwas
yawning. Just beforea b
Figure 12.
—
Diagramsshowing positionsoftomial edges and of particular points (dots) onthetomia during motionsofthetwojaws: a,whencenters ofjawrotationliecloseto tomial planes; b,whencenters ofjawrotation liefarfromtomial planes.Figure 13.
—
Diagramof theavian skullshowingretraction of theupperjaw beyond the normalclosedposition. Becausethelowerjawarticulateswellbehindthe cranialbending axisoftheupperjaw, themanipulativepossibilitiesofthejaware increased. Bitingwith the billtipasshown would notbepossibleifthejawswereofequallength.raising the depressed mandible to its resting position, the bird re- tracted its upper jaw below the resting position with the resultthat only the tips of the jaws were touching
and
thegap between
the bases of the jawswas
clearly visible.Herons have
the postorbital ligament well developedand
are also "coupled" through the jaw-no 3607 KINESIS
OF
AVIANSKULL —
ZUSI25
quadrate articulation. It thus appears that retraction of the upperjaw beyond
the resting position is one of the capabilities of aviankinesis. Its importance lies in the potential for increased diversity of manipulation
by
the bird's beak.In studying the functional significance of the palatine process of the premaxilla,
Bock
(1960) postulated that there aretwo methods
of seed-cracking usedby
heavy-billed finches.The
first he called "the nutcracker method," characterizedby
a kinetic upperjaw and
equal development of the adductors of the mandibleand
the retractors of the palate.He
ascribed thismethod
to the emberizine finchesand
richmondenine finches.By
contrast he claimed that the cardueline finchesemploy
"the vise method,"which
he describes (p. 427) as follows:
In the specialized carduelines, theupperjaw haslostits mobility;it is a nearly stationary block against which the mandible presses. Heavy bosses of bone (thelateral flanges) and rhamphothecadistribute theshocks associated withthe cracking of the seed evenly to all parts of the braincase. Only the adductor musclesare welldevelopedinthe carduelinefinches; in fact,themusclesassociated only with the movement of the upper jaw have become small and are on the vergeof becomingfunctionless.
We have
seen that the evening grosbeak—
one of the heavy-billed cardueline finches— has
akinetic upperjaw
that operates even in the absence of contractionby
its prime protractors, theM.
protractor quadrati et pterygoidei. In order to exert a seed-cracking force, the upperjaw must
bemoved
in opposition to theadduction of the lowerjaw
or held stationary while force is exertedby
both jaws, requiring in eithercasea powerfulM.
p