A Thesis Presented to
The Faculty of Alfred University
Synthesis of Pseudo Lunar Agglutinates from NU-LHT-1M/2M Derivative Simulant
By
Megan Elliott
In partial fulfillment of
The requirements for
The Alfred University Honors Program
May 2022
Under the Supervision of:
Chair and Thesis Advisor: Dr. Holly Shulman
Committee Members
Dr. Alex Clare
Dr. Michele Hluchy
III
Table of Figures
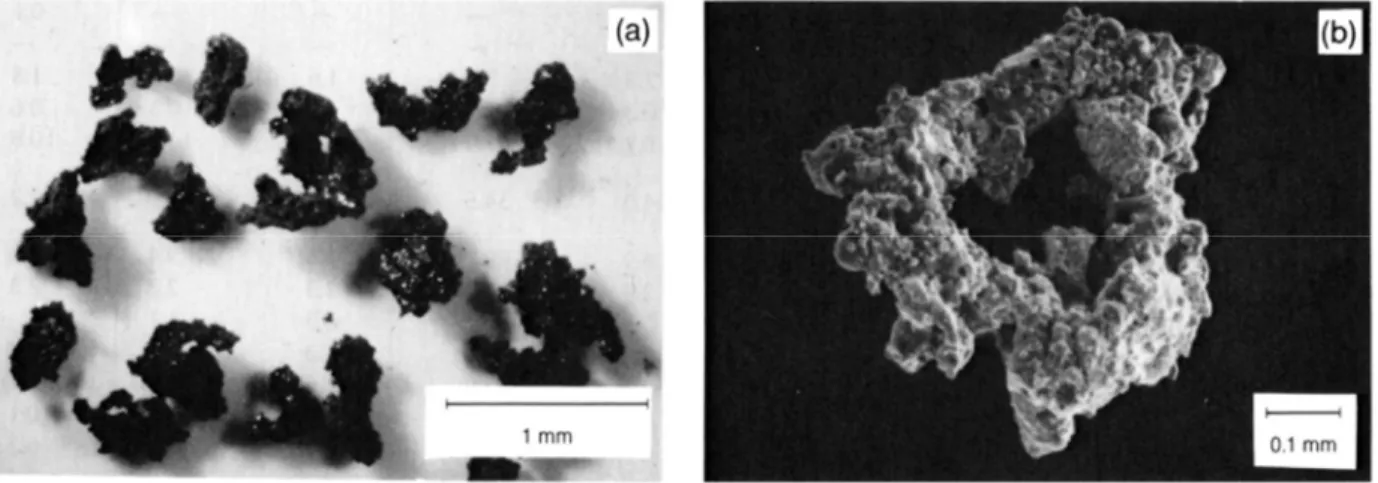
Figure 1: Fig. 7.2. Typical lunar soil agglutinates. (a) Optical microscope photograph of a number of agglutinates separated from Apollo 11 soil sample 10084, showing a variety of irregular agglutinates shapes (NASA Photo S69-54827). (b) Scanning electron microscope photomicrograph of a doughnut-shaped agglutinate. This agglutinate, removed from soil sample 10084, has a glassy surface that is extensively coated with small soil fragments. A few larger
vesicles are also visible (NASA Photo S87-38812)[3] ... 3
Figure 2: quenched glass samples from batch 1(left) and batch 2 (right) ... 8
Figure 3: XRD results from NU-LHT derivative glass batches ... 8
Figure 4: Buchi Rotavap R-300 (left) and Fisher Scientific Isotemp Oven (right) ... 10
Figure 5: Tube furnace ... 11
Figure 6: Gyro mill or Planetary mill ... 12
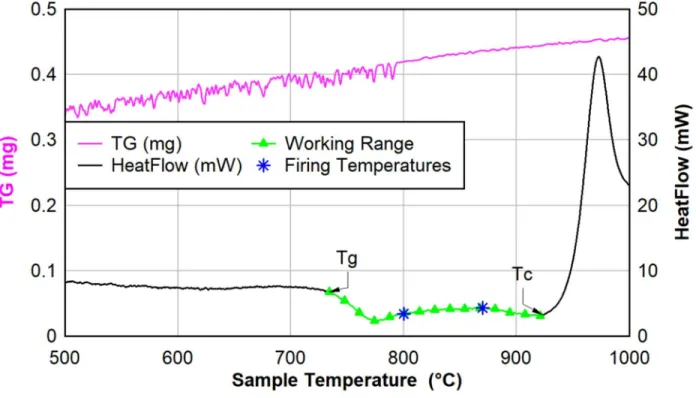
Figure 7: DSC of NU-LHT derivative glass with Tg and Tc marked to show glass working range ... 14
Figure 8: SEM BSE images of USGS pseudo agglutinate taken using backscatter electrons ... 15
Figure 9: SEM BSE images of experimental samples, 800°C no hold (left) and 870°C no hold (right) ... 16
Figure 10: SEM images of experimental sample heated to 870°C with a 20 minute hold at temperature BSE (left) and SE (right) ... 16 Figure 11: Fig. 7.2 (b) Scanning electron micrograph of a doughnut-shaped agglutinate, This agglutinate, removed from soil sample 10084, has a glassy surface that is extensively coated with small soil fragments. A few larger vesicles are also visible. (c) Scanning electron photomicrograph of an irregular agglutinate from soil 10084, showing some vesicular, glassy,
IV
fragment-laden surfaces. Some agglutinates are very delicate and display narrow bridges, necks,
and dendrite-like arms (NASA Photo S87-38811) [3] ... 17
Figure 12: Images of experimental samples taken through an optical microscope. A) 800°C, B) 870°C, C) 870°C 20-minute hold ... 18
Figure 13:Optical microscope images of glass particles from sample heated to 870°C for 3 hours ... 19
Figure 14: Optical microscope image of anorthite particle from sample heated to 870°C for 3 hours with phases circled. A) anorthite, B) glass, C) enstatite and glass, D) diopside ... 20
Figure 15: Anorthite XRD Scan ... 24
Figure 16: Diopside XRD Scan ... 25
Figure 17: Enstatite XRD Scan ... 25
Figure 18: Olivine XRD Scan ... 26
V
Table of Figures
Table 1: Weight percent composition of NU-LHT derivative glass ... 7 Table 2: Mineral proportions for NU-LHT derivative simulant ... 13 Table 3: Glass Batching and Melting Parameters ... 23
VI
Forward
As NASA heads towards new missions to the moon, the need for high accuracy lunar regolith equivalents becomes greater than ever. The next set of lunar missions seeks to create structures on the moon using the materials already there, otherwise known as in-situ resource utilization.
This requires experimentation using something that consistently behaves the same as lunar soil would. While high accuracy mined simulants do exist, they contain elements and minerals that are not present on the moon or are present in different forms. These simulants also are often missing a component of lunar soil that does not occur naturally on earth; Agglutinates.
Agglutinates that are present on the moon do not form naturally on earth because the atmosphere largely prohibits the conditions necessary for their creation. Agglutinates are small (<1mm) aggregate particles found on the lunar surface. They are formed when micrometeoroids, think space sand moving at around 20km/sec, impacts the lunar surface. The energy resulting from these impacts cause clumps of soil particles to go flying away from the surface while partially melting the ejected material. As the moon does not have an atmosphere, the resulting liquid cools extremely rapidly. The end result is glassy particles with minerals stuck to their surfaces and inside the glass matrix, as well as a large amount of both open and closed porosity, resulting in highly unique morphology. The lack of atmosphere on the moon also leads to the formation of metallic iron within and on the agglutinates. The combination of glass and mineral as well as the strange particle shapes means that agglutinate content could have a significant effect on certain properties of the soil, such as packing density, and behavior under applied fields. Thus, a way to recreate agglutinates for testing on earth becomes necessary.
VII
There are processes to make agglutinates currently, the main one by the US Geological Survey utilizing plasma melting. While this process does produce pseudo-agglutinates reasonably similar to real lunar ones, there are some problems with the process. The USGS process is fairly large scale, so making agglutinates with customizable compositions to more closely match specific areas on the lunar surface is close to impossible, unless large batches are desired. The second issue is the material going into the plasma melter, specifically, the leftover rock from creating a specific lunar soil simulant. This creates more difficulty in tailoring the composition, as while it would be possible to add more of one kind than another, you would be restricted by the minerals present in the rocks. The third problem is that this all happens using earthbound minerals in earth atmosphere, which leads to the issue of having elements present in the agglutinates that are not present in lunar soil, or are present in a different form due to the lack of atmosphere on the moon. Thus, the need for a new process becomes evident.
I chose to attempt to replicate the end results of the lunar process as much as possible instead of trying to replicate the how. The proposal was to prepare the glass and minerals separately to more closely match the composition of a specific lunar regolith simulant, NU-LHT, before recombining the glass and minerals and heating them in a furnace that would allow me to control the atmospheric conditions the sample was exposed to. Preparing the glass and minerals separately allows for exact tailoring of their compositions, and means that the form of certain elements, specifically iron, present can be controlled. It also allows for experimentation with the ratios of glass to minerals, something that needs to be explored in future work.
Once the glass and mineral components were completed, they were individually crushed down to a sand-like consistency and mixed together in the appropriate ratios to mimic NU-LHT. This mixture was then split into separate batches for heat treatments in a furnace that allowed for
VIII
atmospheric control so that the samples would not be exposed to oxygen at elevated temperatures. Three samples were initially used, and a fourth was later added to confirm the results. Both optical and scanning electron microscopes were used to look at the particles in the samples after firing and compare them to known images of lunar agglutinates and the USGS version.
When greatly magnified, the sample heated at higher temperature for 10 minutes did start to resemble lunar agglutinates in surface texture. The observations using optical microscopes tracked with this, as there was apparent build up of mineral particles on the surface of the larger glass shards, and more showed up at increased temperatures and increased time. However, when the fourth firing was completed to confirm this, the build up on the glass shards surface was gone and appeared instead on the surface of the mineral particles. This confirmed that the temperature of the firing was too low.
The end result of the experiment was basically that the temperatures that I looked at were too low to allow the glass particles to soften enough for adhesion with other particles, however any higher in temperature and the risk of crystals forming in the glass starts. While this method could still be possible with more work on identifying the specific temperature needed, it is likely that the glass is simply too thick at reasonable temperatures for this to work.
One of the questions that came up while working on this is if I mixed the sample at too high a ceramic to glass ratio. Would adding additional glass powder have changed my results? This would be one of the next pieces to investigate as well as looking at higher temperatures. I would also like to redo the measurements I used to base my selected temperatures on to verify the accuracy of them and look at higher temperature behavior.
IX
During the course of this research, I received significant assistance from a few individuals, especially Dr. Clare and Cooper Howard. Dr Clare was immensely helpful with the process of melting the glass I needed, as were her graduate students. It was Cooper’s methods that were used to prepare the minerals needed to make the NU-LHT derivative simulant, without which this work would not have been possible. He provided significant guidance on synthesizing the minerals, and acted as a sounding board on occasions when I needed assistance on where to go next.
1
Abstract
In this work, a new process for the synthesis of pseudo lunar agglutinates was investigated.
Agglutinates are agglomerates containing glass and minerals. A synthetic lunar mineral blend of anorthite, diopside, enstatite, and olivine was created to mimic Highlands lunar regolith. A derivate glass was prepared from the oxide formula and quenched. The derivative glass and mineral powders were crushed, mixed together and heated in flowing Ar(5%)H2 at several temperatures and times. These samples where then observed under scanning electron and optical microscopes to determine mineral-glass adhesion and compare samples to existing pseudo agglutinates and lunar agglutinates. Particle adhesion was observed by optical microscopy, and SEM images showed similarities to lunar agglutinates. Further experimentation is necessary to determine exact parameters, however feasibility is confirmed.
2
Introduction
As NASA looks to restart space explorations with the 2024 Artemis missions, one of their research focuses is In-Situ Resource Utilization (ISRU). ISRU is the generation of products from local materials while in space[1]. For this, greater understanding of the lunar surface, and higher accuracy simulants for testing of technologies are necessary.
Lunar Surface[2]
The chemical composition of the lunar surface is variable depending on location however the components remain relatively the same with a combination of specific mineral and glass making up the regolith or lunar soil. Silicate minerals make up over 90vol% of most lunar rock, with the most common being pyroxene (Ca,Fe,Mg)2Si2O6, plagioclase feldspar (Ca,Na)(Al,Si)4O8, and olivine (Mg,Fe)2SiO4. Quartz and potassium feldspar as well as minerals containing Fe3+ are fairly rare on the moon. While there are many other minerals on the moon in smaller amounts, these represent the major constituents. This is especially the case for the Highlands region of the moon that is the destination for the Artemis missions. Highlands regolith averages 78vol%
plagioclase, pyroxene and olivine and 18vol% glass. Another important component of lunar regolith is agglutinates. Lunar regolith simulant NU-LHT is a mined simulant designed to mimic highland lunar regolith.
Agglutinates[3]
Agglutinates are aggregates of smaller particles created when micrometeoroids bombard the lunar surface. These impacts cause ejection and melting of regolith grains that clump together and rapidly quench, resulting in heterogeneous glass with irregular morphologies and mineral
3
inclusions, Figure 1. During the melting process, the solar wind element implanted in the regolith grains are liberated, and the hydrogen reacts with the FeO in the regolith grains forming metallic iron nano droplets and H2O. While the exact composition of the agglutinates formed in highly variable due to the small collection of melted particles that form each agglutinate, the average compositions in an area mirror the regolith composition. The agglutinates also contain smaller agglutinates repeated micrometeoroid impacts causes remelting and encasement of already formed agglutinates. Agglutinates are unique to terrestrial planets lacking an atmosphere.
Figure 1: Fig. 7.2. Typical lunar soil agglutinates. (a) Optical microscope photograph of a number of agglutinates separated from Apollo 11 soil sample 10084, showing a variety of irregular agglutinates shapes (NASA Photo S69-54827). (b) Scanning electron microscope photomicrograph of a doughnut-shaped agglutinate. This agglutinate, removed from soil sample 10084, has a glassy surface that is extensively coated with small soil fragments. A few larger vesicles are also visible (NASA Photo S87-38812)[3]
Lunar regolith consists of 25-30vol% agglutinates, making a controllable and reliable method of synthesis for pseudo agglutinates of varying lunar compositions necessary to accurately simulate lunar regolith. The United State Geological Survey (USGS) created one such method[4] by utilizing plasma melting to partially melt the waste feedstock from the Stillwater mill NU-LHT production. This method yields friable particles with irregular internal structures and
4
morphologies at more than 50kg per day. The utilization of waste from the Stillwater mill to feed the plasma melter results in a lack of control over the composition of the ceramic and glass phases, and in the ratio of glass to ceramic within the agglutinates, both of which are important factors in the properties of the produced pseudo agglutinates. The lack of Fe0 nanoparticles also poses and issue, as the presence or absence of these particles could impact certain material properties, especially the dielectric properties. Thus, the development of processes that have a higher degree of compositional control and allow for the formation of nano-metallic iron are necessary.
5
Proposed Process
The proposed process was to prepare glass and minerals of the correct compositions to match the major constituents of NU-LHT. The glass and minerals were then ground and combined, before heat treatment in flowing Ar 5%H at set temperatures between the experimentally determined glass transition of crystallization temperatures. That heat treatment would soften the glass and allow adhesion between the glass particles and between the glass and ceramic particles creating irregular morphologies mimicking those of lunar agglutinates. The flowing Ar 5%H would also ensure the correct oxidation state of the Fe present in the minerals and the glass.
6
Methods
When glass is heated above the glass transition temperature (Tg) it begins to soften and flow allowing adhesion between glass particles and mineral powders. Utilizing this, by combining mineral and glass powders and heating them above Tg the glass and mineral particles should begin to fuse and adhere together creating pseudo agglutinates.
Testing Parameters
For this investigation, differential scanning calorimetry (DSC), scanning electron microscopy (SEM), x-ray diffraction (XRD), and optical microscopy were used.
DSC was performed using a SETSYS Evolution (Setaram Instrumentation, France) using a Pt pan with a heating rate of 10°C/min to 1000°C. Nitrogen was used for the carrier gas, Argon for the protective gas, and chilled water circulation to cool the system.
A JSM-6010PLUS/LA SEM (JOEL Ltd, Tokyo, Japan) was used at 15kV for both backscatter and secondary electron images. Small samples of each powder were attached to metal stud sample holders using carbon tape.
XRD was performed using a Bruker D2 Phaser (Bruker, Billerica, MA). Measurements were performed from 20 to 90°2θ, with 2500 steps at 0.5 sec each. Powder X-ray diffraction was used, specifically in bragg-brentano geometry, with Cu k-α radiation.
Optical microscopy was performed with a Wild M3Z Heerbrugg (Heerbrugg, Switzerland) microscope with attached SPOT Insight 2Mp Firewire Color camera and Fiberlight high intensity
7
illumination series (Dolan-Jenner Industries). Samples were placed on microscope slides with a white paper background to use reflected light for imaging.
Glass and Mineral Preparation
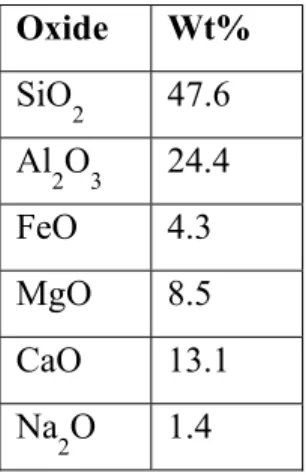
Glass batches were prepared from oxides and carbonates to match a simplified NU-LHT composition[5], discounting the minor constituents (<1%). Table 1 shows the oxide composition used.
Table 1: Weight percent composition of NU-LHT derivative glass
Oxide Wt%
SiO2 47.6 Al2O3 24.4
FeO 4.3
MgO 8.5
CaO 13.1
Na2O 1.4
This oxide composition was achieved by melting SiO2, Al2O3, Fe3O4, MgCO3, CaCO3, and Na2CO3 in 50g batches in a bottom load furnace (Deltech Inc, Denver CO) using a Pt crucible.
The melt remained in the furnace at 1550°C for 1.5 hours. Individual batch information in Appendix I. Batch 1 was water quenched to verify glass formation, while subsequent batches were air quenched, shown in Figure 2.
8
Figure 2: quenched glass samples from batch 1(left) and batch 2 (right)
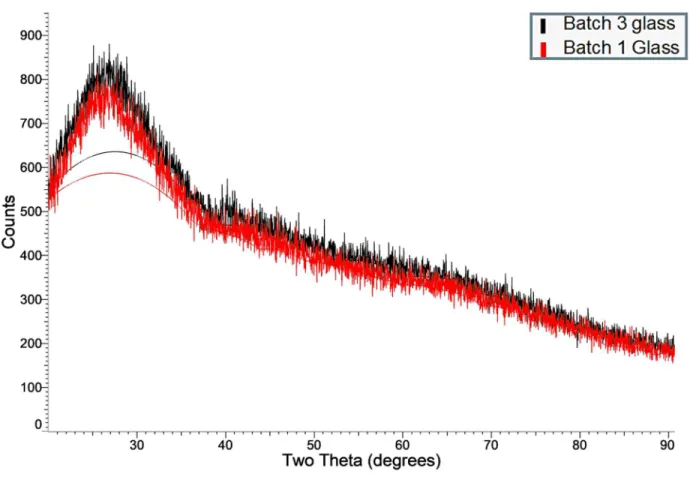
XRD was performed on batches 1 and 3 to determine if the different quenching methods resulted in any significant differences between the glass samples.
Figure 3: XRD results from NU-LHT derivative glass batches
9
As the two XRD patterns were nominally the same, the batches of glass will be treated a identical for the purpose of this report.
Four minerals were used to match NU-LHT composition, Olivine, Anorthite, Diopside, and Enstatite[6]. See Appendix II for XRD scans of each mineral. The Olivine was commercially available at suitable purity, while the remaining minerals had to be synthesized.
Anorthite was batched using Eq1 with fumed silica. The batch was milled in water with DARVAN 821A for 24 hours with zirconia milling media.
1CaCO3 + 1Al2O3 + 2SiO2 -> CaAl2Si2O8 Eq1 The slurry was dried using a Buchi R-300 Rotavapor (Buchi, New Castle, DE) and finished in a Fisher Scientific Isotemp Oven, Figure 4.
10
Figure 4: Buchi Rotavap R-300 (left) and Fisher Scientific Isotemp Oven (right)
Once dry, the anorthite precursor was fired in a MATAIR furnace at 1300°C for 5 hours with a heating rate of 5°C/min.
Preprepared diopside and enstatite precursors were each fired in a tube furnace, Figure 5. in flowing ultra-high purity (UHP) Ar for 5 hours with a heating rate of 5°C/min. The diopside was fired at 1250°C and the enstatite at 1040°C. Both minerals are iron bearing, thus requiring the UHP Ar environment to prevent oxidation.
11 Figure 5: Tube furnace
After firing, the Enstatite remained a fine gray powder, indicating that the iron present remained in a lower oxidation state. The Diopside densified on firing became an orange color indicative of oxygen contamination during firing causing oxidation of the iron present. The resulting sample was ground in a zirconia mortar and pestle until it returned to powder.
The glass and the anorthite were ground to powder using a Glen Creston Gyro Mill (Glen Creston Ltd, Stanmore, UK), Figure 6. Samples were milled in 7 second increments until large pieces were no longer visible.
12 Figure 6: Gyro mill or Planetary mill
The diopside was ground in zirconia mortar and pestle, while the enstatite and olivine required no milling.
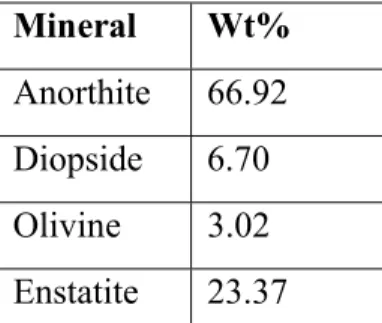
The minerals where then mixed together in the appropriate ratio, Table 2, to create an NU-LHT derivative simulant[7].
13
Table 2: Mineral proportions for NU-LHT derivative simulant Mineral Wt%
Anorthite 66.92 Diopside 6.70 Olivine 3.02 Enstatite 23.37
Anorthite was used for all the plagioclase minerals, and Enstatite was used in place of the Hypersthene as both are orthopyroxenes. The magnetite was discounted as the synthesized Diopside and Enstatite were both iron containing minerals.
Experimental
The samples for the experiment contained 50wt% each of the NU-LHT derivative simulant and the glass powder. Two temperatures were selected between the glass transition temperature (Tg) and crystallization temperature (Tc) of the glass determined by DSC, Figure 7, for the heat treatments.
14
Figure 7: DSC of NU-LHT derivative glass with Tg and Tc marked to show glass working range As literature indicated that 2 hours at temperatures between Tg and Tc would result in a 70-90%
dense sintered sample[8], experimentation was determined to start without a hold at the selected temperature to prevent samples from adhering to the crucible surface. Two samples were individually heated at a rate of 5°C/min to 800°C and 870°C in flowing Ar(5%)H2 in a water cooled tube furnace. The furnace was shut off once it reached temperature to reach the quickest cooling rate possible without removing the sample from the controlled environment of the furnace. A third and fourth sample were then heated to 870°C with the same parameters and a 20-minute and 3 hour hold at temperature respectively. The samples were then analyzed under optical microscope and SEM.
15
Results and Discussion
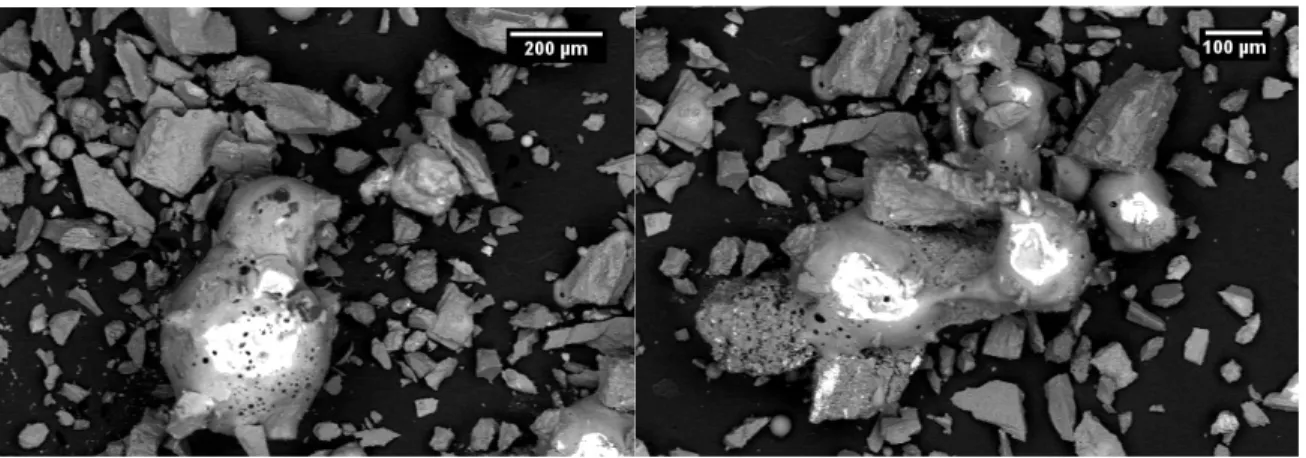
The SEM images from the USGS pseudo agglutinates, Figure 8, and the experimental samples, Figure 9, yielded interesting results. In the USGS sample, the glass and mineral particles were easily distinguishable, as all the glass particles were smooth edged and often had visible open porosity at their surfaces.
Figure 8: SEM BSE images of USGS pseudo agglutinate taken using backscatter electrons The experimental samples did not have this feature as all the particles appeared similar to each other, with hard edges and no obvious porosity. It also became apparent that the particle size distribution of the experimental samples was significantly greater than that of the USGS sample.
16
Figure 9: SEM BSE images of experimental samples, 800°C no hold (left) and 870°C no hold (right)
The small particles visible in the SEM images of the experimental samples were small enough to create difficulty in resolving the surface features of the larger particles. This resulted in lower quality SEM images with less distinct particles. These particles were not present in the USGS samples.
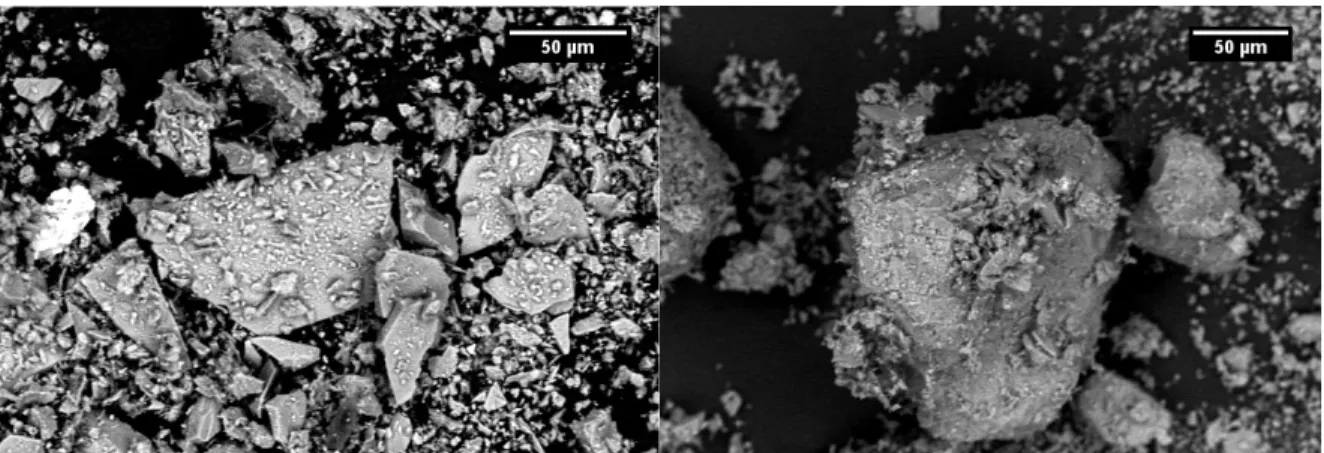
The lack of any apparent rounding of the edges and corners of the glass particles to distinguish them from the mineral ones suggested that the sample was not at a high enough temperature for sufficient time to allow for any significant softening in the glass. This was confirmed by the SEM images from the sample heated to 870°C with a 20 minute, Figure 10.
Figure 10: SEM images of experimental sample heated to 870°C with a 20 minute hold at temperature BSE (left) and SE (right)
17
While the particles seen in those images were still highly dissimilar to the USGS pseudo agglutinates, when compared to the SEM images of real lunar agglutinates Figure 11, some improvement in similarity can be observed.
Figure 11: Fig. 7.2 (b) Scanning electron micrograph of a doughnut-shaped agglutinate, This agglutinate, removed from soil sample 10084, has a glassy surface that is extensively coated with small soil fragments. A few larger vesicles are also visible. (c) Scanning electron photomicrograph of an irregular agglutinate from soil 10084, showing some vesicular, glassy, fragment-laden surfaces. Some agglutinates are very delicate and display narrow bridges, necks, and dendrite-like arms (NASA Photo S87-38811) [3]
The rough surface of the experimental sample began to resemble the surface of lunar agglutinates after the 20 minute hold. It is likely that the added time at temperature allowed the smallest fraction of particles in the sample to begin adhering to the smaller glass and mineral particles.
The optical images of the experimental samples with no hold and a 20 minute hold support that hypothesis. While each particle has clear adhesions on its surface, the samples heated to higher temperatures and the one held at temperature for a longer time clearly have more particle adhesions that the one from a lower temperature.
18
Figure 12: Images of experimental samples taken through an optical microscope. A) 800°C, B) 870°C, C) 870°C 20-minute hold
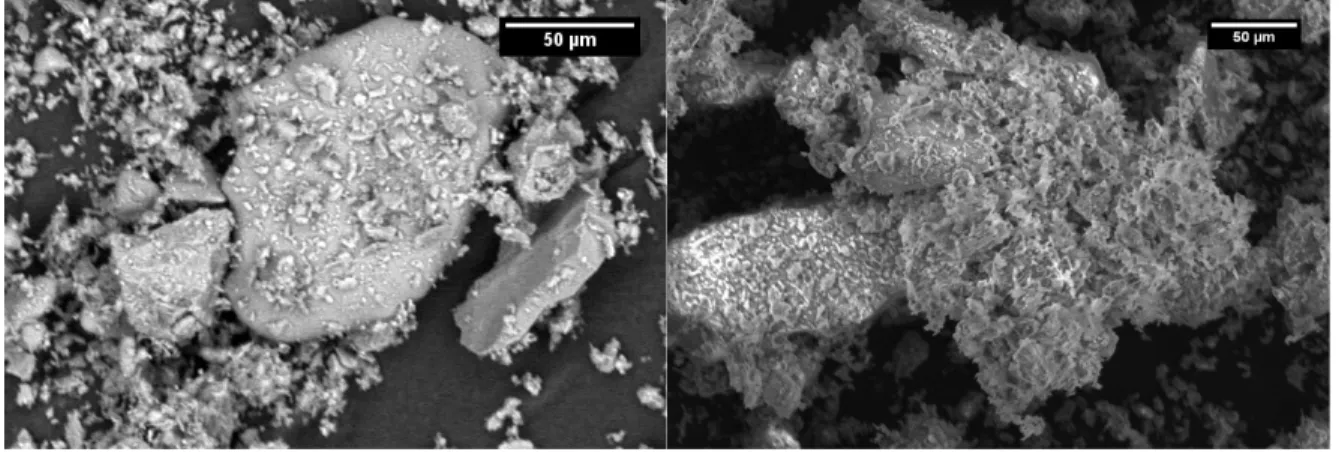
The fracture markings from milling the samples were also still visible on the surfaces of two of the samples, reinforcing that the large glass shards did not soften significantly from the heat treatment. This held true after 3 hours as 870°C, Figure 13, as well.
A B
C
19
Figure 13:Optical microscope images of glass particles from sample heated to 870°C for 3 hours After 3 hours at temperature, the glass shards retained the hard edges from milling and the apparent quantity of particle adhesions on the glass surface decreased. However, the surface of the glass began to appear pitted, possibly due to particles sinking into the surface instead of adhering to it. The fines also began adhering to the large mineral particles, specifically the anorthite ones, Figure 14.
20
Figure 14: Optical microscope image of anorthite particle from sample heated to 870°C for 3 hours with phases circled. A) anorthite, B) glass, C) enstatite and glass, D) diopside
The Anorthite particle had four distinct kinds of particles on its surface, orange diopside, white anorthite, dark gray glass and medium gray enstatite and glass mix. The presence of particles on the anorthite suggests that the glass fines softened sufficiently to adhere this mix of minerals and glass to the anorthite surface. When combined with the lack of apparent rounding of edges and decrease in particles on the surface of the glass particles means that the heat treatment only effects the smallest fraction of glass particles with a minimal effect on the larger shards. Thus, with a reduced particle size, this process for creating agglutinates is feasible.
Further experimentation to determine the exact parameters of effectiveness is needed.
Specifically looking at the needed particle size, and the optimum temperature and time.
Temperatures up to 890°C should be investigated. Increasing the ratio of glass to minerals in the D
A B C
21
samples to greater than 70wt% would also be beneficial. Characterization work to determine the oxidation state of the iron present in each mineral is needed.
22
Works Cited
1. Hall, L. Overview: In-Situ Resource Utilization. 2020 April 2020; Available from:
https://www.nasa.gov/isru/overview.
2. NASA, Lunar Sourcebook A User's Guide to the Moon, ed. D.T.V. Grant H. Heiken, Bevan M. French. 1991.
3. NASA, Agglutinates, in Lunar Sourcebook A User's Guide to the Moon. 1991. p. 296-302.
4. Stoeser, D.W., Steve, NU-LHT-1M Pilot Highlands Soil Simulant.
5. Survey, U.S.G., NU-LHT-2M Materials Safety Data Sheet. 2008: Denver, CO.
6. Howard, C. Synthetic Lunar Minerals. in Lunar and Planetary Science Conference. 2022.
NASA.
7. D.B. Stoeser, D.L.R., S. Wilson Design and Specifications for the Highland Regolith Prototype Simulants NU-LHT-1M and -2M. 2010.
8. Erasmus, E.P., et al., Effects of Sintering Temperature on Crystallization and Fabrication of Porous Bioactive Glass Scaffolds for Bone Regeneration. Scientific Reports, 2017. 7(1).
23
Appendix I Glass Batching
Table 3: Glass Batching and Melting Parameters
Component Batch 1 (g) Batch 2 (g) Batch 3 (g)
SiO2 19.9 19.81 19.82
Al2O3 10.10 10.17 10.12
Fe2O3 1.94 1.93 1.94
MgCO3 7.42 7.42 7.39
CaCO3 9.83 9.88 9.21
Na2CO3 1.06 1.02 1.06
Melt temp 1550 1550 1550
Melt time 1.5 1.5 1.5
Quench Water Air Air
Crucible Pt Pt Pt
24
Appendix II Mineral XRD
Figure 15: Anorthite XRD Scan
25 Figure 16: Diopside XRD Scan
Figure 17: Enstatite XRD Scan
26 Figure 18: Olivine XRD Scan