I want Dr. Tim Keenan for always being available for additional help and contributing to his knowledge in the laboratory. Each HA compound was formulated to reflect the natural calcium-to-phosphorus (Ca/P) ratio of 1.67.
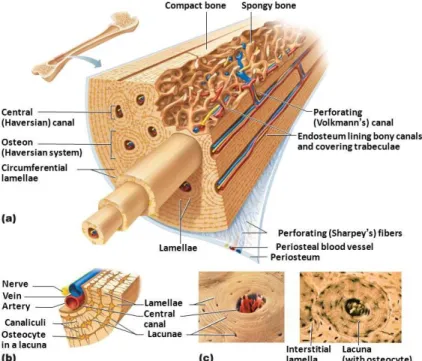
Bone
Importance of the Skeletal System
Types of Bone
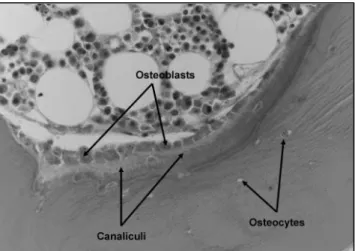
These cells allow bone growth/repair and secrete bone matrix components such as collagen and calcium-binding proteins. Formation of new bone through collagen conversion of the protein matrix that is mineralized in the canalicule [1].
![Figure 1. Regions and components of a long bone. [1]](https://thumb-ap.123doks.com/thumbv2/123dok/10490021.0/10.918.259.708.233.567/figure-1-regions-components-long-bone-1.webp)
Hydroxyapatite (HA)
Synthesizing Hydroxyapatite
For materials that are highly dependent on their amounts of calcium and phosphorus, the Ca/P ratio is used to obtain a simple relative composition of the two main components of the material. The ideal ratio of 1.67 for synthetic HA is based on its suitability for biointegration and also on synthesizing the synthetic analog as close as possible to its natural form, bone tissue.
Biological Testing of HA
There was a random allocation of HA-coated titanium plasma-sprayed implants and control uncoated titanium plasma-sprayed implants. It is also noted that there is greater and faster osseointegration of HA-coated than uncoated implants.
Post Implant Infections
Infection Rates and Risks
Types of Infections
Methods for Fighting Infections with Hydroxyapatite
Antibiotic Integration
Doping
Some studies have been conducted to analyze the effects of metal ions on hydroxyapatite properties. A study showed that doping with Mg2+ increased the grain size, while doping with Zn2+ reduced the grain size. Upon sintering Al 3+ -doped HA at both 1100°C and 1300°C, the samples became thermodynamically unstable, resulting in transformation to TCP and CaO.
It was reported that amounts >5 mol% Al3+ compared to mol% Ca2+ were more likely to cause this phase transformation [24]. Doping of HA by ion substitution continues to develop; however, a significant amount of future research needs to be performed to be able to predict outcomes of doping HA with particular ions and specific concentrations. At this point, hydroxyapatite coatings containing silver (Ag) are most often prepared experimentally by replacing Ca ions with Ag ions by dipping HA in AgNO3.
It works most effectively on the outer surface of the coating and has not been too successful for the full depth of HA coatings. For HA coatings, which have been successfully doped with Ag throughout the coating, sol-gel, co-sputtering, and thermal or cold spraying methods have been used.[25] Synthesizing Ag-doped HA by a precipitation is a new concept and showed promising results when synthesized at 100°C.[25]. Research has shown success in Cu-doped HA removing arsenic from water and soil.[26] In recent years, Cu-doped HA with copper in the form of Cu (CH3COO)2⋅ H2O (copper acetate) has been synthesized to successfully inhibit some bacterial growth; however, no success was achieved when testing with S.
Antibacterial Metals
Silver
Studies indicate that antibiotics used with Ag+ ions in small concentrations increase the effectiveness of killing bacteria by 10 – 1000 times. Vancomycin and Ag+ ions have shown promising results in killing bacteria that have protective outer coats. One of the only negative effects noted is argyria, irreversible discoloration of skin or tissue, when used excessively.
Several case studies show no cellular reactions to silver exposure.[28] Although there is little evidence that silver is toxic to the human body, care should be taken not to use too high a concentration of Ag+ ions when used internally. More recent studies show that silver ions can cause a certain tissue response, especially of the heart, when used excessively.[31]
Copper
Hydroxyapatite (HA) Synthesis
Doped HA Synthesis
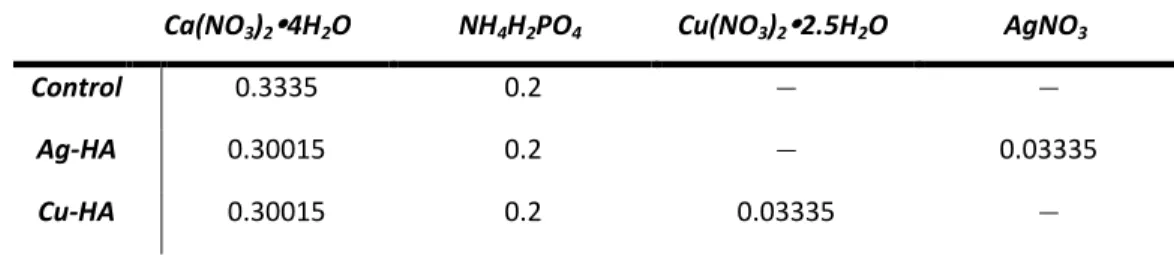
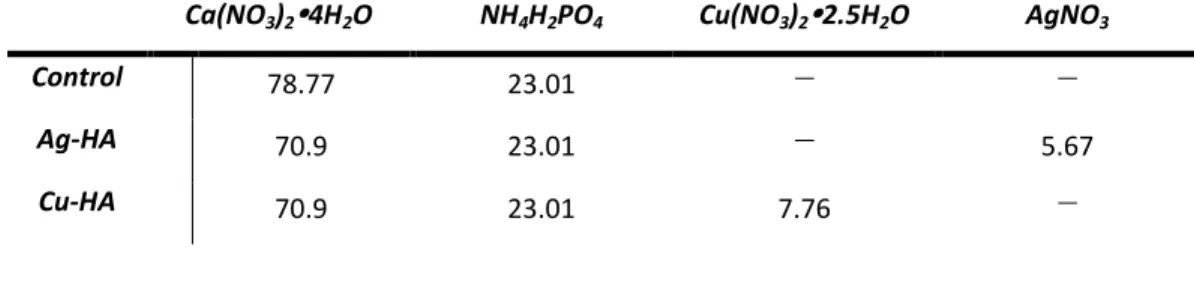
Cu- and Ag-doped HA were synthesized using the same precipitation method as pure HA. The only exception was the replacement of part of calcium (Ca) with copper (Cu) and silver (Ag). Exchanges of copper and silver for calcium were made in the same molar ratio.
For calculations of all synthesized HA mixtures, 0.3335 mol of Ca was considered to be pure HA, where 100% of (X+Ca) was Ca. For each reagent, the total molecular weight was divided by the desired number of moles. Both solutions underwent the same heating and stirring methods as the pure HA, and the processing method remained the same throughout the synthesis.
As in Cu-doped HA, the phosphate source remained the same as pure HA. Both solutions were subjected to the same heating and mixing methods as pure HA, and the processing method remained the same throughout the synthesis.
Sintering
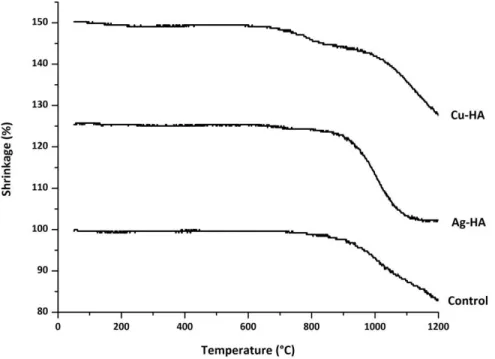
HSM data for HA, Ag-HA and Cu-HA powders used to determine sintering temperatures of the wafers. Before the powders were sintered in the form of disks, the powders were dried at 100°C and allowed to cool in the oven. The dry powders were then ground by hand with a mortar and pestle to break up any agglomerations of powder.
The disks were formed using a Carver Laboratory Press and a 4-ton die to compress 0.052 g of powder for each disk. To ensure that all assemblies were fully sintered, all three assemblies were held at 1100°C for 2 hours. The samples were placed in the oven prior to heating and remained in the oven until completely cooled.
Characterization
- X-ray Diffraction (XRD)
- Particle Size: Dynamic Light Scattering (DLS)
- Surface Area: Brunauer-Emmet-Teller (BET)
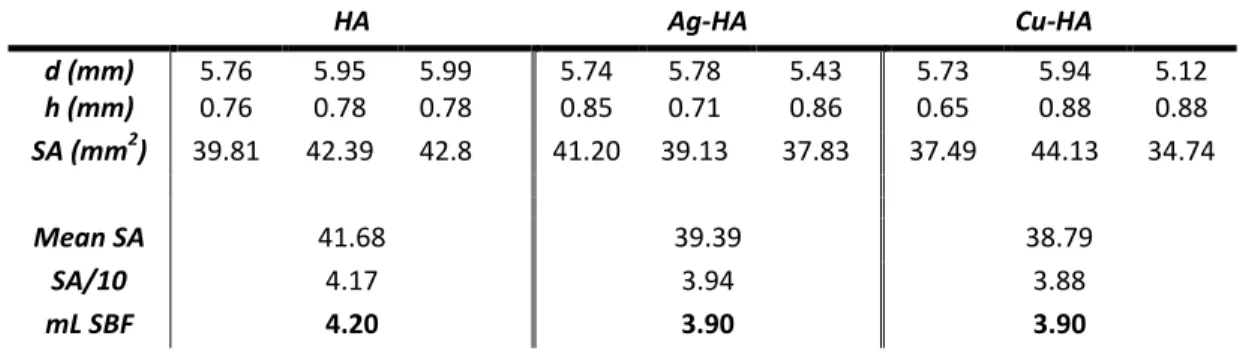
- Surface Crystallization and pH: Simulated Body Fluid (SBF)
- Ion Release: Inductive Coupled Plasma (ICP)
- Scannning Electron Microscopy & Energy Dispersive X-ray Analysis (SEM/EDS)
- Bacterial Testing of Powders
The particle size of each of the synthesized HA powders was determined using a Microtrac NanotracTM ULTRA DLS and Microtrac Flex 10.5.0 software. A sample of each of the 3 HA compounds was measured at each time point in each of the 3 ports, repeating the measurements a total of 3 times. Sintered discs of each of the three HA composites were placed in simulated body fluid and incubated at 37°C.[34] Three samples of each composition were tested for time periods of 15, 30 and 45 days.
To calculate the surface area of the discs, only the surfaces in contact with the SBF, the sides and the top of each disc were included in the calculation. Powder images were taken at a voltage of 15 kV, spot size 5.0 and using backscattered electrons. The antibacterial properties of Ag and Cu doped powders were tested in comparison to pure HA powder with E.
One colony from the appropriate 24-hour culture of bacteria was grown aerobically in liquid broth at 37°C for 24 hours. After incubation, 100 μl of broth was taken from each of the 18 filled wells in the 24-well plate and pipetted into a 96-well plate. The absorbance of the broth was subtracted from each of the wells to determine the relative concentration of bacterial viability of each sample compared to the control bacteria after the 24 hour period.
Characterization of Powders
X-ray Diffraction
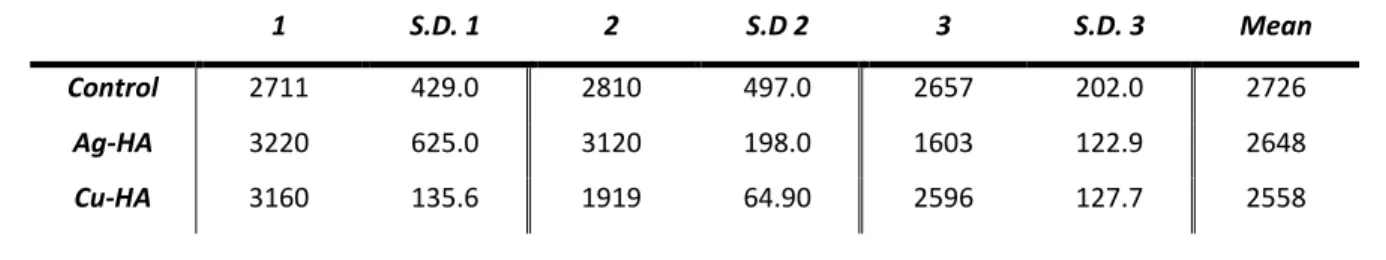
The average particle diameter of each of the three powders all varied in the order of 2,000 nm with the standard deviations all in the order of hundreds of nanometers. The diameter is based on the MV measurement in which the volume distribution is used. The control powder had the largest mean diameter, followed by Ag-HA, and Cu-HA; however, the variation between the mean of the control and the mean of the Cu-HA powder was less than 200 nm.
Similar to the particle diameter, the calculated surface areas of the three powders all had similar results. The specific values for the mean surface area of the powders as well as the standard deviation between the experiments are shown in Table 8. Cu-HA had a higher calculated surface area in m2/g than both the control and Ag-HA.
Scannning Electron Microscopy & Energy Dispersive X-ray Analysis (SEM/EDS)
The composition of the powders determined from the spectra and the calculated (X+Ca)/P ratios are shown in Table 9. The composition of the HA, Ag-HA and Cu-HA powders and the Ca/P ratios determined by EDS.
Characterization of Sintered Discs
Scannning Electron Microscopy & Energy Dispersive X-ray Analysis (SEM/EDS)
All sintered disks showed the same C, O, Ca, P, and Si peaks, with small variations in peak intensity, present in EDS analysis. As shown by the EDS analysis of the powders, the sintered disks of Ag and Cu HA had small Ag and Cu peaks, respectively. The EDS spectra of the disks incubated in SBF showed small peaks of elements present.
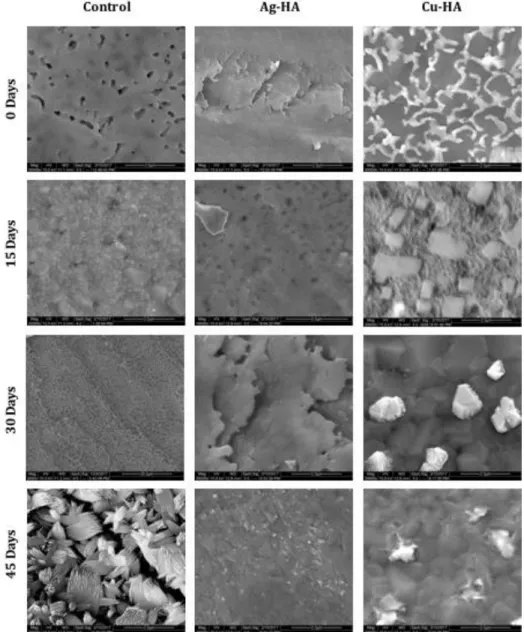
Images of non-SBF control discs show surface differences between individual disc compositions. Changes in surface structure are evident in all discs after incubation in SBF, with Cu-HA showing the most surface deposition. Surface cracks are evident after 45 days in SBF for the HA control disc, indicating that the disc has experienced dehydration.
SEM images taken at 20,000x magnification of the sintered discs after incubation in SBF. note: HA control image after 30 days was taken at 2000x due to surface charge and inability to image at higher magnification).
Ion Release: Inductivly Coupled Plasma (ICP)
Bacterial Testing of Powders
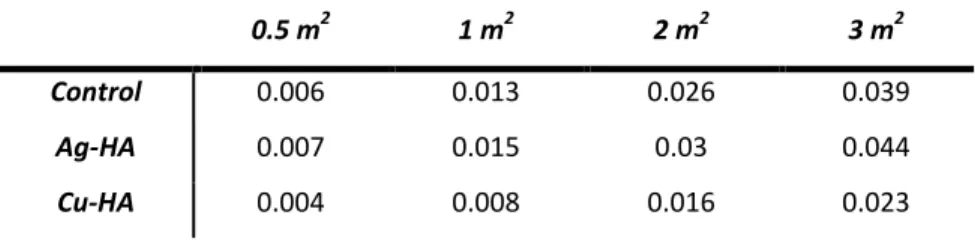
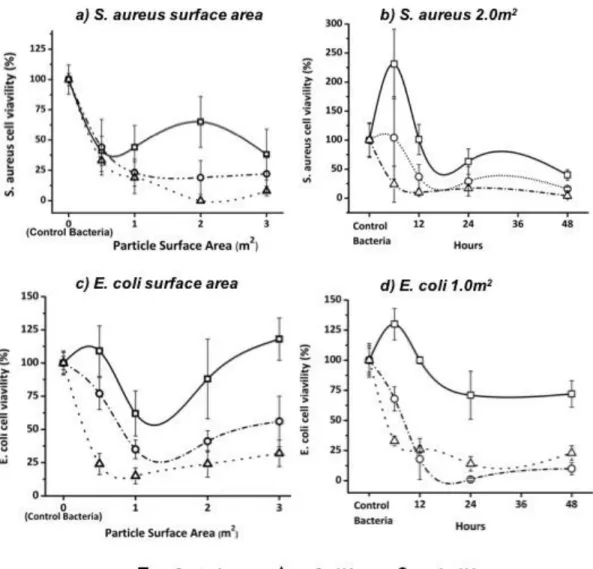
The bacterial inhibition over time of both Ag- and Cu-HA was significantly greater than that of the control, and for both powders bacterial viability increased drastically by 48 hours. Images of the bacterial plates and corresponding colony numbers can be seen in figure 17 for E. coli and S. aureus. Two of the powders were doped with 10 mol% of a metal in the form of nitrates.
Surface mineralization on the surface of discs after incubation in SBF is likely apatite precipitation. Due to the inability to physically count bacterial colonies in the slurry, bacterial viability data were presented as a percentage of control viability. The greatest success was observed for both Ag- and Cu-HA at low concentrations (1 m2 for E. coli and 2 m2 for S. . aureus ), indicating that only small amounts of doped HA powders (10% mol) required to significantly reduce bacterial growth.
These differences in growth optimization may explain the effect of HA control powder on S. In correlation with Cu ion release being significantly greater than Ag, bacterial viability tends to be lower after exposure to Cu-HA powder than Ag-HA powder. Itoh, S., et al., Biocompatibility and osteoconductive activity of a novel hydroxyapatite/collagen composite biomaterial and its function as a rhBMP-2 carrier.
![Figure 5. Antibacterial mechanism of Ag nanoparticles on bacteria. [41]](https://thumb-ap.123doks.com/thumbv2/123dok/10490021.0/19.918.265.708.688.1011/figure-5-antibacterial-mechanism-ag-nanoparticles-bacteria-41.webp)