The aim of this work is the study of the adsorption of dye solution, which is a mixture of three dyes (methylene blue, congo red and malachite green) using commercial activated carbon. As proven by many researchers, the removal of dyes with activated carbon is economically advantageous and technically easier. Activated carbon is widely used as an adsorbent due to its high adsorption capacity, high surface area, microporous structure and high surface degree, respectively.
In 2005 Indra Deo Mall, Vimal Chandra Srivastava, Nitin Kumar Agarwal, Indra Mani Mishra studied the adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon kinetic study and equilibrium isotherm analyses. Kinetics and Equilibrium Studies Bamboo, an abundant and inexpensive natural resource in Malaysia was used to prepare activated carbon by physiochemical activation with potassium hydroxide (KOH) and carbon dioxide (CO2) as the activating agents at 850 ◦C for 2 h. Adsorption isotherm of the methylene blue (MB) on the activated carbon was determined and correlated with general isotherm equations.
The adsorption of methylene blue can be best described by the second-order pseudo-equation. In 2006 Ewa Lorenc-Grabowska, Gra_zyna Gryglewicz studied the adsorption characteristics of Congo Red on coal-based mesoporous activated carbon. Adsorption of Congo Red (CR) dye on coal-based mesoporous bituminous activated carbon (AC) from aqueous solutions was studied. The pseudo second-order kinetic model describes very well the adsorption of CR on mesoporous activated carbon.
Abu-Nameh studied the absorption of phenol and methylene blue by activated charcoal from pecans.
MATERIALS AND METHODS
ADSORBENT
It is a form of carbon that has been processed to be extremely porous and therefore has a very large surface area available for adsorption or chemical reactions. Due to the high degree of microporosity, only one gram of activated carbon has a surface area of 1000 m2, as usually determined by nitrogen gas adsorption.
GLASSWARE AND APPARATUS USED
The samples were washed several times and dried overnight in an oven at 50°C and ground to a fine powder before use.
ADSORBATE
The Congo red dye was first synthesized in 1883 by Paul Bottiger who was then working for the Friedrich Bayer Company in Elberfeld, Germany.
Adsorption Studies
- Effect of contact time
- Effect of initial pH
- Effect of adsorbent dose
- Effect of temperature
- Effect of Contact time
- Effect of adsorbent dosage
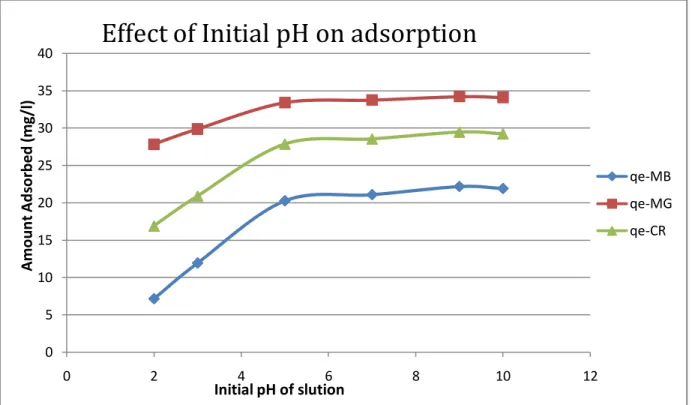
At pH - 2, the removal was minimal, but it increased with increasing initial pH of the dye solution. The adsorption of these positively charged dye groups on the surface of the adsorbent is primarily influenced by the surface charge on the adsorbent, which in turn is influenced by the pH of the solution. The result showed that the availability of negatively charged groups at the adsorbent surface is necessary for the adsorption of basic dyes to continue, which we see at pH -2 is almost unlikely, since there is a net positive charge in the adsorption system due to the presence of H30+ .So as the pH increased, more negatively charged surface was available, facilitating greater color removal. We see that the trend is increasing with increasing pH.
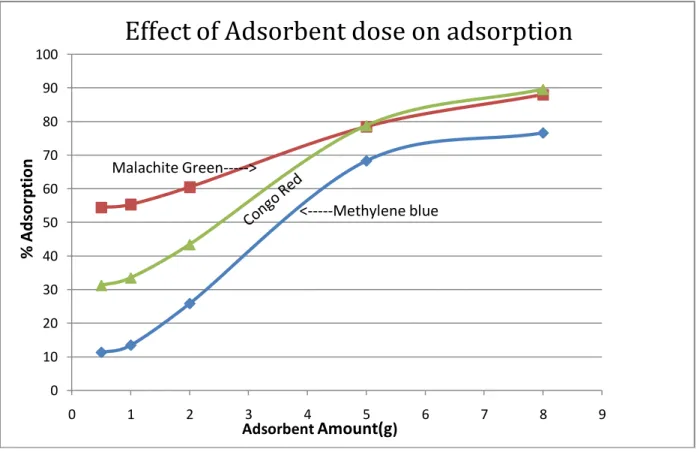
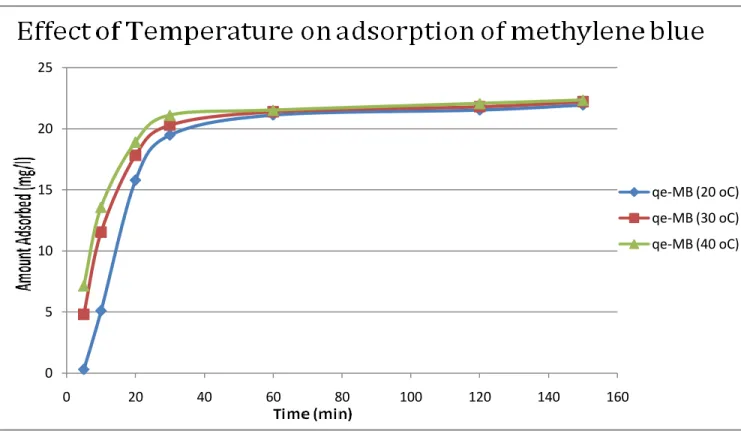
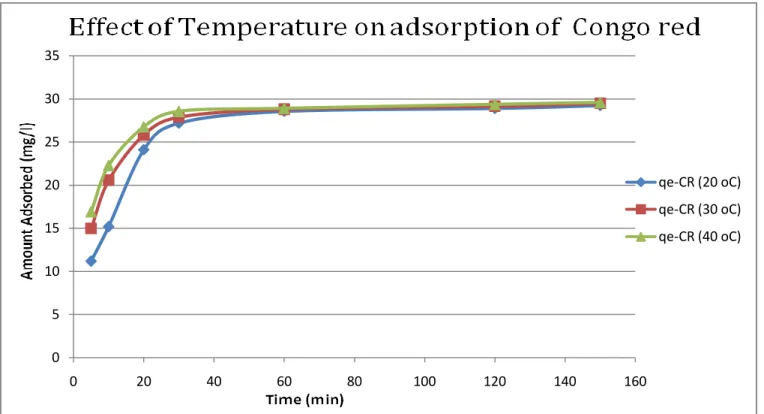
It is clear that with increasing amount, the active sites for adsorption of a mixture of three dyes. The decrease in adsorption capacity with an increase in adsorbent concentration could be attributed to the fact that part of the adsorption sites remained unsaturated during the process. The result of time studies for the adsorption of the three dyes malachite green, methylene blue and congo red at different temperatures is shown in the figures below.
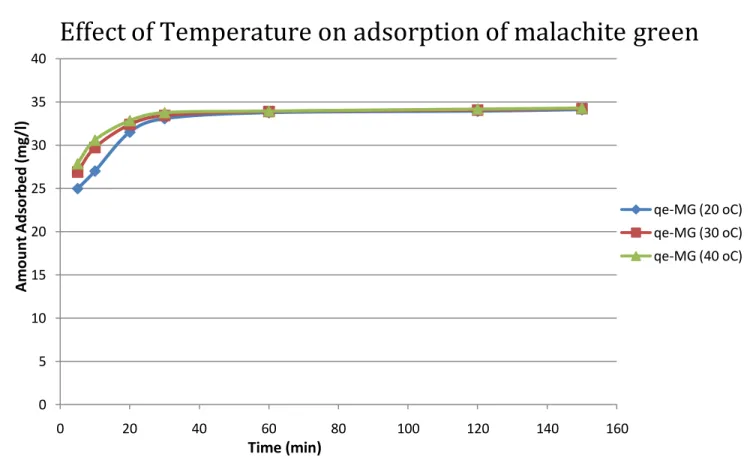
Results indicate that the adsorption capacity of activated carbon for the three dyes (methylene blue, malachite green and congo red) increased with temperature. This may be the result of the increase in the mobility of the large dye ion with temperature. Furthermore, increasing temperature can cause a swelling effect within the internal structure of the activated carbon that allows large dyes to penetrate further.
The result showed that availability of negatively charged groups at the adsorbent surface is necessary for the adsorption of basic dyes to proceed, what we see at pH -2 is almost unlikely as there is a net positive charge in the adsorption system due to of the presence of H30+ .As the pH increased, more negatively charged surface was available thus facilitating greater color removal. It is clear that with increasing amount, the active sites for adsorption of mixture of three dyes increases resulting in increase in removal efficiency. The decrease in adsorption capacity with an increase in the adsorbent concentration can be attributed to the fact that some of the adsorption sites remained unsaturated during the process and agglomeration of activated carbon as a result of which all the surface area is not available for adsorption process.
The optimum temperature is 30 oC. The adsorption capacity of activated carbon for three dyes (methylene blue, malachite green and Congo red) increases with temperature. Kavitha (2001) Removal of Congo red from water by adsorption onto activated carbon prepared from coir kernel, agricultural solid waste, dyes and pigments. Walker (2006) Adsorption of methylene blue onto activated carbon produced from steam-activated bituminous coal, Chemical Engineering Journal.
Grabowska Ewa Lorenc, Gryglewicz Gra_zyna (2005) Adsorption characteristics of Congo Red on coal-based mesoporous activated carbon, dyes and pigments. Sya Rizal (2005) Adsorption properties of malachite green on activated carbon derived from rice husks prepared by chemical-. Indra Deo Mall, Vimal Chandra Srivastava, Nitin Kumar Agarwal, Indra Mani Mishra, Adsorptive removal of malachite green dye from aqueous solution using bagasse fly ash and activated carbon kinetic study and equilibrium isotherm analysis.
Martin Deva Prasath (2009) Adsorption of malachite green on carbon prepared from borassus bark, The Arabian Journal for Science and Engineering, Volume 34, Number 2A, July 2009.