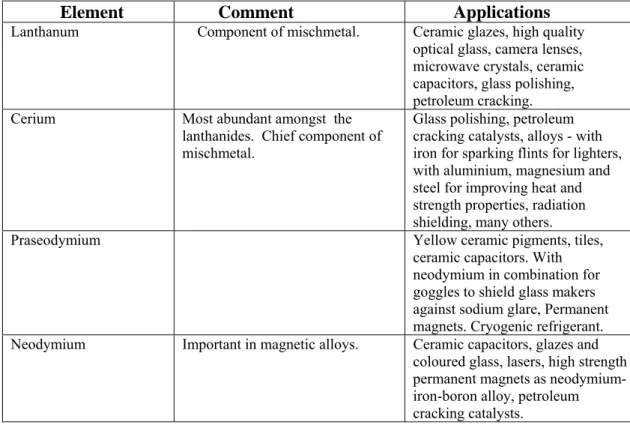
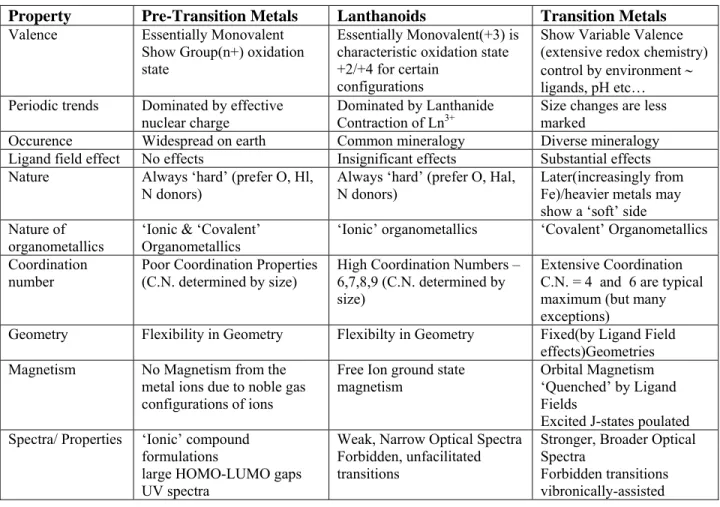
Inorganic Chemistry
Chemistry of Lanthanoids
Dr. Reena Jain Dept. of Chemistry
Hindu College Delhi -110052 (26.10.2006)
CONTENTS
Introduction
Position of lanthanoids in the periodic table Terrestrial abundance and distribution
Extraction of lanthanoids from minerals
Electronic structure Atomic and ionic radii : Lanthanoid contraction
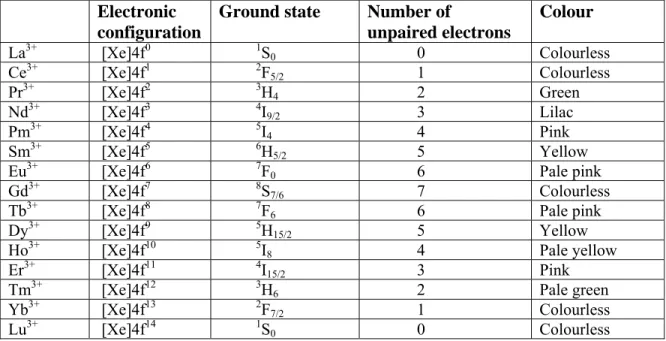
Oxidation states Separation of lanthanoid elements Magnetic properties Colour and spectra Chemistry of “0” oxidation state – The metal Chemistry of lanthanoid ions in +2 oxidation state Chemistry of lanthanoid ions in +3 oxidation state Chemistry of lanthanoid ions in +4 oxidation state Complex formation Uses of lanthanoids and their compounds Some comparisons and contrasts
Introduction
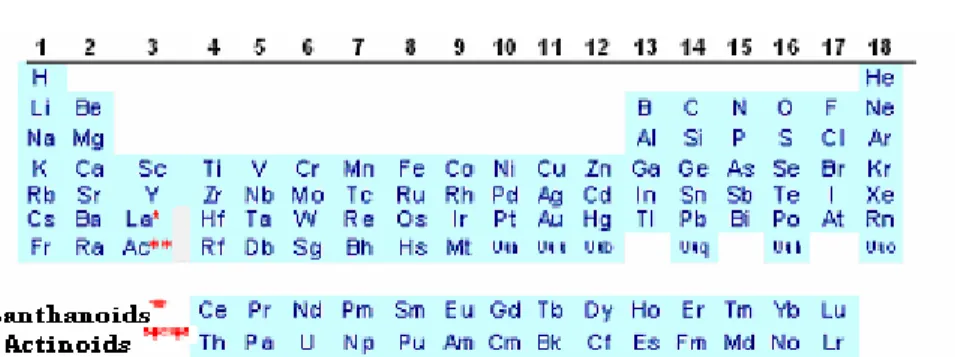
Group 3 of the periodic table contains scandium , yttrium and lanthanum . Strictly speaking , actinium should also be included , but in practice , it is studied separately . There are fourteen elements that follow lanthanum and these are called lanthanides . The lanthanides comprises of the largest occurring group in the periodic table . These lanthanides are placed below the main body of the periodic table in the manner of a footnote . The full - width version of the periodic table shows the position of the lanthanides more clearly (Table 1) .
Table 1: Modern Periodic Table
These lanthanides are associated with a major confusion regarding their terminology.
Whether the term “Lanthanides” refers to the fifteen elements from La to Lu or the fourteen elements from Ce to has been debated for long . “Rare Earth Elements” and
“ Rare Earth Metals” are trivial names sometimes applied to a collection of these elements in the periodic table . Earth is an obsolete term for “oxide” . At the time of their discovery , the oxides of these elements were believed to be scarce in abundance , as minerals . This terminology is no longer appropriate , as these elements are no longer rare , except promethium , with a t1/2 of 2.6 years . To avoid any confusion , now the term “lanthanoid” rather than “lanthanide” , is used to represent these elements as the suffix “-ide” is generally used to indicate anions . However , even now there is a confusion regarding the position of La , i.e. , whether the group is made up of fifteen elements , La to Lu , or fourteen elements , Ce to Lu . Lanthanides are chemically similar to each other , to scandium as well as yttrium . Currently, the general symbol, Ln, is used for the fourteen elements ( Ce - Lu ) and group III elements Sc , Y and La.
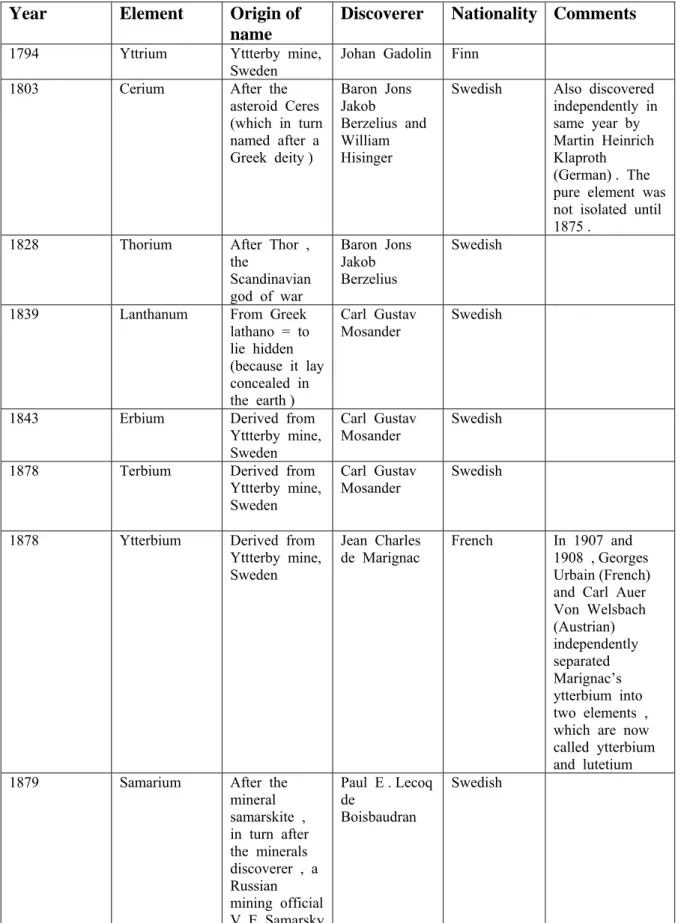
The story of the lanthanoids begins in 1787 when a young Swedish artillery officer, Lieutenant Carl Axel Arrhenius , who was a keen amateur geologist , was exploring a quarry at a small town called Ytterby , near Stockholm . He found a new , very dense black mineral which he named ytterbite . Its chemical analysis carried out by Johan Gadolin , a Finnish chemist in 1794 showed that the new mineral contained oxides of iron , beryllium, silicon and a new , previously unidentified 'earth' which he named 'yttria' . Yttria was later shown to be a mixture of the oxides of six rare earth elements . The history of discovery and naming of the lanthanides are summarized in Table 2 .
Table 2: Discovery & origin of names of lanthanoids, including yttrium, thorium &
scandium
Year Element Origin of
name
Discoverer Nationality Comments
1794 Yttrium Yttterby mine,
Sweden Johan Gadolin Finn
1803 Cerium After the
asteroid Ceres (which in turn named after a Greek deity )
Baron Jons Jakob
Berzelius and William Hisinger
Swedish Also discovered
independently in same year by Martin Heinrich Klaproth (German) . The pure element was not isolated until 1875 .
1828 Thorium After Thor ,
the
Scandinavian god of war
Baron Jons Jakob Berzelius
Swedish
1839 Lanthanum From Greek
lathano = to lie hidden (because it lay concealed in the earth )
Carl Gustav
Mosander Swedish
1843 Erbium Derived from
Yttterby mine, Sweden
Carl Gustav
Mosander Swedish
1878 Terbium Derived from
Yttterby mine, Sweden
Carl Gustav Mosander
Swedish
1878 Ytterbium Derived from
Yttterby mine, Sweden
Jean Charles
de Marignac French In 1907 and 1908 , Georges Urbain (French) and Carl Auer Von Welsbach (Austrian) independently separated Marignac’s ytterbium into two elements , which are now called ytterbium and lutetium
1879 Samarium After the
mineral samarskite , in turn after the minerals discoverer , a Russian mining official V. E Samarsky
Paul E . Lecoq de
Boisbaudran
Swedish
1879 Scandium After
Scandinavia Lars Fredrik
Nilson Swedish
1879 Holmium After the
Latin word for Stockholm, Holmia
Per Teodor Cleve
Swedish Also discovered
independently by Jacques Louis Soret and Marc Delafontaine (Swiss)
1879 Thulium From the
Latin Thule, an ancient name for Scandinavia
Per Teodor
Cleve Swedish
1880 Gadolinium In the honour
of Johan Gadolin , a Finnish chemist
Jean Charles
de Marignac Swiss of
French origin Paul E . Lecoq de Boisbaudran independently isolated the element from Mosander’s yttria in 1886
1885 Praseodymium From Greek
prasios = green , in reference to the colour of the salts and didymos = twin , because the earth didymia was separated into two salts ; Pr and Nd
Carl Auer
Von Welsbach Austrian
1885 Neodymium From Greek
neo = new and didymos
= twin , because the earth didymia was separated into two salts;
Pr and Nd
Carl Auer
Von Welsbach Austrian Not isolated in relatively pure form until 1925
1886 Dysprosium From Greek
dys = bad and prositos
= aproachable, , dysprositos means hard to get because of the difficulty involved in its detection and isolation
Paul E . Lecoq de
Boisbaudran
French
1901 Europium After Europe Eugene
Demarcay French
1907 Lutetium After Lutetia , Latin name for the place where Paris was founded
Independently by Georges Urban and Carl Auer Von Welsbach
French and Austrian
1947 Promethium After
Prometheus , in greek mythology , who brought fire to mankind in reference to harnessing of the energy of the nuclear fission and warning against its dangers
Charles DuBois Coryell Lawrence E . Glendenin and Jacob A . Marinsky
American
On the basis of their separablility , the lanthanoids were conveniently divided into the
“cerium group minerals” or “light earths” (including light lanthanoid elements , from La to Euro) and the “yttrium group minerals” or “heavy earths” (including heavy lanthanoid elements from Gd to Lu , along with Y) . Yttrium is lighter than other “yttrium group minerals” , but is still grouped with them , as it has a comparable ionic radius and occurs in nature associated with the ores of heavier lanthanoids. This unit shall deal with the general chemistry of lanthanoids, including the implementation of the conceptual approach . The unit also provides the background essential to understand the problems of their recovery and separation , along with their applications.
Position of Lanthanoids in the Periodic Table
The lanthanoids have atomic numbers between those of barium (Z = 56) and hafnium (Z = 72) , and hence must be placed between these two elements . Ba is an alkaline earth metal belonging to group 2 , below Sr . Hf is present is group 4 , below Zr , thus leaving only one place between them , which lies exactly below Υ ( Z = 39 , group 3) . Since all the lanthanoids resemble each other in many aspects , therefore it become necessary to accommodate all of them together at one place . This problem is solved by placing the first element i.e. La below Υ and the remaining elements separately in the lower part of the periodic table (Table 1).
Terrestrial Abundance and Distribution
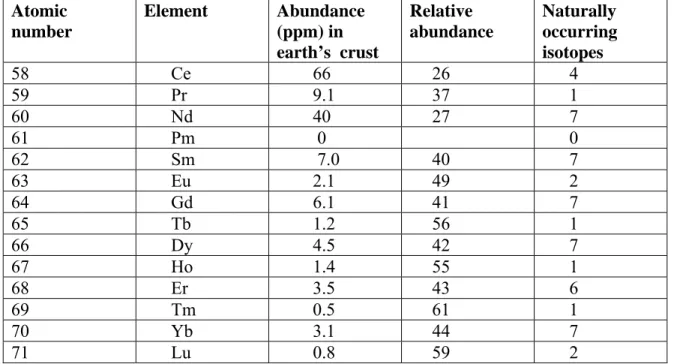
The lanthanoid elements are not particularly rare. Apart from the unstable 147 Pm (half life 2.6 years) of which traces occurs in uranium ores , all the lanthanoids are actually more abundant than iodine . Cerium is the twenty - sixth most abundant of all elements , being half as abundant as Cl and more abundant than lead . Even Tm , the rarest after Pm , is more abundant than iodine , and Lu is more abundant than gold . The abundance of these elements and the number of naturally occurring isotopes vary regularly , in accordance with Harkin′s rule (Table 3) .
Table 3: Abundance of the lanthanoides in the earth’s crust by mass and number of natural isotopes
Atomic number
Element Abundance (ppm) in
earth’s crust
Relative abundance
Naturally occurring isotopes
58 Ce 66 26 4
59 Pr 9.1 37 1
60 Nd 40 27 7
61 Pm 0 0
62 Sm 7.0 40 7
63 Eu 2.1 49 2
64 Gd 6.1 41 7
65 Tb 1.2 56 1
66 Dy 4.5 42 7
67 Ho 1.4 55 1
68 Er 3.5 43 6
69 Tm 0.5 61 1
70 Yb 3.1 44 7
71 Lu 0.8 59 2
According to this rule , the elements with even atomic numbers are more abundant and have more stable isotopes , than those with odd atomic numbers . The graphical representation of their abundance is given in fig 1 .
Fig. 1: Abundance of Lanthanoids in Earth’s crust
The non - existence of promethium in nature may be explained by Mattauch′s rule . This states that if each of the two elements with consecutive atomic numbers have an isotope of the same atomic mass , one of the isotopes will be unstable . Since Nd (Z = 60) has stable isotopes with mass numbers 142 , 143 , 144 , 145 , 146 , 148 , 150 and 152 and Sm (Z = 62 ) has the isotopes with mass numbers 144 , 147 , 148 , 149 , 150 , 152 and 154 , there are not many stable mass numbers available for promethium (Z = 61) . If Pm is to have a stable isotope , it must have a mass number outside the range 142 – 150 . The isotopes of Pm which have been identified so far are radioactive .
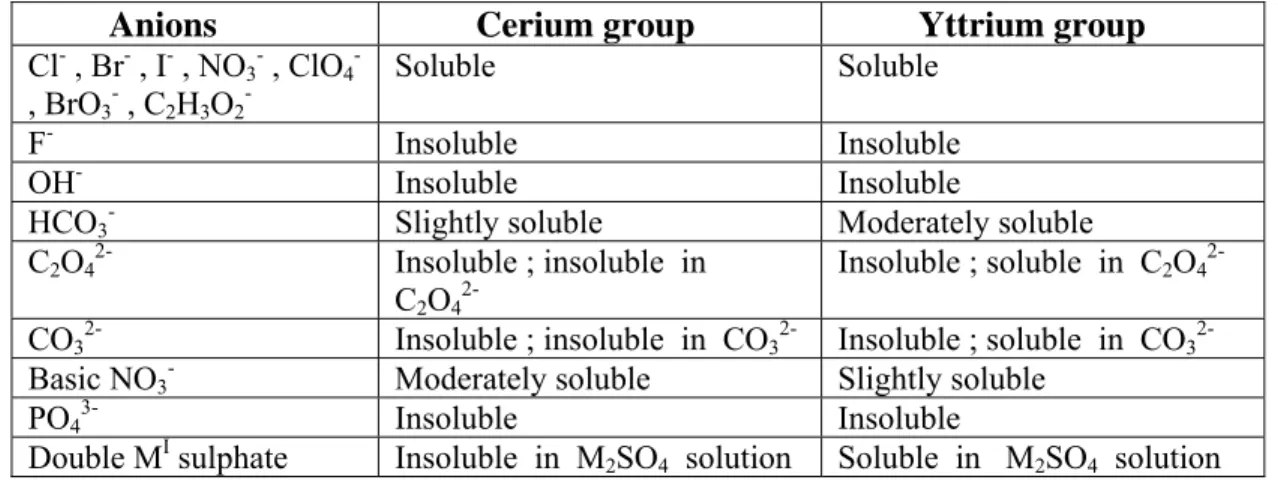
Many minerals are known to contain lanthanoids (Table 4) . The symbols Ce and Υ in the table represent the elements in cerium group and yttrium group , respectively . Out of the list of the mentioned minerals , only two, namely, monazite and bastnasite are of commercial importance . Monazite is sparsely distributed in various rocks but , due to its high density and inertness , it is concentrated by weathering into sand or beaches , usually in presence of other similar minerals such as cassiterite (SnO2) . Their rich deposits occur in Travancore , South Africa , Brazil , Malaysia , the U.S.A and Australia . In fact , before 1960 , monazite was the only source of lanthanoids . However , a vast deposit of bastnaesite was explored in the Mountain Pass , California has since then become the most important single source of lanthanoids . Apart from the U. S. A. it is also found in Madagascar .
Table 4: Important Minerals of Lanthanoids
Minerals Composition Location of significant deposits
(1) Cerium group minerals (i) Monazite Sand- Mixture of ortho-
phosphates of Ce-earths, (Ce)PO4
50-70% Ce-earths(i.e.
elements of at. no. 57 to 62 calculated as oxides) 1-4% Y-earths (i.e.
elements of at. no. 63 to 71 calculated as oxides) 5-10% ThO2
1-2% SiO2
22-30% P2O5
Traces of U
Occurs in the sand beaches of Travancore(India)
Brazil South Africa U.S.A.
(ii) Bastnaesite-cerium earth fluoro- carbonate,(Ce)FCO3
65-70% Ce-earths ,
< 1% Y-earths Sweden, California, New Maxico (iii) Cerite-A hydrated silicate of the
composition,
(Ce)3 MII H3Si3O11(M-Ca,Fe)
Traces of thorium 51-72% Ce-earths 7.6% Y-earths Traces of Th, U, Zr
Sweden Caucausus (2)Yttrium group minerals
(i) Gadolinite or Ytterbite- A ytterium- earth , iron and beryllium silicate, (Fe, Be)3 (Y2) Si2 O10
35-48% Y-earths (Calculated as oxides) 2-17% Ce-earths Upto 11.6% BeO Traces of ThO2
Sweden, Norway
USA(Texas and Colorado)
(ii) Xenotime –An orthophosphate of Y- earth(analogous to Monozite), (Y), PO4
54-65% Y-earths
∼ 0.1% Ce-earths Upto 3% ThO2, upto 3.5%U3O3 2-3% ZrO2
Norway Brazil
(iii) Euxenite- Mixture of titanates, niobates and tentalates of Y-earths, (Y) (Nb, Ta) TiO6. XH2O
13-35% Y-earths (Calculated as Oxides) 2-8% Ce-earths (Calculated as Oxides) 20-23% TiO2, 25-35%
(Nb, Ta)2O5
Australia, Idaho(U.S.A.)
At present , China is estimated to have the world’s largest deposits of lanthanides (43%) and is now the largest producer of these elements . India contributes only 3%
towards the production of lanthanoids. Promethium is not available from rare earth ores. It occurs only in traces in uranium ores where it is formed by spontaneous fission of 238U . It was first isolated as 147Pm by exchange methods from products of nuclear fission reaction .
Extraction of Lanthanoids from Minerals
The distribution of the lanthanoids in the two commercially important minerals , monazite and bastnaesite , is quite similar . Both these minerals contain metals such as Ce , La , Nd and Pr . However , monazite typically contains 5 – 10% ThO2 and 3%
yttrium earths , which are almost absent in bastnaesite . The complex composition makes the chemical treatment of monazite very extensive and lengthy . Moreover , though thorium is only weakly radioactive , it is contaminated with daughter elements such as
228Ra which are more active and hence require careful handling during the processing of monazite .
Processing includes cracking the minerals , recovering the lanthanoids (along with thorium) , removing thorium if present and separating the lanthanoids . The concentration of the mineral usually begins with gravity separation on Wilfley table. Since these minerals are heavy , their sand gets caught up on the riffles and the gangue material is washed off and dried . The magnetic impurities are removed by magnetic separation.
The concentrated mineral is then subjected to chemical treatment , which is technically known as “opening up” or “cracking” . These treatments depend on the ore being used and the extent to which the metals are to be separated from each other . The chemical treatment of monazite is done either by NaOH or concentrated H2SO4 solution . The principle underlying the cracking is the difference in solubilities of Ln2 (SO4)3 . Na2 SO4 . x H2O for the light and the heavy lanthanoids and also the low solubility of the hydrous oxide of thorium.
(i) Cracking of monazite by conc. H2SO4 – The finely powdered and concentrated ore is treated with 93% H2SO4 at 2000 C for several hours . The reaction is exothermic and the resulting viscous paste is leached with cold water . Th , La and the lanthanoids dissolve as sulphates , leaving behind the insoluble residues which mainly contain radioactive 228Ra . The solution of sulphates of Th , La and Ln , on partial neutralization with NH4OH , precipitates out ThO2 . The remaining solution is then treated with Na2SO4 , to salt out La and the light lanthanoids as sulphate , leaving the heavy lanthanoids in solution . This scheme is summarized in fig.2 (a). The solution containing the sulphates of heavy lanthanoids can then be used to separate the individual components by various methods , such as valency change, ion exchange and solvent extraction , discussed later in the unit .
(ii) Cracking of monazite by NaOH - The finely powdered and concentrated ore is treated with 65% NaOH solution at 1400C , followed by extraction with water . A slurry of impure hydrous oxides is obtained , which is treated with boiling aq. HCl until the pH is 3.5 . Crude ThO2separates out leaving behind a solution of impure lanthanoid
chlorides. The solution is then treated with a solution of BaCl2 and Ln2 (SO4)3 in stoichiometric amounts. BaSO4 precipitates out, along with radioactive 228Ra as RaSO4 . This scheme is summarized in fig.2 (b). The remaining solution containing Ln and lanthanoid chlorides can be used to separate the individual components by special techniques.
Symposium on Symposium on Symposium on “ Emerging Areas In Chemistry”
Dec. 1 – 3 , 2005 Hindu College University of Delhi
Symposium on Symposium on
Fig. 2: Cracking of Monazite / xenotime by (a)conc. H2 SO 4; (b) NaOH
Out of the two cracking methods, the conc. H2SO4 treatment is more economical than the NaOH process. However, the latter process gives higher yields and a cleaner
separation .
(iii) Cracking of bastnaesite - The bastnaesite mineral contains very small amounts of Th and heavy lanthanoids and requires a comparatively simpler treatment . The concentrated ore is treated with conc. H2SO4 at 2000 C , when CO2 , HF and SiF4 are evolved . The dried product is then treated with water to obtain a solution containing lanthanum and the light lanthanoids , which are then separated out via special techniques discussed later in the unit . A schematic representation of the procedure followed is given in fig 3.
Fig.3: Cracking of Bastnaesite Electronic Structure
The electronic structure of the members of group 3 , in the modern periodic table , indicates that the elements usually listed in this family are the first members of the four d – type transition series .
Sc , Z = 21 1s2 2s2 2p6 3s2 3p6 3d1 4s2 Or , [Ar] 3d1 4s2
Y , Z = 39 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d1 5s2 Or , [Kr] 4d1 5s2
La , Z = 57 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d1 6s2 Or , [Xe] 5d1 6s2
Ac , Z = 89 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d106s2 6p6 6d1 7s2 Or , [Rn] 6d1 7s2
In elements succeeding scandium and yttrium , the electrons are added to the 3d and 4d levels respectively , giving the first and the second transition series . However , after lanthanum , the energy of the 4f level falls below that of the 5d and thus the electrons are added to the inner , well - shielded 4f orbitals before entering into the 5d subshell . Hence, the lanthanoid series is defined by the progressive filling of the 4f
orbitals , namely , z3 , xz2, yz2 , xyz , z ( x2 - y2 ) , x ( x2 - 3y2 ) and y ( 3x2 - y2 ) . The general set f orbitals are illustrated in fig. 4. Since there are seven such orbitals , each with a capacity of two electrons , a total of fourteen elements of this f - type series may result before the 5d orbitals starts filling up again . This accounts for the elements cerium through lutetium (Z = 58 to 71).
Fig.4: Shape of seven f orbitals
The electronic configuration is established on the basis of the emission spectra of the element under consideration . If the spectrum is simple , containing only a few lines , its interpretation becomes easier and the correct ground state configuration can be established for the atom in question . However , the emission spectra for many lanthanoids is highly complex , making the establishment of an absolutely correct configuration extremely difficult . The difficulty arises due to the fact that the 5d and 4f orbitals have comparable energy , so that the distinction between the two is not easy . The configuration of the lanthanoids is summarized in table 5.
Table 5: Ground state electronic configuration of Lanthanoids Element Atomic
number (z)
Electronic configuration Idealized observed La 57 [Xe]5d16s2 [Xe]5d16s2 Ce 58 [Xe]4f15d16s2 [Xe]4f15d16s2 Pr 59 [Xe]4f25d16s2 [Xe]4f3 6s2 Nd 60 [Xe]4f35d16s2 [Xe]4f4 6s2 Pm 61 [Xe]4f45d16s2 [Xe]4f5 6s2 Sm 62 [Xe]4f55d16s2 [Xe]4f6 6s2 Eu 63 [Xe]4f65d16s2 [Xe]4f7 6s2 Gd 64 [Xe]4f75d16s2 [Xe]4f75d16s2 Tb 65 [Xe]4f85d16s2 [Xe]4f9 6s2 or
[Xe]4f85d16s2 Dy 66 [Xe]4f95d16s2 [Xe]4f10 6s2 Ho 67 [Xe]4f105d16s2 [Xe]4f11 6s2 Er 68 [Xe]4f115d16s2 [Xe]4f12 6s2 Tm 69 [Xe]4f125d16s2 [Xe]4f13 6s2 Yb 70 [Xe]4f135d16s2 [Xe]4f14 6s2 Lu 71 [Xe]4f145d16s2 [Xe]4f145d16s2
It clearly indicates that the 4f orbitals are not occupied regularly , mainly in case of Ce , Gd and Lu . Hence , the general electronic configuration for lanthanoids is [Xe] 4f1-
145d06s2 , with the exception of Ce , Gd and Lu . In cerium , even the sudden contraction and reduction in energy of the 4f orbitals immediately after La , is not yet sufficient to avoid occupancy of the 5d orbital . Gd has a 5d1 arrangement , leaving a half filled 4f , which leads to increased stability . Lu has a 5d1 arrangement , as the f shell is already full .
On the basis of the similarity in the outer electronic configuration , scandium , yttrium and actinium should be placed along with the lanthanoids . However , due to the physical limitations of the modern periodic table , the lanthanoids and actinoids are placed separately from the main body of the periodic table . Neither scandium nor yttrium as well as lanthanum can be called properly as lanthanoides , since the 4f orbital does not have any electron . Property wise , yttrium and lanthanum are better discussed with the lanthanoids than with any other elements . On the other hand , scandium is markebly different , even though it was first isolated from yttria sources .
Atomic and Ionic Radii : Lanthanoid Contraction
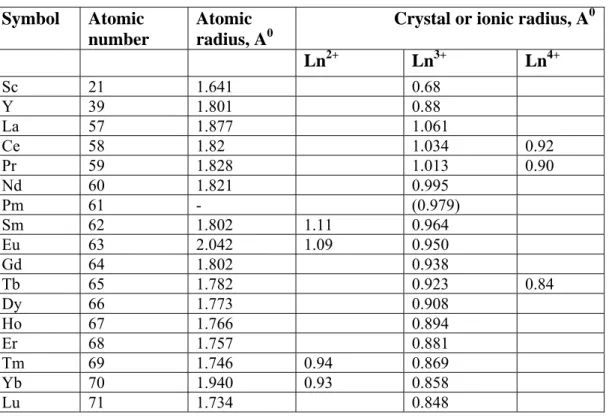
In the periodic table , the atomic as well as ionic radii normally increase on descending down a group , due to the inclusion of extra filled shells of electrons . However , on moving from left to right across a period , the atomic and ionic radii decrease . This is due to the fact that the extra orbital electrons are not able to shield the extra nuclear charge completely and hence the increase in the effective nuclear charge is responsible for the decrease in size . The atomic and ionic radii of lanthanoids are given in Table 6.
Table 6 : Size relationship Symbol Atomic
number
Atomic radius, A0
Crystal or ionic radius, A0
Ln2+ Ln3+ Ln4+
Sc 21 1.641 0.68
Y 39 1.801 0.88
La 57 1.877 1.061
Ce 58 1.82 1.034 0.92
Pr 59 1.828 1.013 0.90
Nd 60 1.821 0.995
Pm 61 - (0.979)
Sm 62 1.802 1.11 0.964
Eu 63 2.042 1.09 0.950
Gd 64 1.802 0.938
Tb 65 1.782 0.923 0.84
Dy 66 1.773 0.908
Ho 67 1.766 0.894
Er 68 1.757 0.881
Tm 69 1.746 0.94 0.869
Yb 70 1.940 0.93 0.858
Lu 71 1.734 0.848
The table clearly shows that on moving from Sc to Y to La , there is a steady increase in the atomic as well as the ionic radii . This is due to the fact that addition of electrons to higher energy levels overcome the increasing contractive effects resulting from the enhanced attraction produced by larger nuclear charge. However, as we move along the lanthanoid series , there is a decrease in atomic as well as ionic radii . A similar but more limited trend characterizes the non - tripositive ions. The contraction in size from one element to another is fairly small , so that the additive effect over the lanthanoid elements from Ce to Lu is just 0.2 A0 . This limited contraction in the
atomic and ionic radii of the lanthanides is known as Lanthanoid Contraction.
The shielding effect of the electrons decreases in the order s > p > d > f . In lanthanoids the additional electron enters 4f sub - shell and not the valence shell , namely , sixth shell. The “Lanthanoid Contraction” , therefore , occurs because , although each increase in nuclear charge is balanced by a simultaneous increase in electronic charge , the directional characteristics of the 4f orbitals cause the 4fn electrons to shield themselves and other electrons from the nuclear charge only imperfectly . Thus each unit increase in nuclear charge produces a net increase in attraction for the whole extranuclear electron charge cloud and each ion shrinks slightly in comparison with its predecessor . On the other hand , although a similar overall reduction is seen in the atomic radii , the trend for Eu and Yb is spectacularly irregular . Mathematically , lanthanoid contraction could be understood in terms of effective electron potential , Veff , which is expressed as :
Veff = r Zeff
− + 2 2
) 1 (
r l l +
Or, Veff = columbic potential + centrifugal potential
The angular momentum for an f orbital (l = 3) is large and hence the centrifugal potential , which tends to keep the electrons away from the nucleus , is also large . Increase in the atomic number increases the columbic attraction to a large extent for a smaller value of n , due to a proportionately greater change in Zeff . This can be viewed empirically , to differing penetration effects , 4f orbitals (and the atoms in general) steadily contract across the lanthanoid series . Fig. 5 shows that the graph has two peaks , one at Eu and the other at Yb , which possess exceptionally high values for their atomic radii. This is regarded to be due to the difference in their metallic bonding . Most of these metals are composed of a lattice of Ln3+ ions with a 4fn configuration and three electrons in the 5d / 6s conduction band . Metallic Eu and Yb , however , are composed predominantly of larger Ln2+ ions with 4fn+1 configurations and only two electrons in the conduction band , thus leaving behind half - filled and completely filled 4f orbitals , respectively . The rough parallel between these metals and barium supports this contention. Correspondingly , the slightly reduced atomic radius of cerium is due to the presence of ions in an oxidation state somewhat above +3 . Similar discontinuities are found in other properties of the metals , particularly at Eu and Yb . The decrease in the crystal or ionic radii is indicated in Fig 6 . The general decrease in crystal radius from Ln2+ to Ln3+ to Ln4+ reflects the effect of increasing cationic charge.
Fig.5: Atomic radii of lanthanoides, barium and Hafnium
Fig.6: Crystal radii of Ln2+ , Ln3+ and Ln4+ ions
It is to be noted that a contraction resulting from the filling of the 4f electron shell is of course not exceptional . Similar contraction occurs in each row of the periodic table.
In the d – block for instance , the ionic radii decrease by 20.5 pm from Sc3+ to Cu3+ ,
and by 15 pm from Y3+ to Ag3+ . However, the importance of the lanthanide contraction arises from its consequences:-
1] Basic character of oxides , Ln2O3 and hydroxides , Ln(OH)3 : The atomic and ionic radii affect those properties of the metal and their respective cations , which mainly reflect the attraction or lack of attraction for the electrons . This includes basicity . In broad terms , basicity is a measure of the ease with which a species loses electrons and it decreases with decrease in the atomic or ionic radii . In terms of ionic radii , basicity may be expected to decrease in the order :
La3+ > Ce3+ > Pr3+ > Nd3+ > Pm3+ > Sm3+ > Eu3+ > Gd3+ >Tb3+ > Dy3+ > Ho3+ > Y3+ >Er3+ >Tm3+
> Yb3+ >Cu3+ > Sc3+
Amongst the tripositive ions , due to the decrease in size from La3+ to Lu3+, the covalent character in Ln(III) hydroxides increases . This is in accordance with Fajan’s rule . Hence , the basicity of the hydroxides decreases from La to Lu . Thus La(OH)3 is the most basic while Lu(OH)3 is the least basic hydroxide . The hydroxide of more highly charged Ce4+ is even more basic than any tripositive ion . Basicity differences are reflected in the hydrolysis of ions – more basic ion hydrolyses less readily ; solubilities of salts ; thermal decomposition of oxy - salts – more basic oxy - salts decompose with difficulty ; and the formation of complex species .
2] Occurrence of yttrium with heavy lanthanoids :The magnitude of lanthanoid contraction is such that the ionic radii of Y3+ (0.88 A0) is reached in the holmium - erbium region ( 0.894 - 0.881A0 ) . This similarity in ionic radii , along with the equality in ionic charge (i.e. +3) accounts for the invariable natural occurrence of yttrium with these heavier lanthanoids . The marked similarity in the crystal structure , chemical properties and solubility between yttrium compounds and those of the heavier lanthanoids make it difficult to separate yttrium from heavy lanthanoids . In fact , the behaviour of yttrium is considered to be so characteristic of these elements that these elements are referred to as yttrium earths . It is to be noted that the marked differences observed between the chemistry of scandium and that of the lanthanoids are consistent with the fact that Sc3+ is much smaller than even the Lu3+ ion .
3] Melting and boiling point : The hardness and melting and boiling points of the elements increase from Ce to Lu . This is because of the increase in attraction between the atoms , with decrease in size .
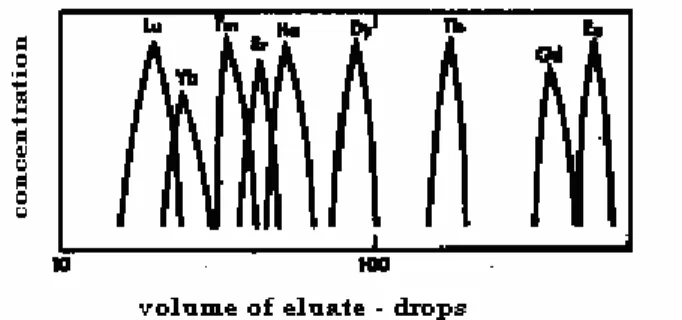
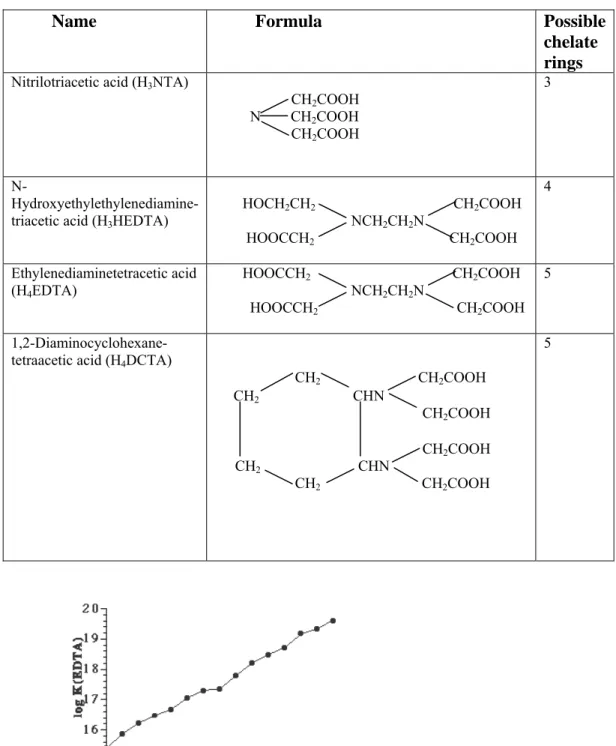
4] Separation of lanthanoids : The chemical properties of an ion are mainly dependent on its size and charge . For the various Ln3+ ions , since the charge remains the same and the decrease in size is just marginal , their chemical properties are very similar , making their separation difficult . But , it is this small variation in properties that permits the separation of the lanthanoids by fractional means .
5] Effect on the post - lanthanoid elements : The elements which follow lanthanoids in the third transition series of the periodic table , are called post - lanthanoid elements . Due to the lanthanoid contraction , the post - lanthanoid elements are considerably smaller than expected .
Normally , the covalent radii increase with increase in the atomic number , on moving down the group . This , in fact , holds good when we compare the covalent radii values of the elements of the first , second and the third transition series (Table 7 ) . Table 7 : Covalent radii of the transition elements (Αο)
Sc 1.61
Ti 1.32
V 1.22
Cr 1.17
Mn 1.17
Fe 1.17
Co 1.16
Ni 1.15 Y
1.80 Zr 1.45
Nb 1.34
Mo 1.29
Tc -
Ru 1.24
Rh 1.25
Pd 1.28 La
1.87 Hf 1.44
Ta 1.34
W 1.30
Re 1.28
Os 1.26
Ir 1.26
Pt 1.29
On comparing the covalent radii values of the elements of the second transition series with those of the third series , it is observed that the normal increase in the covalent radii values from Y to La disappears after lanthanoids . Thus pairs of elements such as Zr / Hf , Nb / Ta and Mo / W are almost identical in size . The close similarity of properties in such a pair makes their chemical separation difficult . The sizes of the third row of the transition elements are very similar to those of the second row of transition elements . Thus , the second and the third rows of transition elements resemble each other more closely than do the first and second rows . These similarities between members of the second and third transition series continue through the platinum metals to at least silver and gold . As a consequence of the contraction in the atomic radii of the elements of the third transition series , the packing of atoms in their metallic crystals become so much compact that their densities become very high . Thus , while the densities of the elements of second transition series are only slightly higher than those of the elements of first series , the densities of the elements of third transition series are almost double than those of the elements of the second transition series . Hence , the increase in atomic mass from 91.2 to 178.4 g/mol and almost similar radii leads to an increase in density from 6.51 to 13.35 g/cm3 , on moving from Zr to Hf . Indeed , due to similar chemical behaviour , Hf (1923) was discovered 134 years later than Zr(1789) .
Oxidation States
The ionization enthalpy and standard electrode potential values for the lanthanoids are listed in table 8 . The sum of the first three ionization enthalpy values is low.
Thus , the oxidation state ( + 3 ) is ionic and the stability of Ln3+ dominates the chemistry of these elements . In just the same way as for other elements , the higher oxidation states occur in the fluorides and oxides and the lower oxidation states occur in the other halides , mainly bromides and iodides . Oxidation numbers (+2 ) and (+4) do occur , particularly when they lead to a noble gas configuration (f0 in Ce4+) , or a half - filled f shell (f7 in Eu2+ and Tb4+) , or a completely filled f shell (f14 in Yb2+) . In addition , (+2 ) and (+4) states also exist for elements that are close to these configurations . Thus , Sm2+ and Tm2+ occur with f6 and f13 configurations , while Pr4+
and Nd4+ have f1 and f2 configurations , respectively (table 9 ) . However , of the non - tripositive state , only tetrapositive cerium , praseodymium , and terbium and dipositive samarium , europium and ytterbium have sufficient chemical stability .
Table 8 : Some properties of the lanthanoid elements Element Ionization
enthalpy / kJmol-1
E0(M4+/M3+) /V
E0(M3+/M2+) /V
E0(M3+/M) /V
∆atmH /kJmol-
1
∆hydH /kJmol-
1
1st 2nd
3rd
Ce 541 1047 1940 1.61 - -2.483 419 -3370
Pr 522 1018 2090 ∼2.860 - -2.462 356 -3413
Nd 530 1034 2128 - - -2.431 328 -3442
Pm 536 1052 2140 - - -2.423 301 -3478
Sm 542 1068 2285 - -1.000 -2.414 207 -3515
Eu 547 1085 2425 - -0.360 -2.407 178 -3547
Gd 595 1172 1999 - - -2.397 398 -3571
Tb 569 1112 2122 2.7 - -2.391 389 -3605
Dy 567 1126 2230 - - -2.353 291 -3637
Ho 574 1139 2221 - - -2.319 301 -3667
Er 581 1151 2207 - - -2.296 317 -3691
Tm 589 1163 2305 - - -2.278 232 -3717
Yb 603 1175 2408 - -1.205 -2.267 152 -3739
Lu 513 1341 2054 - - -2.255 - -3760
Table 9 : Distinguishing electronic configuration for observed oxidation states
Symbol
Configuration
+2 +3
+4
La [Xe]4f0(La3+)
Ce [Xe]4f2(CeCl2) [Xe]4f1(Ce3+) [Xe]4f0(Ce4+)
Pr [Xe]4f2(Pr3+) [Xe]4f1(PrO2 ,
Na2PrF6)
Nd [Xe]4f4(NdI2) [Xe]4f3(Nd3+) [Xe]4f2(Cs3NdF7)
Pm [Xe]4f4(Pm3+)
Sm [Xe]4f6(Sm2+) [Xe]4f5(Sm3+)
Eu [Xe]4f7(Eu2+) [Xe]4f6(Eu3+)
Gd [Xe]4f7(Gd3+)
Tb [Xe]4f8(Tb3+) [Xe]4f7(TbO2 ,
TbF4)
Dy [Xe]4f9(Dy3+) [Xe]4f8(Cs3DyF7)
Ho [Xe]4f10(Ho3+)
Er [Xe]4f11(Er3+)
Tm [Xe]4f13(TmI2) [Xe]4f12(Tm3+) Yb [Xe]4f14 (Yb2+) [Xe]4f13(Yb3+)
Lu [Xe]4f14(Lu3+)
The direct correlation between oxidation state and electronic configuration is the exception rather than the rule . Since , 6s2 configuration is the most characteristic configuration for the lanthanoids, a uniform dipositive state might be expected . However, the dominance of the +3 oxidation state results from the stabilizing effects of increasing positive charge on different orbitals . When electrons are removed from a lanthanoid atom , the orbitals are stabilized in the order 4f > 5d > 6s . This is the same order in which the orbitals penetrate through the inert core of electrons towards the nucleus . By the time an ionic charge of +3 is reached , the 4f have become core - like , with any remaining electrons occupying the 4f , leaving 6s and 5d orbitals empty . Removal of further electrons from the core then becomes energetically unfavourable. This explanation can further be expanded by examining the relative stabilities of other oxidation states from a thermodynamic view point with the help of fig. 7 , 8 , and 9 .
Fig.7: Plot of I1 , I2 , I3 , I4 and ∆Hatom for lanthanoids
Fig. 8(a): Plot of [ I1 + I2 + I3 + ∆atomH] Fig. 8(b): Plot of (b) [ ∆hydH(Ln3+)]
Fig. 8(c): Plot of (c) [I1 + I2 + I3 +∆atomH + ∆hydH(Ln3+)] Fig. 9 : Plot of [ I1 + I2 + I3 + I4 + ∆atomH]
Stability of +2 oxidation state - a so called “ anomalous” state
The stability of the +2 oxidation state can be studied by examining ∆rG for a process such as :
Ln2+ (aq) + H + (aq) Ln3+ (aq) + ½ H2 (g) A thermodynamic cycle for this reaction can be constructed as:-
Ln2+ (aq) + H+ (aq) ∆rH Ln3+(aq) + 1/2 H2(g)
+∆hydH(Ln2+) -∆hydH(Ln3+)
Ln2+(g) + H+(aq) Ln3+(g) + 1/2 H2(g) I3 + (∆hydH(H+))
According to the Hess’s law of constant heat summation :
∆rH = ∆hydH(Ln2+) + I3 + ∆hydH(H+) + (-∆hyd H(Ln3+))
also , ∆rG = ∆rH - T∆rS
Hence , ∆rG = [ ∆hydH(Ln2+) + I3 + ∆hydH(H+) + (-∆hyd H(Ln3+) )] - T∆rS
∆hydH(Ln2+) , ∆hydH(H+) and (-∆hyd H(Ln3+)) represent the enthalpy of hydration for Ln2+ , H+ and Ln3+ , respectively . I3 is the third ionization enthalpy for the lanthanoid ,. ∆rH is the enthalpy of reaction , T is the temperature , ∆rG is the Gibb’s free energy and
∆rS is the entropy change for the reaction . Here , ∆hydH(H+) remains constant and can be ignored , as variation in ∆rG across the Ln series is sought . Due to the lack of data , it is assumed that ∆rS is constant across the series – as for the 3d metals . Hence,
∆rG = I3 + [ ∆hyd H(Ln2+) + (-∆hyd H(Ln3+) )] + constant
Since the ionic radii moves smoothly along the series, ∆hydH(Ln2+) and ∆hydH(Ln3+) are also expected to vary regularly . ∆hydH(Ln3+) is larger in magnitude than
∆hydH(Ln2+) due to the higher charge and smaller radius of the ion with higher oxidation state . The term in square brackets is thus negative and favours the +3 oxidation state. As the ions gets smaller across the series , this term smoothly increases so that the +3 state becomes more favoured . On the contrary , I3 increases across the series , disfavouring the existence of +3 oxidation state . However , the dominance of the +3 oxidation state arises from the balance between the increasing enthalpy of hydration and the increasing ionization enthalpy . The occurrence of the +2 state for some of the lanthanoids arises from the non - smooth changes in the third ionization enthalpy values .
The overall trend across the series, in general, is for the increase in I3 values. This is due to the increase in nuclear charge across the f – block. This outweighs the increasing repulsion between the electrons. However , the ‘break’ between f7(Eu2+ ) and f8(Gd2+) cannot be explained easily . This break is actually due to the extra stability associated with the f7 (half – filled) configuration. Similarly the higher than expected I3 for Yb2+ with f14 (fully - filled) configuration can also be explained on the same basis . This can be explained even on the basis of the Hund’s rule , according to which the electrons prefer to adopt arrangements in degenerate orbitals so as to have maximum number of parallel spins . Destroying pairs of such parallel spins requires extra energy. In Gd2+(f8) , there are seven electrons with parallel spin and one with anti - parallel spin . A pair of electrons with anti - parallel spins repels more than ‘expected ’ and so is destabilized , leading to a low I3 , despite the increase in nuclear charge . A similar decrease in I3 is observed on moving from Yb2+ to Lu2+. To understand small irregularities in the third ionization energy values, a detailed inspection of the electronic configuration is required. For example , for Pr2+ (4f3 ) and Pm2+(4f5 ) , Hund’s
ule predicts the lowest energy by way of arranging the electrons as : r
ml 3 2 1 0 -1 -2 -3
All the three electrons have ml values of the same sign and can thus be taken to be orbiting in the same direction . There is slightly less repulsion between electrons which are orbiting in the same direction as they come across one another less frequently . The electrons are thus repelling one another slightly less than expected and hence ionization enthalpy is higher than expected. Now consider Pm2+(4f5) , for which the lowest energy arrangement is :
ml 3 2 1 0 -1 -2 -3
The electron that is removed upon ionization is that with ml = -1 . As this is orbiting in the opposite direction to the other electrons, it is repelled slightly more than ‘expected’ and its ionization becomes easier. Thus, the ionization enthalpy of Pm2 + is less than expected. This is known as quarter - shell effect. An identical situation occurs between f10 and f12 in the second half of the series leading to the three quarter - shell effect. The I3 values of Nd and Dy are thus larger than expected, leading to the occurrence of their compounds in the +2 oxidation state.
Amongst the various lanthanoids in dipositive state, samarium, europium, and ytterbium have sufficient chemical stability to be of importance to exist in aqueous solution and to form series of compounds. Standard electrode potential values indicate that in aqueous solution, these ions are all strong reducing agents, with reducing strength decreasing as :
Sm2+ > > Yb2+ > > Eu2+
Stability of +3 oxidation state – the “characteristic” state
The observed oxidation states of the lanthanoids noted either in solution or in insoluble compounds (Table 9) , clearly indicates that whatever is the electronic configuration of the lanthanoids in the ground state , they all form the tri - positive ions . This can be examined more closely by considering the following thermodynamic cycle :
Ln (s) ∆H0 Ln3+ (aq)
∆atmH - ∆hyd H(Ln3+)
Ln (g) I1 + I2 + I3 Ln3+ (g)
According to the Hess’ s law of constant heat summation :
∆H0 = ∆atmH + I 1 + I2 + I3 + (-∆hyd H(Ln3+))
where ∆atomH is the enthalpy of atomization of the lanthanide ; I1, I2 and I3 are the first, second and third ionization enthalpies respectively and ∆hydH(Ln3+) is the enthalpy of hydration for Ln3+(g) . The standard electrode potential, E0(Ln3+
(aq) / Ln (s) ) can be calculated using a thermodynamic relation
∆rG0 = ∆H0 - T∆rS0 = - n FE0
The value of standard electrode potential of a lanthanoid thus depends on the balance between the energy supplied in the form of enthalpy of atomization and the sum of the first three ionization enthalpies [ ∆atm H + I1 + I2 + I3 ] , and the energy released in the form of enthalpy of hydration for Ln3+(g) . These enthalpy terms are graphed in Fig 8. The production of Ln3+ (g) shows a smooth trend, based on size effects. Shell structure effects superimposed with clear maxima at half shell (f 7 ) and full shell (f 14) are also reflected . The enthalpy of hydration of Ln3+(g) shows only a smooth ionic size based trend and no shell structure effects . Balance of trends in ionization enthalpy along with enthalpy of atomization and hydration reflects the dominance of the shell effects over the size effects.
It can also be shown that the tri-positive state is a more preferred state in comparison to +2 and +4 state. In other words, in aqueous solution, oxidation represented by the equation:
Ln3+(aq) +
4
1 O2 (g) + H+ (aq) Ln4+ (aq) +
2
1 H2O (l)
or reduction represented by the equation :
Ln3+ (aq) +
2
1 H2 (g) Ln2+ (aq) + H+ (aq)
are not very favourable . The conversion from one oxidation state to another in aqueous solution is controlled by the magnitudes of the energy required to remove an electron from the gaseous ion in its lower oxidation state (i.e. , ionization