I acknowledge the Indian Institute of Technology, Guwahati for providing institute scholarships for the entire period of the Ph.D. The fifth chapter is devoted to the synthesis and application of chiral backbone polymers with optically active (1R,2R)-1,2-diamines for the asymmetric synthesis.
Copper(I) Oxide Nanoparticles Catalyzed N-Arylation of Amides and Imidazoles
Palladium Catalysts
Copper Catalysts
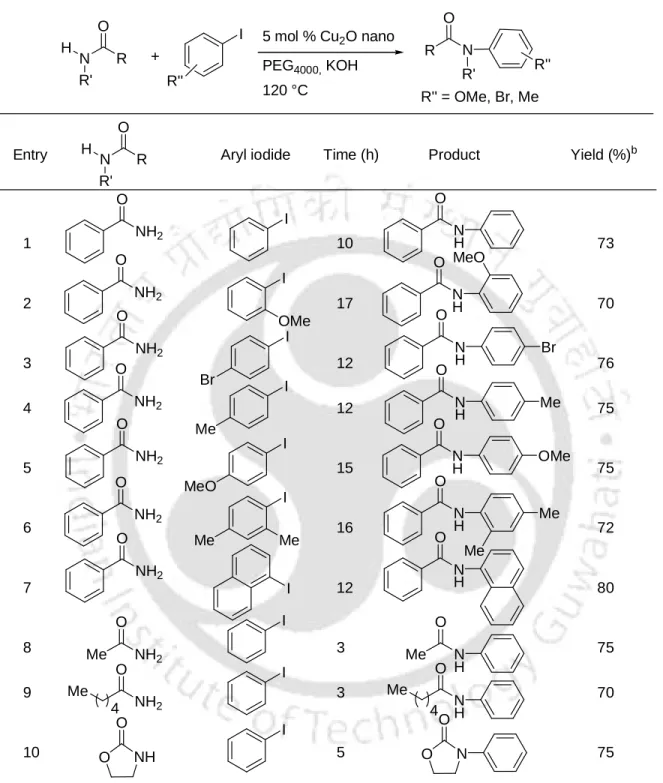
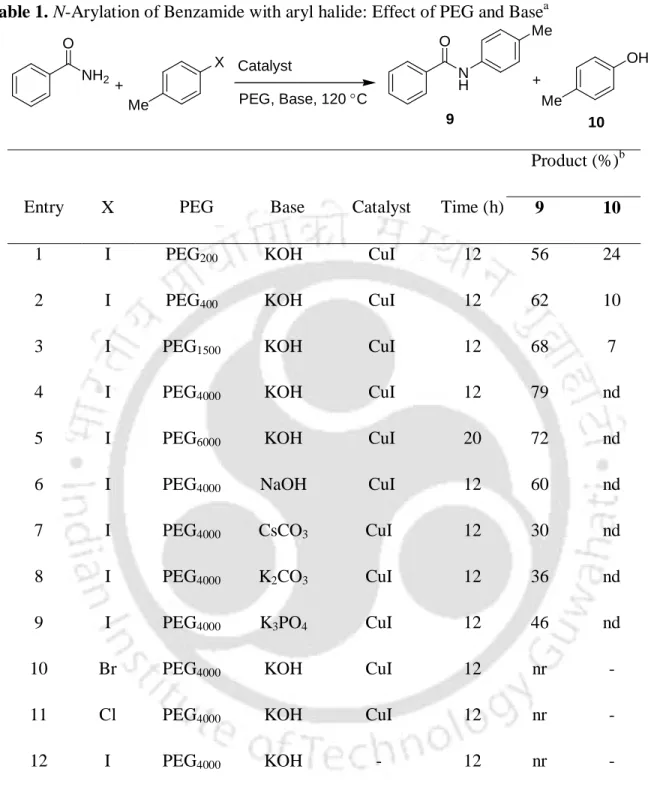
The reaction has occurred efficiently to provide the cross-linked products at 100 °C in the presence of K3PO4 in dioxane (Scheme 6).6a. Recently, we have reported CuO nanoparticles catalyzed N-arylation of amides and imidazoles with aryl iodides at 110 °C in the presence of KOH (Scheme 9).7b–c The procedure is simple, general, ligand-free and efficient to give cross-linked products in high yield.
Present Study
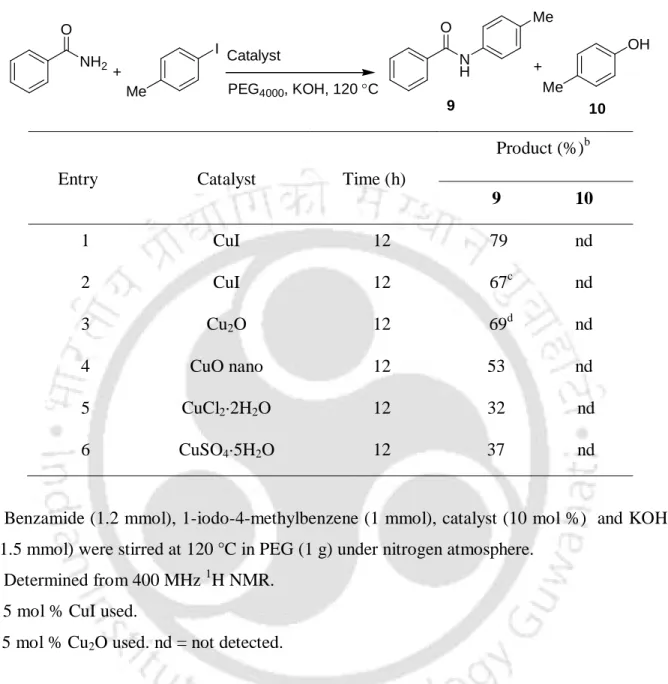
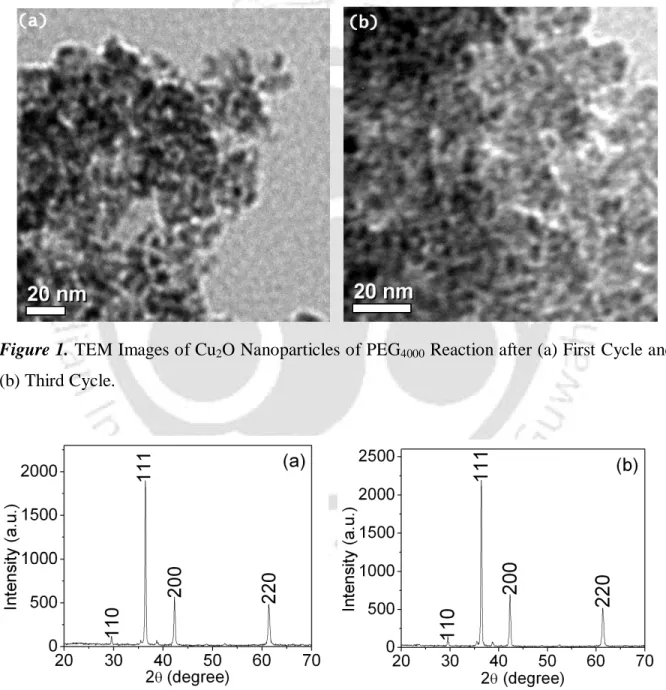
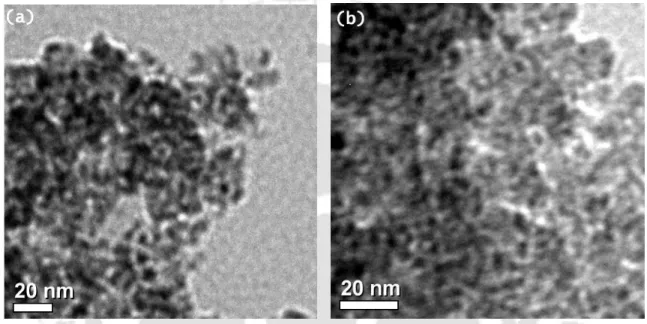
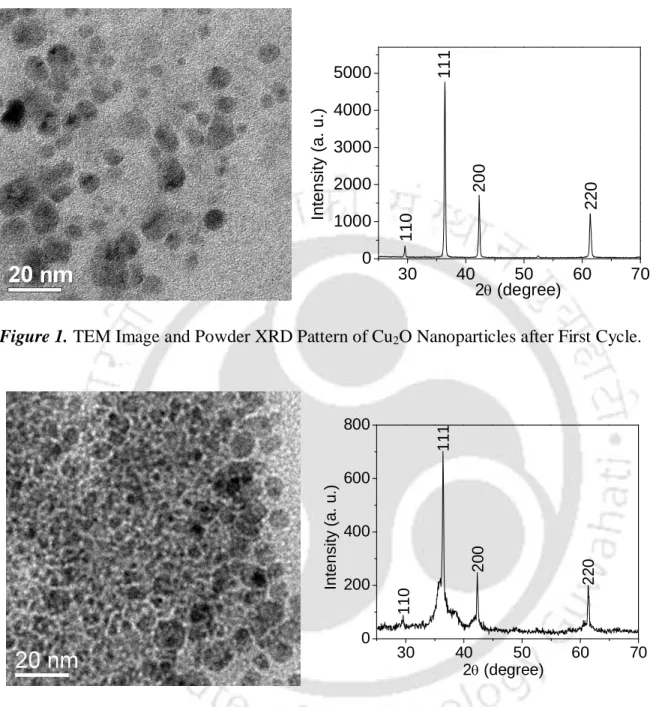
The 7-10 nm sizes of the Cu2O nanoparticles were determined by transmission electron microscopy (TEM) (Figure 1) and their identities were determined using X-ray powder diffraction analysis (Figure 2). After completion of the reaction of benzamide with 1-iodo-4-methylbenzene, the reaction material was treated with ethyl acetate (10 mL) and water (5 mL).
Copper(I) Oxide Nanoparticles Catalyzed S-Arylation of Thiols
Palladium Catalysts
Subsequently, the coupling of thiols with aryl chlorides and bromides is studied using palladium(II) complex 1 in the presence of NaOt-Bu (Scheme 3).3c These reaction conditions are also suitable for the reactions of aryl halides with amines and alkenes. Hartwig and colleagues showed the coupling of thiols with aryl halides using Pd(OAc)2.
Copper Catalysts
Nickel Catalysts
Cobalt Catalyst
Present Study
The sizes 7-10 nm of the Cu2O nanoparticles were determined by transmission electron microscopy (TEM) (Figure 1-2) and their identities were established using the powder X-ray diffraction analysis (Figure 1-2). Elemental analysis of the cross-linked products was performed using the Perkin Elmer-2400 CHNS analyzer.
Chiral Binuclear Copper(II) Catalyzed Asymmetric Nitroaldol Reaction
Asymmetric Nitroaldol Reaction using Unmodified Nitroalkanes
- Chiral Metal Catalysis
- Chiral Rare Earth Metal Catalysts
- Chiral Copper Catalysts
- Chiral Zinc Catalyst
- Chiral Cobalt Catalysts
- Chiral Magnesium Catalyst
- Chiral Chromium Catalysts
- Organocatalysis
- Biocatalysis
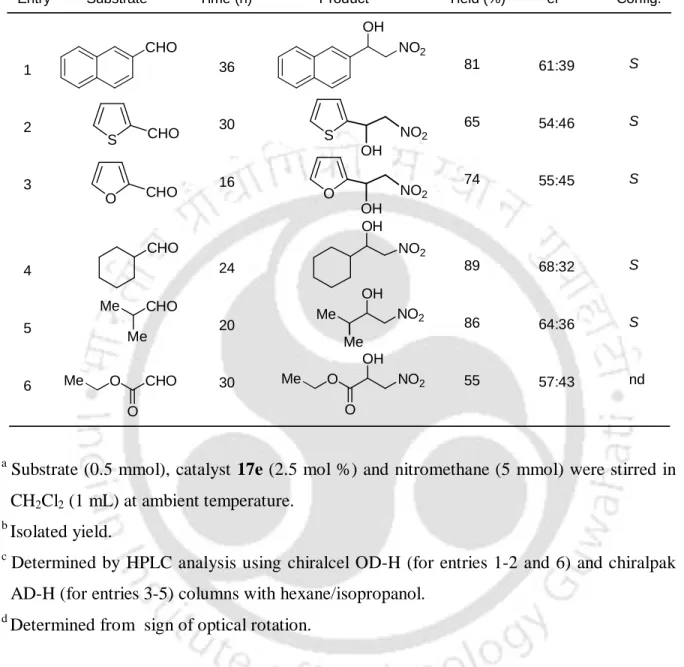
Recently, Saa and co-workers described the lanthanum-based bifunctional catalyst 2 for nitroaldol reaction (Scheme 5).8h In this method, aliphatic aldehydes show the best results compared to aromatic and -unsaturated aldehydes. Chiral copper-catalyzed asymmetric nitroaldol reaction is an active topic.9 Evans and co-workers used copper(II) bisoxazoline 3 for the reaction of aldehydes with nitromethane at room temperature with up to 94 % ee (Scheme 6).9e. Trost and Yeh studied the chiral dinuclear zinc complex 5 for the asymmetric nitroaldol reaction.
Choudary and colleagues have described the nanocrystalline MgO-BINOL as a heterogeneous catalytic system for the nitroaldol reaction at -78 °C in THF. Schulz and colleagues have studied chiral chromium(III)-salen 7 in its monomeric form as a soluble catalyst for nitroaldol reaction with up to 85% ee. Griengl and colleagues have developed the first example for a biocatalytic asymmetric nitroaldol reaction using hydroxynitrile lyase from Heavea brasiliensis (HbHNL).
Asymmetric Nitroaldol Reaction with Modified Nitroalkanes
Present Study
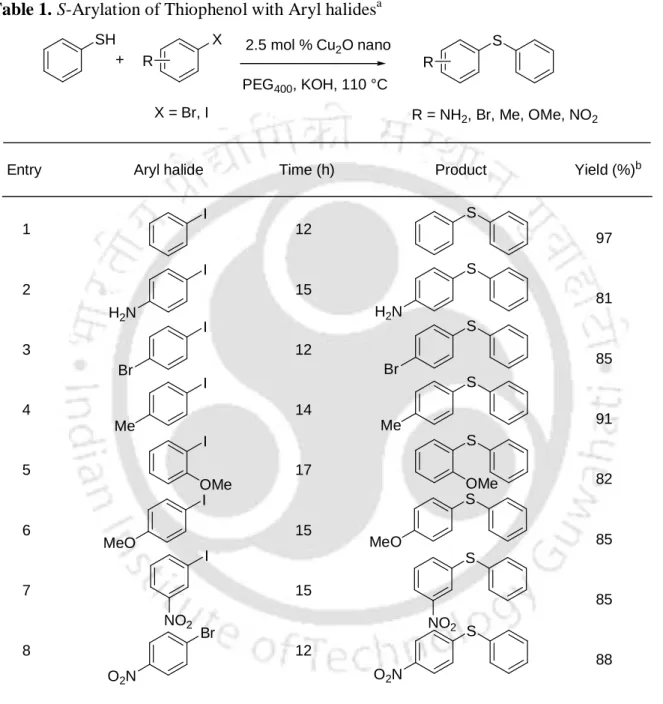
The reactions are free of the addition of an additive or base and the complexes 17a-g act as a bifunctional catalyst. The latter were then treated with Cu(OAc)2·1H2O in EtOH to give the complexes 17a-g as a green powder (Scheme 18). The catalytic activity of the complexes 17a-g was studied for the addition of nitroalkanes to aldehydes.
Removal of the solvent afforded a residue, which was purified on silica gel column chromatography using ethyl acetate and hexane (1:19) to give 16a–g as a yellow compound. The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography using ethyl acetate and hexane to give 17a–g as a green colored powder. After completion, the solvent was evaporated and the residue was purified by silica gel column chromatography using ethyl acetate and hexane as eluent.
Self-Assembly and Application of Copper(II) Complexes for Acceleration of Nitroaldol Reaction “On Water”
Diels-Alder Reaction
The Diels-Alder reaction has proved to be of great synthetic value, forming a key step in the construction of compounds containing six-membered rings. In 1939, Hopff and Rautenstrauch discovered that the Diels–Alder reaction can be carried out efficiently in an aqueous environment.10a In 1980, Rideout and Breslow achieved both the increased rate and excellent selectivity of the Diels–Alder reaction between cyclopentadiene and of butanone in water. These reactions were performed at a higher concentration (0.15 M for both reactants) to keep the reaction mixture as heterogeneous in water.
This could be the first example of a water reaction where a significant rate increase and a much higher selectivity was achieved simply by using water to support the reaction of two insoluble substances. In 2005, Sharpless and co-workers demonstrated the "on water" effect on the Diels–Alder reaction of trans,trans-2,4-hexadienyl acetate with N-propylmaleimide. Kobayashi and co-workers have reported aza-Diels-Alder reaction of silver triflate activated imines with Danishefsky's diene in water in good yield (Scheme 3).10e.
Transformations Catalyzed by Transition Metals
Chen and Li reported an "on-water" Pd-catalyzed 1,4-addition of terminal alkynes to -unsaturated ketones. The procedure is experimentally simple, works well in water, and can be carried out in the presence of oxygen, leading to a broad spectrum of γ,δ-alkynyl ketones (Scheme 5).12.
Oxidation and Reduction Reactions
This single atom transfer reaction was successfully performed on water in moderate to good yield (Scheme 6).13a,b. Li and co-workers reported a catalyst-free on water oxidation of aromatic silyl enol ethers. The substrates are converted to -hydroxyketones in good to excellent yields simply by stirring water in the presence of air.
Under these conditions, the water-insoluble aldehyde 3 is quantitatively reduced, while the water-soluble aldehyde 4 showed no reaction (Scheme 8).13d.
Nitroaldol Reaction
Recently, Li and co-workers used DNA as a catalyst for the nitroaldol reaction in water.
Present Study
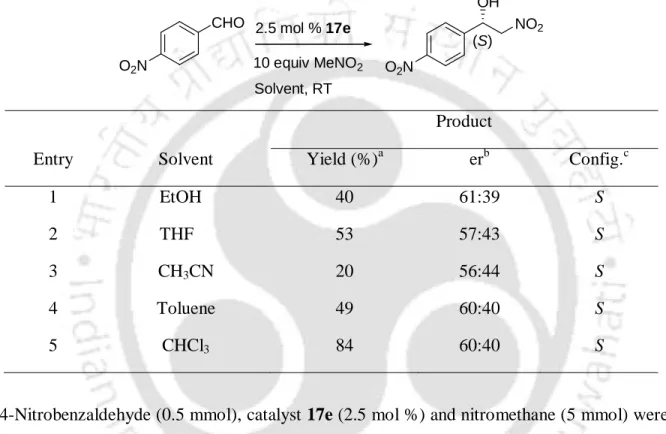
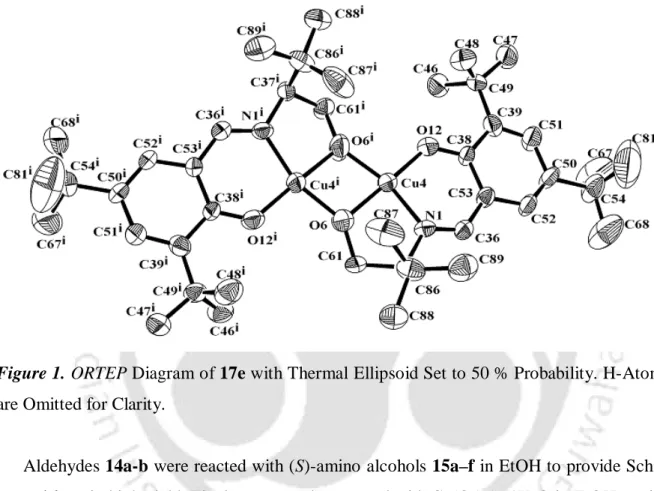
Optimization of the reaction conditions was pursued with 4-nitrobenzaldehyde and nitromethane as model substrates (Table 1). After the addition of nitromethane to 4-nitrobenzaldehyde was complete, the catalyst was recovered by filtration and reused for the reaction of fresh nitromethane with 4-nitrobenzaldehyde. These results clearly suggest that no leaching of the metal complex is involved and the reaction is a heterogeneous process.
In conclusion, the synthesis, crystal structure and application of a self-assembled aqueous copper(II) complex 8 and an open tetranuclear copper(II) cubane complex with an alxoxo bridge 9 are described for catalyzing the nitroaldol reaction in water. Drying (Na2SO4) and evaporation of the solvent gave a residue which was passed through a short pad of silica gel using ethyl acetate and hexane as eluent to afford an analytically pure nitroaldol product. Drying (Na2SO4) and evaporation of the solvent gave a residue which was passed through a short pad of silica gel using ethyl acetate and hexane as eluent to provide analytically pure nitroaldols in high yield.
Synthesis of New Chiral Main Chain Polymers with (1R,2R)-1,2- Diamines
Binapthyl Based Chiral Main Chain Polymers
In these polymeric complexes, the catalytic sites are expected to be highly organized along the rigid and sterically ordered backbone. This is different from most polymer-supported catalysts, where flexible and sterically disordered polymers are used. The rigidity and stereoregularity of chiral conjugated binaphthyl polymers enable systematic tuning of the microenvironment of catalytic sites for the development of enantioselective polymeric catalysts.
For example, polymer 3, which contains two markedly different catalytic sites, BINOL and BINAP, has shown excellent stereoselectivity in tandem asymmetric reactions involving the diethylzinc addition to the aldehydes and hydrogenation of the ketones (Scheme 2).8n It shows that the rigid polybinaphthyl structure can not only retain the catalytic properties of a monomeric catalyst, but can also allow distinctly different catalytic sites to function independently in the polymer chain to perform various asymmetric reactions. After the reaction, the polymer complex was recovered from the organic solution by precipitation with methanol. For example, the polymer 4 with rhodium catalyzes the asymmetric hydrogen transfer to ketone with increased selectivity of (58% ee) compared to the monomer (32% ee) (Scheme 4).6d.
Soluble Macromolecular Stereoselective Catalysts Containing Salen Metal complexes derived from chiral salen are the most successful enantioselective catalysts
The most common approach to synthesize a soluble polymer with salen repeating units 7 is the Schiff's base formation of bis(o-hydroxybenzaldehyde) with diamine (Scheme 5).10. Zheng and co-workers studied the chiral manganese(III)-salen 8 as a soluble catalyst for asymmetric epoxidation of unfunctionalized olefins together with 4-phenylpyridine N-oxide (4-PPNO) with up to 97 % ee (Scheme 6). 10a The catalyst 8 is recoverable without loss of activity and selectivity. In addition, the catalyst 8 exhibits activities and enantioselectivities as good as the Jacobsen homogeneous catalyst.10b.
Yu and co-workers have used chiral manganese(III) salen 9 for the epoxidation of styrene (Scheme 7).11 However, the recovered catalyst shows less reactivity and selectivity compared to the fresh catalyst. Weck and co-workers have developed cobalt(III)-salen 10 for the kinetic resolution of racemic terminal epoxides (Scheme 8). The catalyst is recyclable without loss of activity and selectivity. 12.
Present Study
The organic layer was separated and the aqueous layer was extracted with CH2 Cl2 (2 x 100 mL). The organic layer was separated and the aqueous solution was further extracted with CH2 Cl2 (3 x 5 mL). After cooling to ambient temperature, the solvent was evaporated and the residue was dissolved in CHCl3 (50 ml).
The solvent was then evaporated and the residue was dissolved in Et 2 O (5 mL) and passed through celite. The filtrate was concentrated and the residue was purified by flash column chromatography using hexane and ethyl acetate (19:1) as eluent to give 25 as a yellow solid in g). The organic layer was separated and the aqueous solution was further extracted with CH2Cl2 (2 x 5 mL).
To a stirred solution of
- References
The solid was filtered and the filtrate was evaporated to give a residue which was treated with a 1:3 mixture of H 2 O and CH 2 Cl 2 (15 mL). The solvent was then evaporated under reduced pressure and the residue was dissolved in CHCl3 (20 mL). The solution was passed through a short pad of silica gel and concentrated under reduced pressure and purified by flash chromatography using hexane and ethyl acetate (19:1) as eluent to afford 14 as an orange solid (mg).
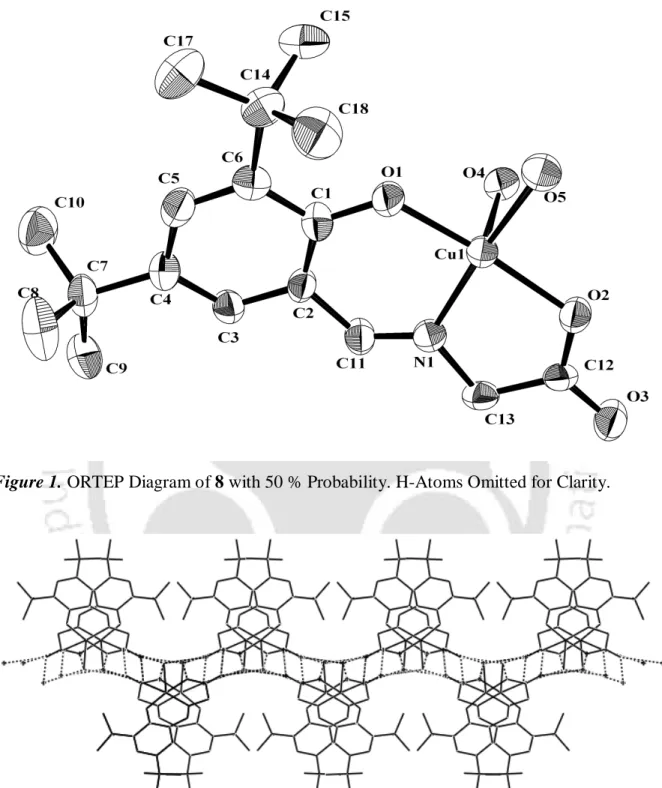
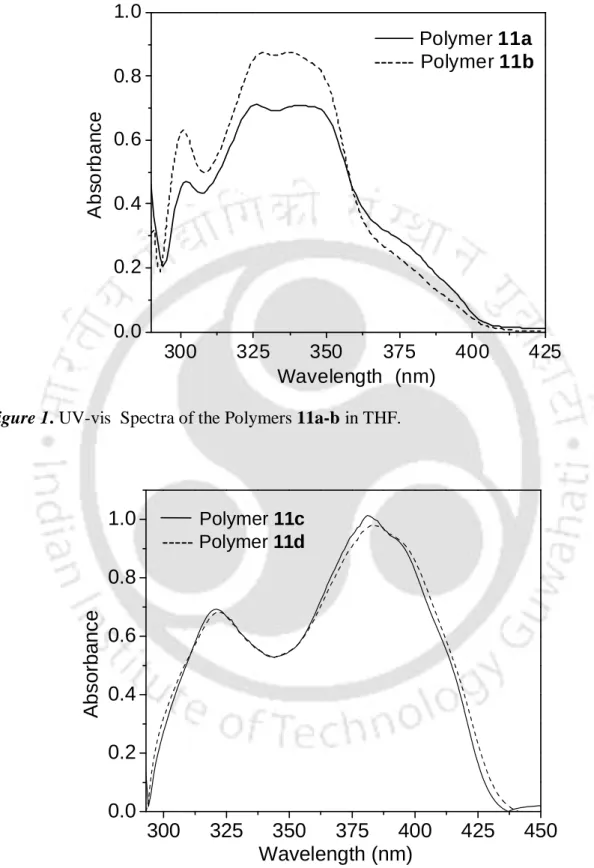
The solution was then treated with MeOH (2 ml) and the resulting precipitate 11b was collected as a yellow powder in mg) yield. To a solution of polymer 11a or 11b (1 mol%) in dry toluene, Et 2 Zn (1 mol%) was added and the resulting mixture was stirred at ambient temperature for 1 hour. The resulting solution was warmed to room temperature and then stirred for an additional 18 hours.
List of Publications
12 Cu2O nanoparticles catalyzed N-arylation of amides and imidazoles and S-arylation of thiols with aryl iodides.
Conferences