For practical purposes, the environment in the immediate vicinity of the system is called the surroundings. A property that does not depend on the size of the system is called an intensive property. The change of state property depends only on the initial and final state of the system.
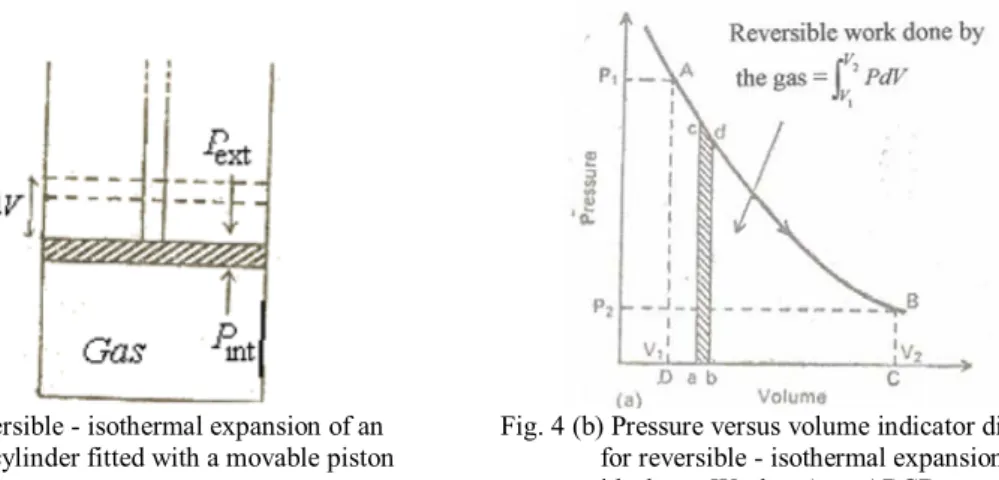
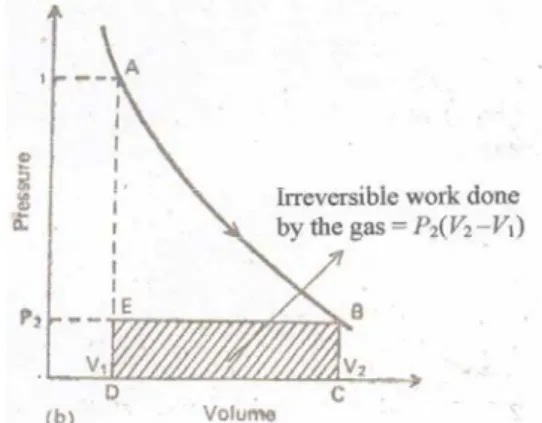
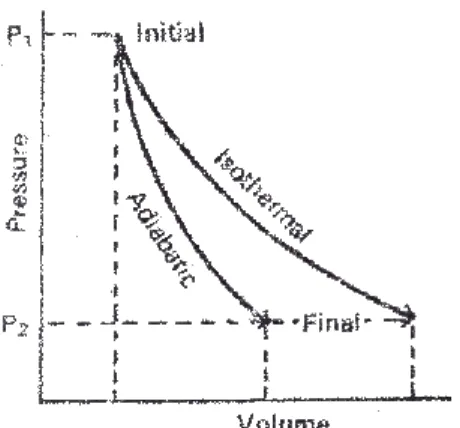
A process is said to be isothermal when the temperature of the system is kept constant. The total energy of all the molecules of the system is called internal energy or internal energy. The change in the internal energy of the system due to the temperature difference between the system and its surroundings is called heat.
Thus, heat flows from the system to the environment and the internal energy of the system decreases. In this equation, ∆U is the change in the internal energy of the system, q is the heat absorbed by the system, and w is the work done on the system. The change in internal energy is equal to the heat exchanged between the system and the environment at constant volume.
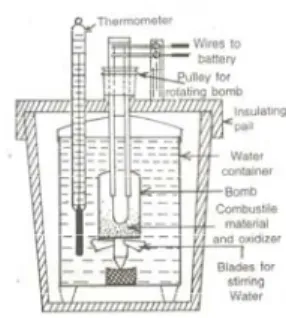
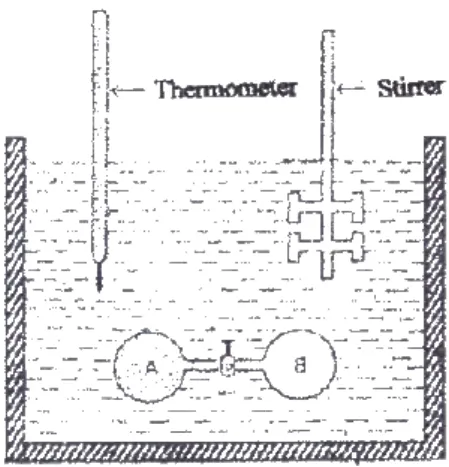
The heat exchanged between the system (reaction) and the environment (water + calorimeter) is calculated from the equation.
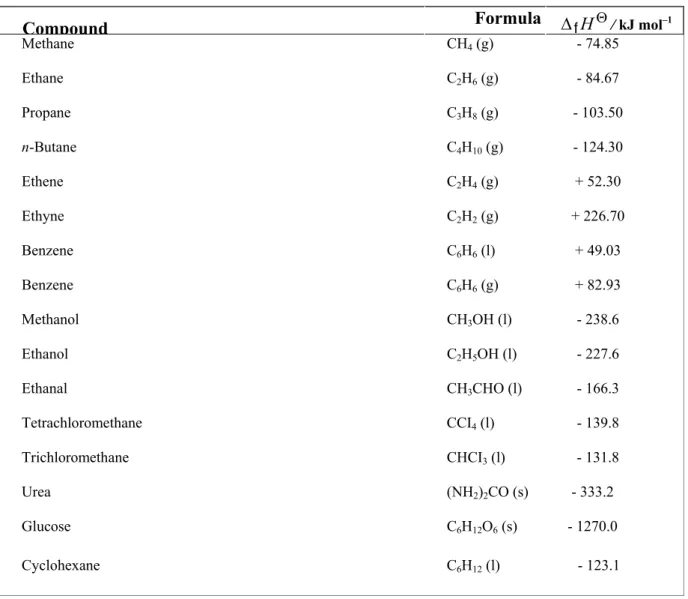
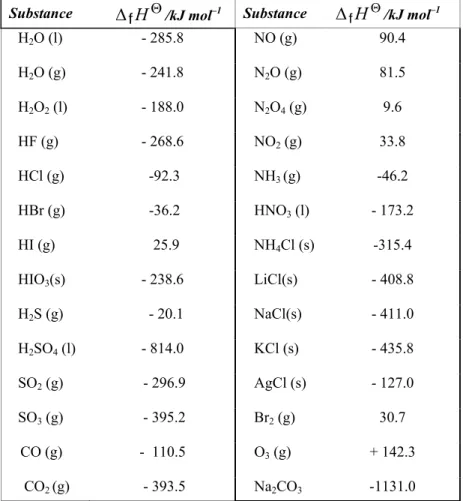
The standard enthalpy of formation is denoted by ∆fHυ and is also called the standard heat of formation. The standard enthalpy of formation of a gaseous hydrogen atom is equal to the enthalpy change for the following reaction. The standard enthalpy of formation of a gaseous carbon atom is equal to the enthalpy change for the following reaction.
The enthalpy of combustion is the heat released at constant pressure when one mole of fuel is burned completely in oxygen. The enthalpy change associated with the formation of one mole of a compound from its constituent elements in their respective standard stable states is called standard enthalpy of formation. The enthalpy change in any type of chemical reaction namely combination, combustion, hydrogenation, dissociation of bonds or neutralization is called the enthalpy of reaction.
Important Statements: The enthalpy of formation is always the same as the enthalpy of reaction representing the formation of that compound. However, the enthalpy of each reaction is not necessarily the enthalpy of formation. For example. Therefore, the enthalpy change of this reaction is equal to the enthalpy of combustion of hydrogen and also to the standard enthalpy of formation of H2O (l).
The H–H bond enthalpy is equal to the enthalpy change for the breaking of one mole of H–H bonds in H2 molecules and the formation of free gaseous H atoms. ii) O bond enthalpy. Thus, the enthalpy of bond dissociation is defined as the change in enthalpy until the breaking of a given bond in a certain chemical environment . The change in enthalpy during the conversion of one mole of a substance into its constituent atoms in the gaseous state is called atomization enthalpy or heat of atomization of this substance.
The atomization enthalpy of a diatomic molecule is equal to its dissociation enthalpy ∆dH. So:. But the enthalpy of atomization of methane is equal to the enthalpy of reaction (A) ∆aH (CH4) = ∆rH (A). B) Calculation of C-C bond energy from thermochemical data. The reaction enthalpy (D) is the enthalpy of atomization of ethyne, which is the energy required to break one mole of C≡C bonds and two moles of C-H bonds in one mole of ethyne.
The enthalpy of fusion is denoted by ∆fusH It is also called heat of fusion.
When molar heat capacity depends on temperature as
From this equation the value of the reaction enthalpy at any temperature T2 can be calculated provided that its value at a temperature T1 is known and the molar heat capacities of the reactants and products are also known. Determine the following (a) Enthalpy of sublimation (b) Enthalpy of combustion. c) Standard enthalpy of formation of a compound (d) Enthalpy of neutralization. The enthalpy of formation is the enthalpy of reaction, but each enthalpy of reaction needs represents the enthalpy of formation.
How are bond dissociation enthalpy and C–H bond enthalpy related in methane (CH4). Illustrate the following with suitable examples: (i) Exothermic process. ii) Endothermic process (iii) Standard state of gas (iv) Thermochemical equation. v) Hess's law of constant heat addition (vi) Enthalpy of hydrogenation. The enthalpy of neutralization of CH3COOH with NaOH is –51.63 kJ mol–1 Calculate the enthalpy of ionization of CH3COOH.
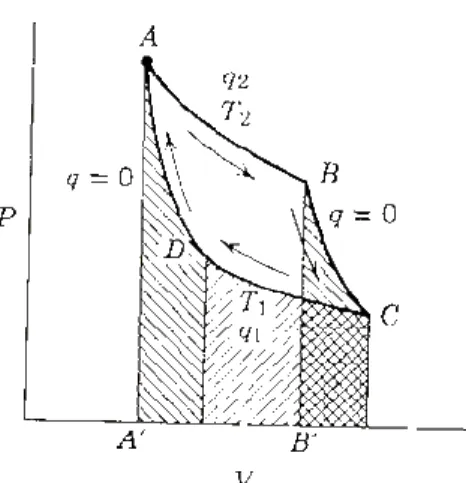
Find an expression for the change in heat capacity of a reaction with temperature if its enthalpy change with temperature is expressed by ∆rHΘ= A – BT –CT2. The first law of thermodynamics allows us to calculate energy changes (∆U) and enthalpy changes (∆H) for various types of chemical reactions and physical transformations. The entropy function of a system of bodies tends to increase in all possible processes occurring in nature, if we include in the system all such bodies that are affected by changes.
Thus, the entropy of a substance in its gaseous state is greater than in its liquid state, and it is greater than in its solid state. The entropy change of the system (∆S system) does not depend on the path followed by the change, but the entropy change of the surroundings (∆Ssurronnd1ngs) does. The total entropy change of an isolated system is equal to the sum of the entropy change of the system and the entropy change of the surroundings.
The total entropy change of an isolated system is also called the entropy change of the universe (∆Suniverse). If the amount of substance is one mole, then entropy and its changes are expressed as joules per kelvin per mole (J K–1 mol–1). ii) Calories per kelvin = cal K–1 is the non-SI unit of entropy. But in the present discussion we will give another logical basis of introducing the definition of entropy in terms of Euler's reciprocity relations for the exact differentiation of the state function.
First we will prove that dqrev is inexact differential and then show that dqrev divided by T i.e. dqrev /T is an exact differential.
Calculate the entropy of vaporization of a liquid which boils at 110.6 °C when
- Error! Bookmark not defined.Calculate the standard Gibbs energy change for the reaction CH 3 OH (1) + 3/2O 2 (g) → CO 2 (g) + 2H 2 O (1) that can be converted into electrical
The entropy of gaseous mercury is greater than that of liquid mercury (b) For the process AgNO3(s) ⎯⎯→ AgNO3(aq). Therefore, the entropy change of an isolated system is equal to the entropy changes of the system and its surroundings. The total entropy change of an isolated system is called the entropy change of the universe.
The entropy change of the system can be calculated based on the mathematical statement of the second law of thermodynamics. For irreversible processes, the entropy change of the system is the same as that for reversible processes. Entropy change of the environment (∆Senvironment) The entropy change of the environment is calculated as.
The change in Gibbs energy of the system is related to the entropy change of the universe. The decrease in the free energy of the system is equal to the network that can be obtained from the system. At constant pressure and constant temperature, the change in the free energy of the system is related to the enthalpy change (∆H) and the entropy change (∆S).
At constant pressure, the heat exchanged between the system and the surroundings is equal to the change in enthalpy of the system. Conclusion: A decrease in the free energy of a system is equal to an increase in the entropy of the universe multiplied by the Kelvin temperature. This means that at constant temperature and constant pressure, the free energy of the system must decrease in a spontaneous process and.
At constant volume and constant temperature, the change in Helmholtz energy of the system is related to the internal energy change (∆U) and entropy change (∆S) by. At constant volume, the heat exchanged between the system and the surroundings is equal to the internal energy change of the system. Conclusion: The decrease in the Helmholtz energy of the system is equal to increase in the entropy of the universe multiplied by the kelvin temperature.
That is, at a constant temperature and a constant volume in a spontaneous process the Helmholtz energy of the system must decrease. For a finite change in the state of the system. and ∆G = ∆A for an ideal gas at constant temperature.