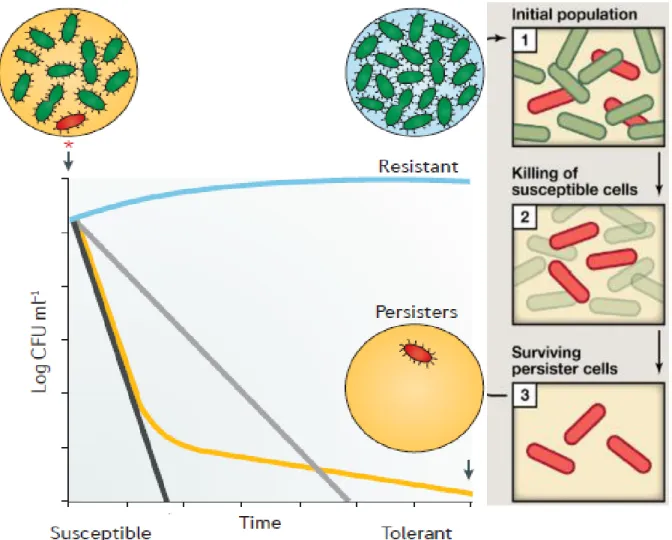
And in a long-term predation in the continuous system was carried out to characterize this remaining sub-population of prey. The bacterial persisters are a small sub-population of the whole culture that exists within a dormant state and, although they have no genetic mutations or modifications, they survive longer during exposure to antibiotics due to this temporary state. Since these persister cells survive in the presence of different antibiotics, their existence is considered important as they can cause chronic or recurrent infections.
In this study, I characterized both persistent cells induced by an antibiotic pretreatment, i.e., antibiotic persisters (AP), and the subpopulation of prey cells that survive predation by B. BALOs: Bdellovibrio and organisms of similar USA: United States of America SCV : Small colony variant ppGpp : Guanosine tetraphosphate pppGpp : Guanosine pentaphosphate RPM : Revolution per minute PFU : Plaque forming unit CFU : Colony forming unit LB : Luria-Bertani broth DNB : Cell optimality : Optional.
INTRODUCTION
Predatory bacteria, Bdellovibrio bacteriovorus
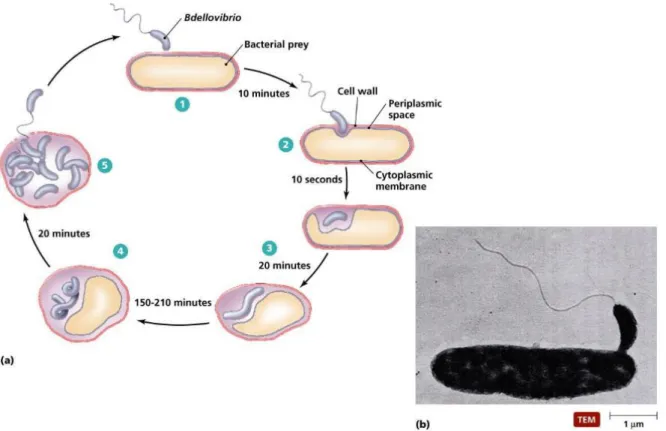
- The life cycle of Bdellovibrio bacteriovorus
- Residual prey cell after predation
- Small colony variants (SCVs)
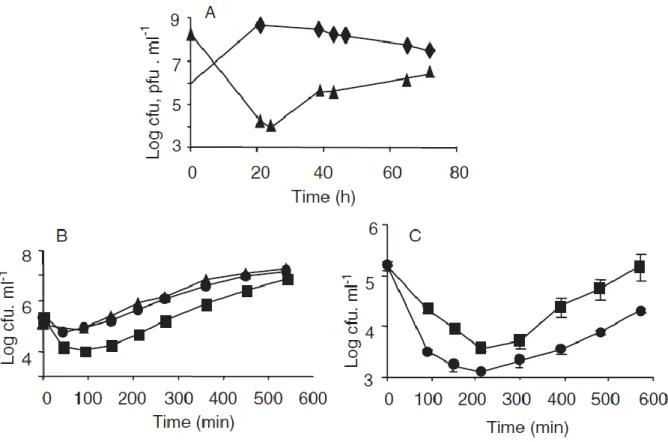
The small subpopulation of prey cells that remained after the predator is considered to be cells resistant to predation by Bdellovibrio bacteriovorus. 106-107 and 108-109 pfu ml-1) Therefore, it contains different levels of the remaining prey cell from the carried predator. However, predation was more efficient at a higher concentration of predatory bacteria when 0.45 μm filtered predatory cell lysate was added to the fresh prey cell.
And the naive prey cell quickly formed bdelloplast, while the remaining cell became longer and less attached to the predator after exposure to predatory bacteria. Therefore, increased resistance to predation was not a stable trait but appeared to be transient as the remaining cell becomes sensitive to predation like a naive prey cell. In conclusion, it is shown that failure to eradicate the remaining prey population in BALO-prey interactions does not arise from the inability of the predator to encounter prey cells in low concentration, as previously suggested.
Predation of fresh Ecc prey cells by a different concentration of predator containing residual prey cell. high - filled circles / low - filled squares). If the remaining prey population was resistant to predation, then the B. bacteriovorus HD100 count in the chemostat should decrease due to a washout effect.
Persister cell & Dormancy
- Toxin-antitoxin system in persister cell formation
Typically, toxin-antitoxin systems act on important cellular processes such as translation, reproduction, cytoskeleton formation, membrane integrity, and cell wall biosynthesis. Through this toxin-antitoxin system, the bacterial cell can adapt to stress by slowing cell growth or inhibiting cell growth. Toxin-antitoxin systems consist of the set of two genes that encode the stable toxic protein and the unstable antitoxin.
The toxin could disrupt the essential mechanisms for the cellular process, including DNA replication, mRNA stability, protein synthesis and cell wall biosynthesis, and the antitoxin mediates the effects of the toxin. There are several types of toxin-antitoxin systems and they are classified according to how toxin is neutralized by antitoxin. And type III occurs when the antitoxin inhibits toxin activity by binding the toxin protein.
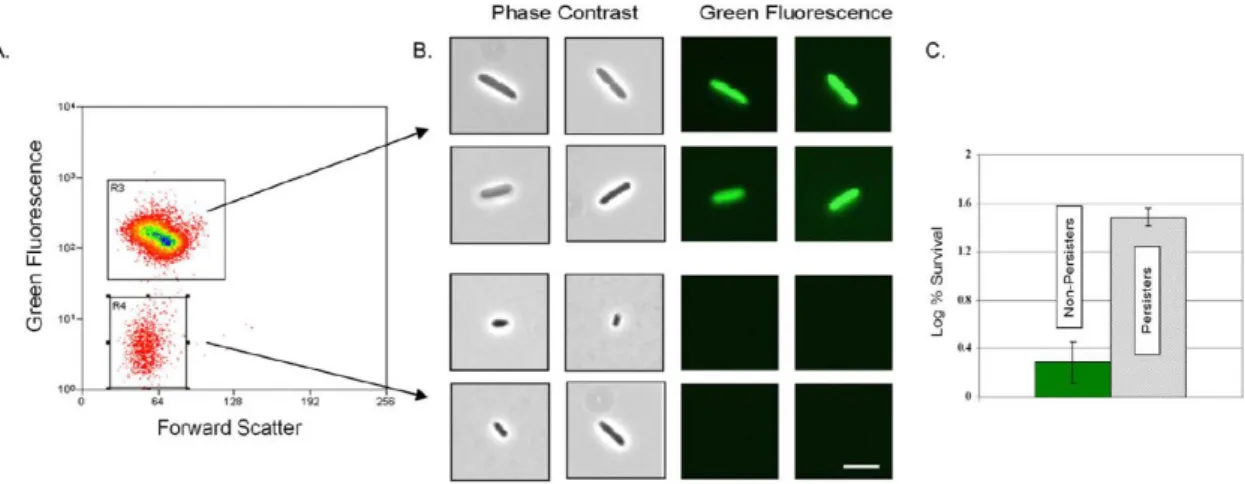
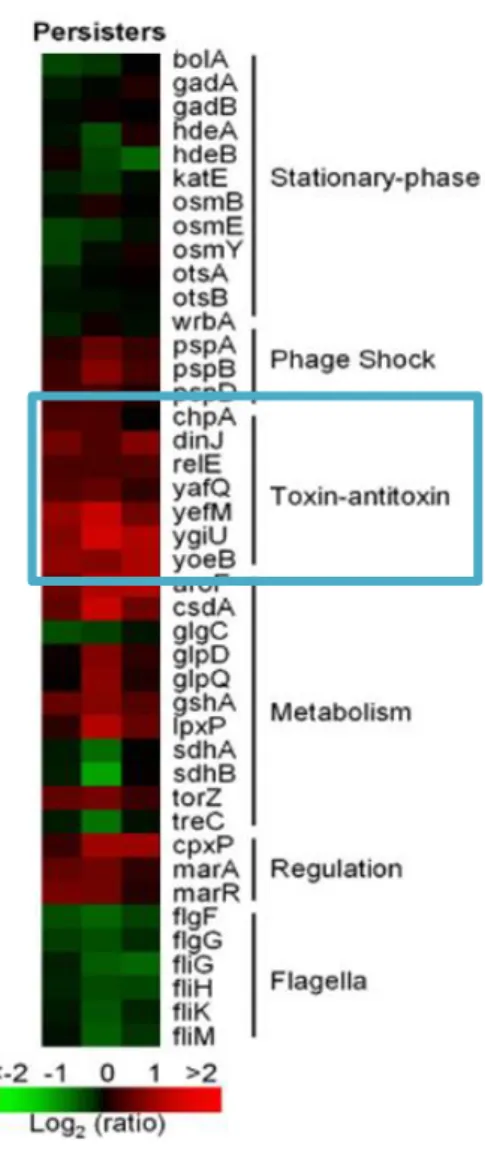
However, the toxin-antitoxin systems are taken into account because they are involved in the formation of persistent cells to induce a dormant state. Toxin-antitoxin systems were first considered as a genetic basis for the formation of persistent cells from normal cells, as they induce a dormant state via toxin overproduction, leading to an increase in persistence. In 2006, DNA microarrays were performed on carefully isolated quiescent cells using a green fluorescent protein (GFP) reporter downstream of a ribosomal promoter. Metabolically inactive cells were isolated via fluorescence-activated cell sorting (FACS) based on reduced fluorescence. (Figure 1.7., Figure 1.8.) [39] These quiescent cells showed tolerance to ofloxacin compared to normal growing cells and showed that TA-related genes with differential transcription included dinJ, yoeB, yefM, yafQ,dinJ, relE and maze, compared to the non-persistent cell.
As noted above, the proposed mechanisms by which toxin-antitoxin systems cause persistence are related to dormancy. In the MqsR/MqsA type II TA system, the MqsR toxin cleaves most of the transcripts in the cell, so that the toxin leads the cell to a quiescent state by reducing translation. For the TisB/IstR-1 type I system, the toxin induces a quiescent state of the cell by reducing proton motive force and ATP levels.
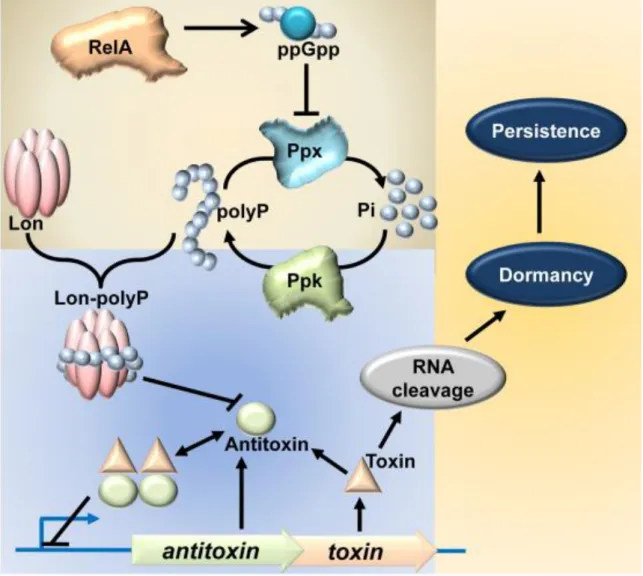
Confirming the importance of TA systems for persistent cell formation, Lon protease was shown to be required for persister cell formation when Lon protease is degraded. As the antitoxin that can neutralize the effects of the toxin is degraded, the toxin is free to act on its target RNA. And one experiment showed that (p)ppGpp is involved in inducing persister cells with relA and spoT deletions showing extremely low levels of persister cells in exponentially growing cultures.
EXPERIMENTAL METHOD & MATERIALS
- Bacterial strain
- Used media
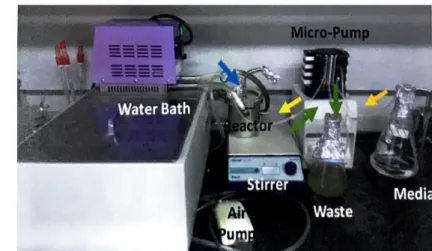
- The culture of predatory bacteria, Bdellovibrio bacteriovorus HD100
- Induce persister cell formation by pre-treatment of antibiotics
- Development of rifampin-resistant cell
- Persister cell predation by Bdellovibrio bacteriovorus HD100
- Antibiotic susceptibility test of induced persister cell
- SDS toxicity test
- Isolate residual prey cell using 0.1 % SDS
- Antibiotic susceptibility test of the residual cell from predation
Escherichia coli MG1655 pUCDK, was grown overnight in 15ml LB broth at 37°C in the shaking incubator. And it was centrifuged (2,000 × g, 15 minutes) and the optical density adjusted to 1.0 of liquid cultured Escherichia coli MG1655 pUCDK with HEPES buffer. When it reaches the optical density (OD600) of 0.7-0.8, treat with antibiotics (rifampin or tetracycline 100μg/ml, 50μg/ml for each antibiotic) and incubate for 1 hour with shaking at 250rpm at 37°C in the shaking incubator.
Wash the persister cell to remove residual antibiotic 1 time by centrifugation (2,000 × g, 15 min) with HEPES buffer. The number of viable cells measured by CFU at this time was set as an initial number of induced persister cells in the further experiments. Spread 100μl of the cultured cell on LB plate containing rifampin (100μg/ml) and incubate at 37°C incubator.
Take the single colony from the rifampin LB plate and inoculate into fresh LB media containing rifampin. After 24 hours, 1 ml of the sample was taken and inoculated into fresh 9 ml of LB medium with rifampin added. Repeat these processes 3 more times and the surviving cell from the culture with rifampin was stored in 25% glycerol stock at -80°C.
After 18 and 24 hours from the point where the predatory bacteria were added, OD and CFU were measured. After antibiotic pretreatment, cultures were centrifuged down and resuspended with HEPES to remove residual antibiotics, then adjusted to a turbidity of 1.0 at OD600. First, different concentration of detergent was prepared in 96-well plates by dilution before addition of prey cell alone.
Overnight cultured Bdellovibrio bacteriovorus is filtered with a 45 μm syringe filter (Merck Millipore LTD., USA) to remove prey cell and added as 1/100 diluted in 100 ml prey with turbidity of 1.0 at OD600. Treat the prey-predator mixture with the selected concentration of 0.1% sodium dodecyl sulfate (SDS) for 1 h at 30 °C in a shaking incubator. After 1 h SDS treatment, wash the SDS from the prey by centrifugation (2000 × g, 15 min) and resuspend once with HEPES buffer.
RESULTS
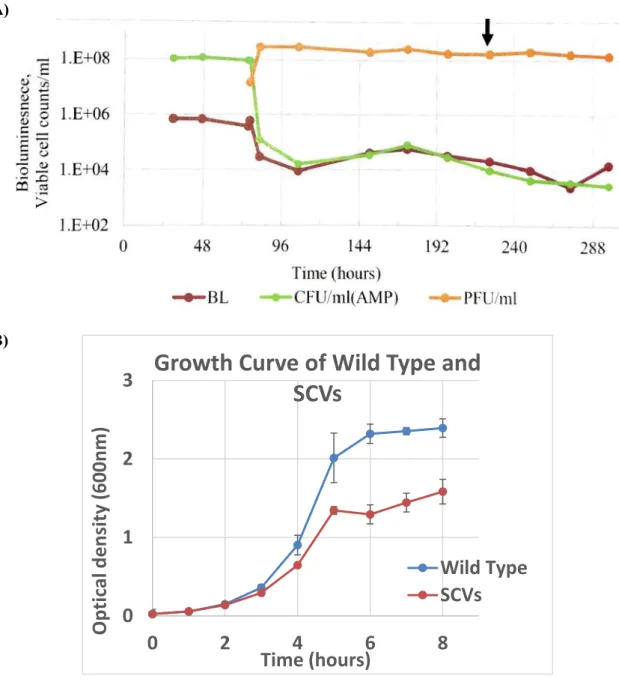
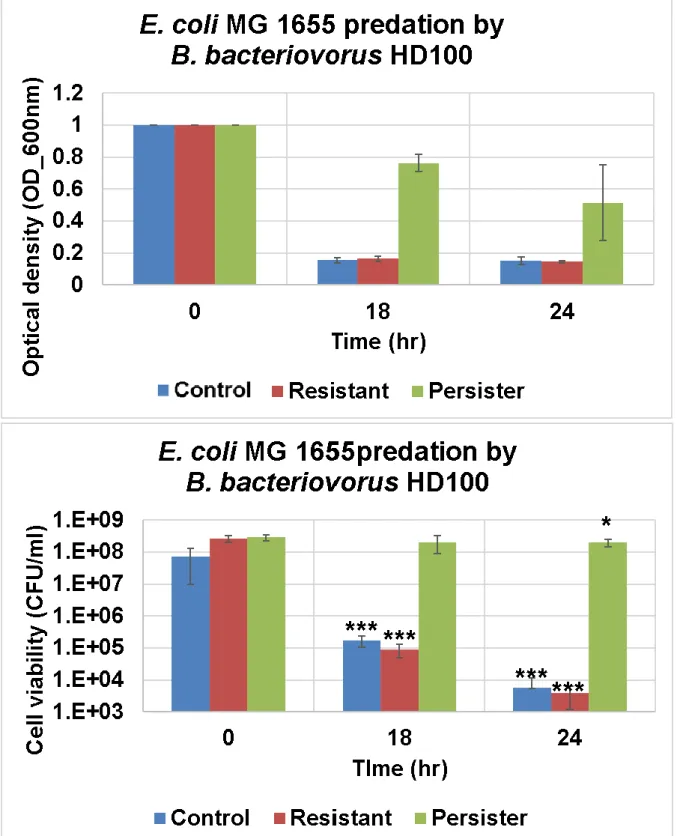
Antibiotic persister is resistant to predation by Bdellovibrio bacteriovorus
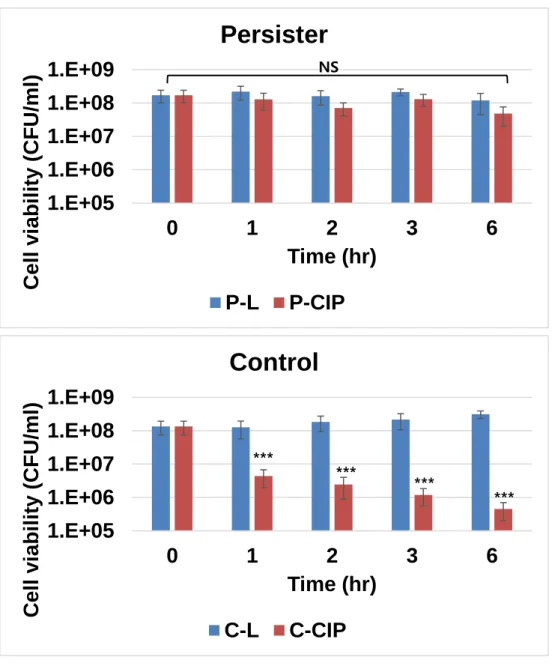
Predation persister show resistant to antibiotics
Transcriptomic analysis of antibiotic persister and SCVs
CONCLUSION
34;Seasonal and geographic distribution of luminescent bacteria in the eastern Mediterranean Sea and the Gulf of Elat." Applied and Environ. 34;Plastic phenotypic resistance to predation by Bdellovibrio and similar organisms in bacterial prey." Environmental Microbiology. 34; Toxin-antitoxin systems influence biofilm and cell persistence and the overall stress response." Applied and Environmental Microbiology.
34;Persistence increases in the absence of the alarmone guanosine tetraphosphate by reducing cell growth. “Scientific reports. 34;Variable persistent gene interactions with (p)ppGpp for persistence formation in Escherichia coli.” Frontiers in microbiology. 34;Toxins Hha and CspD and small RNA regulator Hfq are involved in persistent cell formation via MqsR in Escherichia coli." Biochemical and Biophysical Research Communication.
34;Ranking of persister genes in the same Escherichia coli genetic background demonstrates varying importance of individual persister genes in tolerance to different antibiotics." Frontiers in Microbiology. 34;Stationary phase genes upregulated by polyamines are responsible for making Escherichia coli persister cells tolerant against netilmicin." FEMS microbiology letters. 34; Role of Global Regulators and Nucleotide Metabolism in Antibiotic Tolerance in Escherichia coli." Antimicrobial Agents and Chemotherapy.