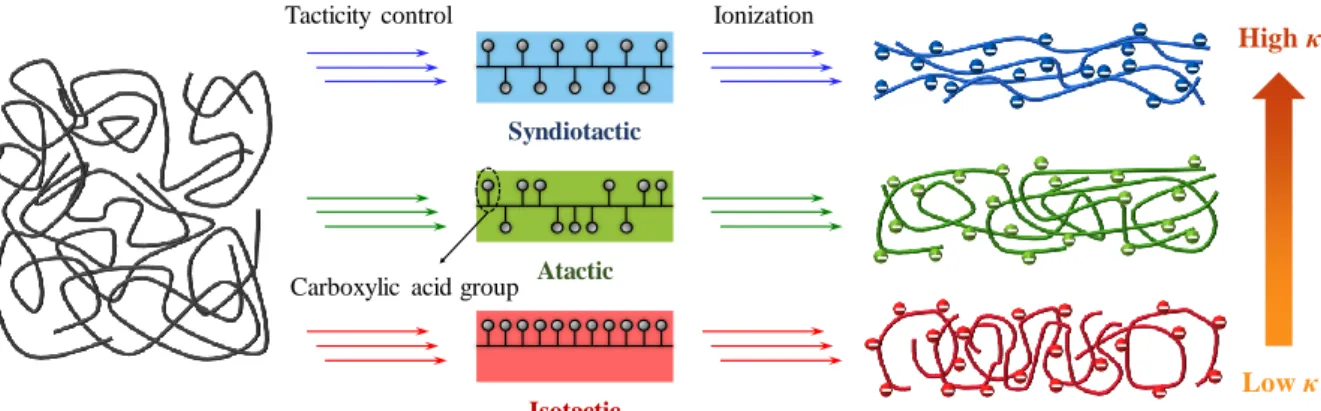
High thermal conductivity of polymers is important to improve the functionality and reliability of products by effectively dissipating heat in polymer products. Here we demonstrate that the tacticity of polymers changes the chain conformation in ionized polymers due to the difference in the Coulombic interaction between the functional groups. Depending on their tacticity, polymers with the same degree of ionization were observed to have significantly different cross-plane thermal conductivities, as high as 1.14 W/m∙K in ionized atactic poly(acrylic acid)(PAA) and 0.69 W /m∙ K in ionized syndiotactic poly(methacrylic acid)(PMAA), but only 0.55 W/m∙K in ionized isotactic PAA and 0.48 W/m∙K in ionized isotactic PMAA and atactic PMAA.
The modulus of elasticity, viscosity data affecting the conformation of the polymer chain suggested a validating effect on the thermal conductivity of polymers. In addition, we systematically investigated the effect of annealing temperature on the interplanar thermal conductivity of amorphous natural biopolymer. This high thermal conductivity is mainly achieved by improving the crystallinity of the polymer and the low interplanar spacing, which resulted in efficient phonon transport.
Introduction
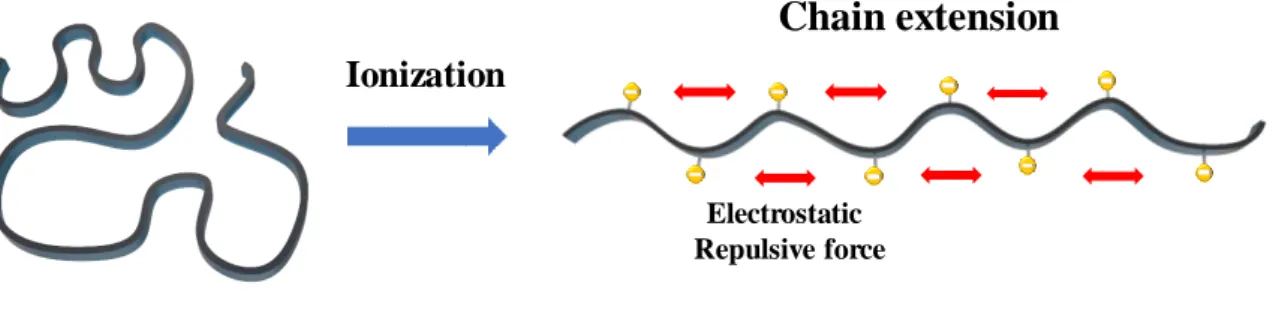
Meanwhile, extended chain conformation provides longer spatial paths for heat transfer with a greater persistence length, and leads to high thermal conductivity. They showed a dramatic increase in cross-plane thermal conductivity as polymer pH increased, as high as ∼1.2 W/m∙K, which is nearly six times higher than the original polymer. Consequently, polymer tacticity affects the conformation of the polymer chain, resulting in flexibility, stiffness, crystallinity, viscosity, 20, 21 elastic property, 22-24 and thermal conductivity of the polymer.
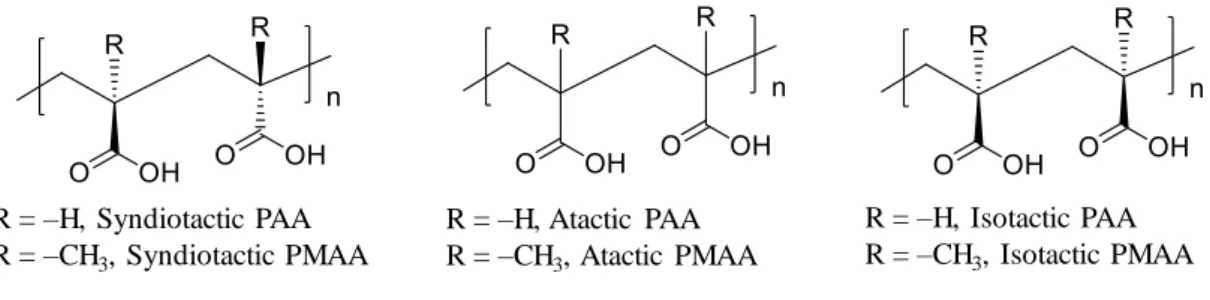
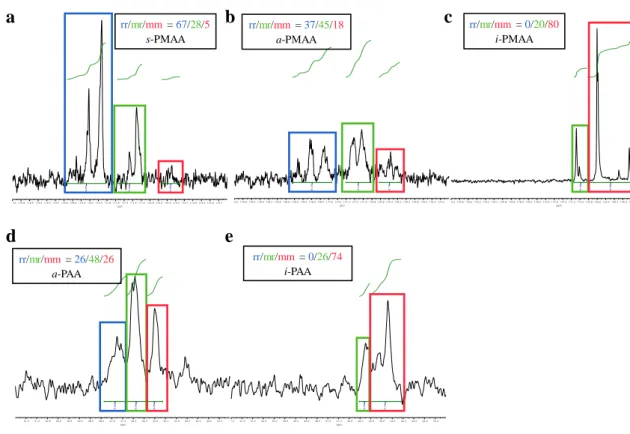
Thus, the difference in tacticity affects the improvement of the thermal conductivity of the polymer. Here, we experimentally demonstrated the effect of tacticity on the thermal conductivity of the polymer. We specifically studied the effect of tacticity on the thermal conductivity of ionized poly(methacrylic acid) (PMAA) and poly(acrylic acid) (PAA) (Figure 4).
We further measured the viscosity and elastic modulus representing chain elongation to investigate the relationship with thermal conductivity. We observed that the polymer tacticity has a strong effect on the thermal conductivity of the ionized polymer. In addition to the method of increasing the thermal conductivity of amorphous polymers by changing the chain conformation, there is also a study of increasing the crystallinity of polymers.
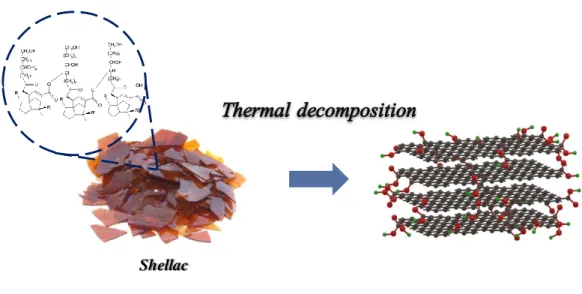
Thermally reduced shellac film at 900 ℃ shows a significant increase in thermal conductivity across the plane up to 1.22 W/m∙K, which is six times higher than natural polymer. Furthermore, the structural characterization was measured by X-ray diffraction (XRD) and Raman spectroscopy, revealing the correlation with across-plane thermal conductivity.
Experimental Method
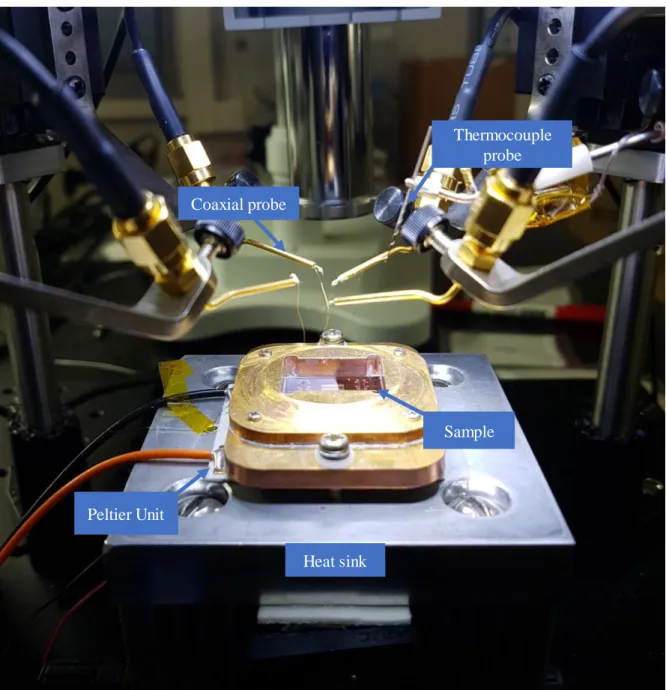
- Thermal conductivity measurement (Differential 3-omega method)
- Theory of 3-omega method
- Measurement setup
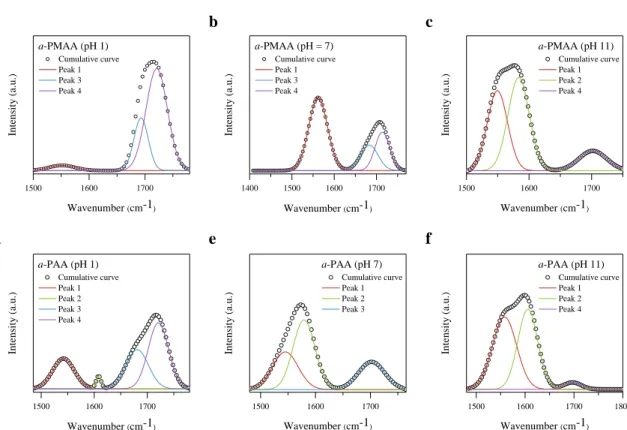
- FT-IR spectroscopy
- Elastic modulus measurement
- Viscosity measurement
𝐼ℎ,0 𝑡 =𝐼ℎ,0𝑐𝑜𝑠 𝜔𝑡 (1) This harmonic current generates the Joule heating and generates the temperature change (∆𝑇𝐴𝐶) in the heat pipe and the underlying substrate. Temperature oscillation of the heating line occurs, which causes the resistance of the heating line to oscillate at ω. The third harmonic voltage signal is provided by the ω current modulation and the 2ω change in heater line resistance.
This model is satisfactory because the width of the heating line (50 μm) is much larger than the thickness of the polymer film (0.15 μm ~ 1.8 μm). The geometry of the heating lines was carefully controlled by using a deposition shadow mask. To meet the 1D heat diffusion model, the thermal penetration depth must be much greater than half the width of the heating line (w/.
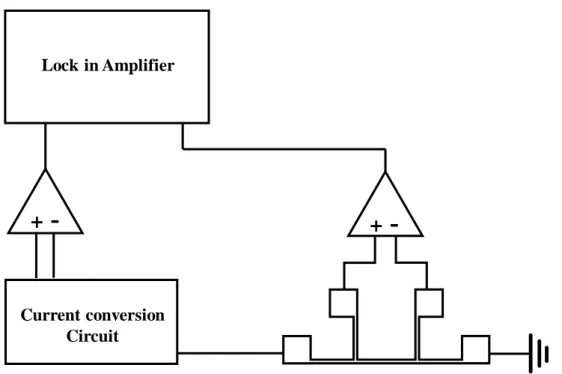
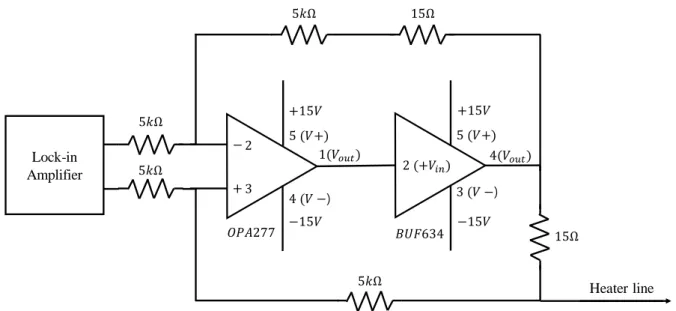
Then the lock-in amplifier detects and measures the first harmonic and third harmonic voltage signals between the inner pads of the heating line. Accurate information about the width, length, temperature coefficient of resistance and thickness of the heating line film is needed to calculate the thermal conductivity across the plane of thin polymer film. The resistance temperature coefficient of the heating line is calculated by the ratio of the resistance change during the temperature difference.
The heater line is deposited with 5 nm adhesive layer (Cr) and 300 nm heater line material (Ag) using an e-beam evaporator on the polymer film. The temperature coefficient of resistance (𝛽ℎ) of the heater line is calculated by the following equation. a) The thickness of the near heater line sample was measured by the surface profiler.

Sample preparation
- Synthesis of polymer
- Synthesis of s-PMAA 32
- Synthesis of a-PMAA 1
- Tacticity analysis
- Polymer film fabrication
- Polymer Solution Preparation
- Thin Film Preparation
- Thermal annealing of shellac film preparation
After evaporation of the solvent, the product was dried under reduced pressure and dissolved in CDCl3 for NMR analysis. First, for PMAA samples, the required amount of polymer was dissolved in DI water by heating to 80℃ to ensure complete dissolution. Finally, the final concentration of the solution was adjusted to 1 wt % by adding DI water.
Then, the surface of the silicon wafer is treated with O2 plasma treatment for 3 minutes at a power of 100 W. The prepared solutions with a weight percentage of 1 wt% were spin coated for 1 hour at 300 rpm on a completely cleaned silicon wafer substrate (spincast with low rotation speed). This low speed led to a slow drying of the polymer solution (60 minutes), which is expected to reduce the anisotropy in the polymer film, as well as the large film thickness µm), improving the accuracy of the differential 3-omega measurement.
After spin coating, the sample was annealed at 100℃ for 30 minutes to evaporate the remaining solvents. For the differential 3-omega method, half of the sample was carefully scratched out with a steel blade, and residual polymer above the wafer was clearly removed by wiping with a cotton swab soaked in DI water and acetone each. Polymer solutions (120 µl) were coated by drop casting on cleaned silicon wafers with 100 nm SiO 2 layers and the resulting films were left to air dry for 1 h.
The prepared drop cast film is thermally annealed at a target temperature for 30 minutes with a tube furnace. For the sample for thermal conductivity measurement, half a part of the cast film was removed using a steel knife, and the cleared area was further cleaned with a cotton swab soaked in water and ethanol to obtain a clean polymer-free reference area .
Results and discussion
Validation of the differential 3-omega method results
Tacticity dependent cross-plane thermal conductivity in electrostatically engineered amorphous
- Cross-plane thermal conductivity of PMAA and PAA by different tacticities
- Degree of Ionization
- Elastic modulus and viscosity
As shown in previous studies, highly ionized a-PAA generates the repulsive force between negatively charged ionized groups, leading to chain elongation and thermal conductivity enhancement. However, the ionization of a-PMAA, i-PMAA and i-PAA increased with higher pH, but the thermal conductivity improvement effect was negligible. Therefore, it is assumed that the difference of tacticity, a difference of side chain arrangement, will play an important role in thermal conductivity enhancement.
In addition, the elastic modulus and viscosity of the polymer were measured to investigate the correlation between the measured thermal conductivity and ionization-induced chain elongation. Modulus of elasticity and viscosity of PMAA and PAA films coated with polymer solutions of different pH depending on tacticity. As shown in Figure 22a, s-PMAA shows an increase in elastic modulus as the pH increases, such as a tendency for thermal conductivity.
However, in a-PMAA and i-PMAA, there was a negligible enhancement of the elastic modulus as polymer ionization increased. Due to this conformation, elastic modulus and thermal conductivity show great improvement at higher pH. In a-PMAA and i-PMAA, these obstructing effects were dominant, leading to a negligible increase in elastic modulus.
However, despite the chain elongation evidenced by elastic modulus on both tactics, thermal conductivity improvement of i-PAA is negligible. We assume that the discrepancy between elastic modulus and thermal conductivity of i-PAA generates from the relatively large torsion between the i-PAA polymer chain inhibits the heat propagation, thus reducing the improvement of thermal conductivity.
Thermal annealed shellac thermal conductivity
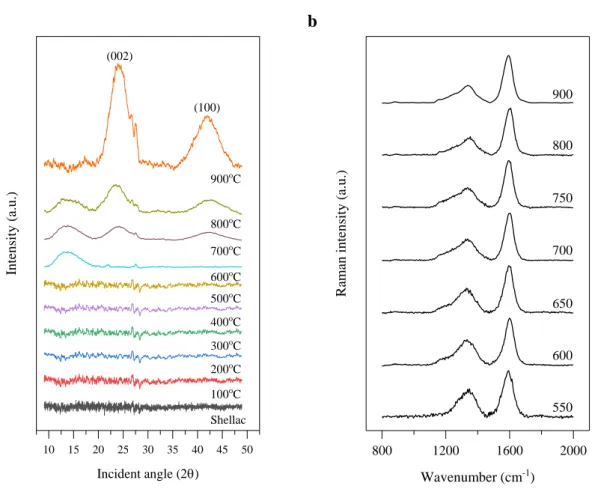
We hypothesize that the inconsistency between the elastic modulus and thermal conductivity of i-PAA, which is caused by the relatively large torsion between the i-PAA polymer chain, inhibits heat propagation, therefore reducing the increase in thermal conductivity. a) X-ray diffraction spectra for TRGO films at different temperatures. In order to determine the cause of the increase in thermal conductivity between the planes due to the increase in the thermal reduction temperature, we investigate the structural properties of the annealed sample using XRD and Raman spectroscopy. In addition, when the thermal reduction was gradually increased to 900℃, we observed that the GO peak intensity decreased and finally disappeared at 900℃, showing the development of reduced graphene oxide peak at 25°.
As a result, the higher the annealing temperature above a certain level, the higher the RGO characteristic. As the annealing temperature rises to 900℃, the intensity of the D band decreased, and the G band showed development. From the above analysis, the development of RGO and improvement in crystallinity was observed as the thermal reduction temperature increased, which seems to be a factor in the increase of thermal conductivity between the planes.
Conclusion
The crystallization on shellac was started when we annealed above at a temperature higher than 600◦C. As the annealing temperature increases, the cross-plane thermal conductivity of polymer film increases, and thermal reduction of shell films at temperature 900◦C shows a significant increase in cross-plane thermal conductivity (ƙmax = 1.22 W/m∙K), which is six times higher than natural polymer. As the annealing temperature increases, ID/IG ratio is decreased, which indicates that the graphene property increases and the crystallinity of polymer film also increases.
The improvement in thermal conductivity in this study was attributed to heat reduction at high annealing temperature, which leads to the crystallization and with the characteristic of graphene, which also reduces interlayer space facilitating thermal transport.
F; Salagnac, P.; Glouannec, P., Enhancement of thermal conductivity of electrically insulating syndiotactic poly(styrene) matrix for two-phase conductive polymer composites. R; Lee, J.; Park, Y.-B.; Kim, G.-H., Direct growth of thermally reduced graphene oxide on carbon fiber for improved mechanical strength. Pierre, T., Determination of the tacticity and analysis of the pH titration of poly(acrylic acid) by proton and carbon-13 NMR.
32] Ishitake, K.; Satoh, K.; Kamigaito, M.; Okamoto, Y., Stereospecific free radicals and RAFT polymerization of bulky silyl methacrylates for tacticity and molecular weight controlled poly(methacrylic acid). 33] Couvreur, L.; Lefay, C.; Belleney, J.; Charleux, B.; Guerret, O.; Magnet, S., First nitroxide-mediated controlled free radical polymerization of acrylic acid.
Acknowledgements