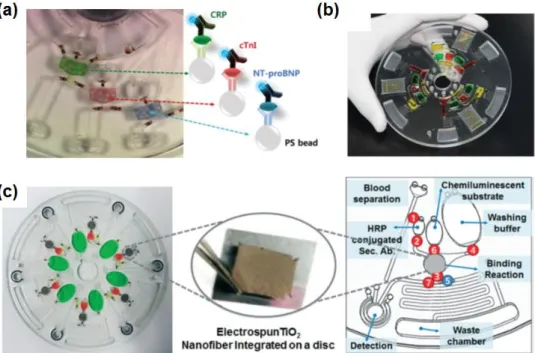
The spinning speed was obtained from the encoder connected to the servo motor of the spinning device. One disk is used per patient due to the limitation of blood sample volume.
Introduction
Liquid biopsy
- Circulating tumor DNA
- Extracellular vesicles
- Clinical significances
Perhaps the combinatorial analysis of ctDNA and EVs has widely reported the detection of EGFR mutations from NSCLC patients due to the FDA-approved cobas® EGFR v2 mutation test. This study also further reported that low mutation allele frequency (MAF) in exoNA is an independent prognostic factor for longer survival.
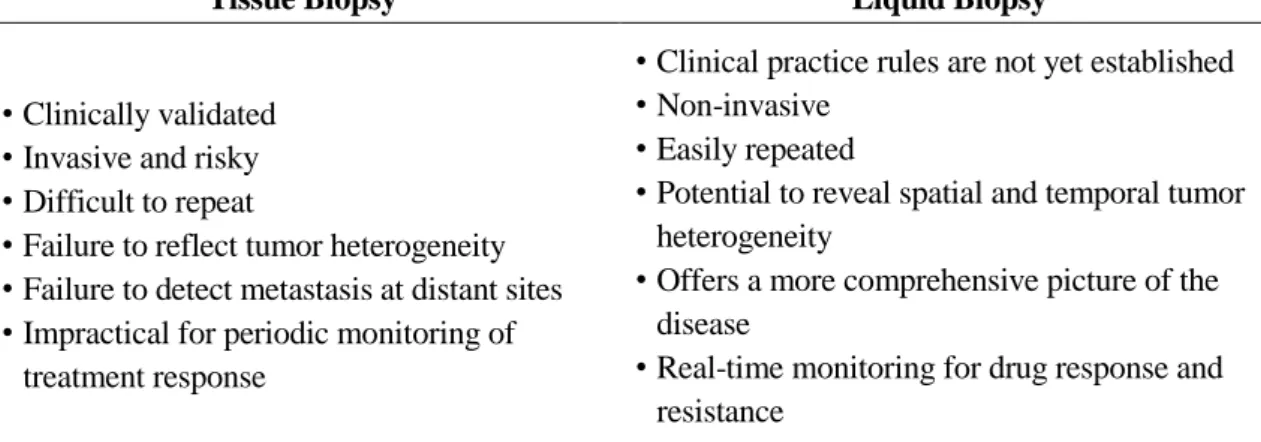
Lab-on-a-chip
The pressure-driven system, on the other hand, can complete complex analyzes on a single device, meaning it has been widely used by many people working in the microfluidic research field. Therefore, in the matter of integration and automation, centrifugal microfluidic systems, also called lab-on-a-disc (lab-disc), have arisen.
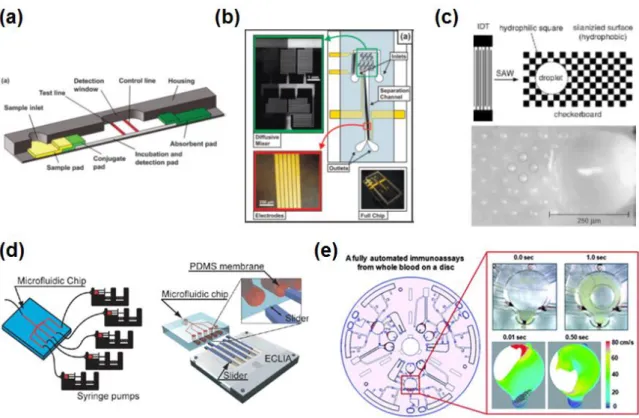
Lab-on-a-disc
- General physics
- Microfluidic functions
- Applications
The average flow rate (U) in a microchannel can be derived from the parameters; L is the length of the fluid in the microchannel, 𝑟̅ is the average distance of the fluid in the channels from the center of the disk. Although the volume of the dosing chamber must be determined in advance, accurate dosing can still be achieved.
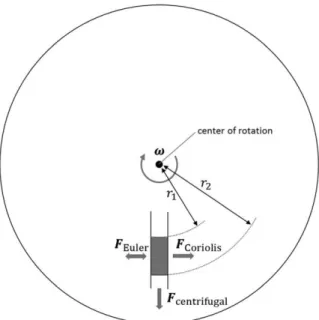
Sample preparations on microfluidics for liquid biopsy
- cell-free DNA purification on microfluidics
- EV isolation on microfluidics
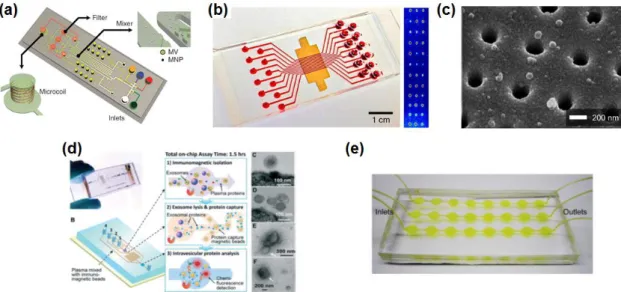
-EV isolation based on a microfluidic device. a) Magnified size of magnetic bead-decorated microvesicle can be isolated by size-shift-based filtration prior to on-chip NMR detection,14 (b-c) immunoaffinity-based EV isolation directly on the SPR substrate.15-. Therefore, many studies have focused on EV isolation based on size variation in microfluidics (Figure Woo et al.
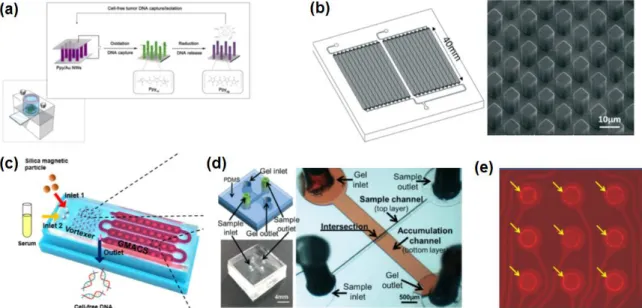
Research motivations
Recent trends in isolation technology for circulating cell-free DNA and extracellular vesicles in microfluidics for liquid biopsies are reviewed in this section. Along with the rapidly increasing clinical importance of liquid biopsy, the microfluidic approach is expected to play an increasingly important role in this field.
Research aims
Fully automated, on-site isolation of cfDNA from whole blood on a disc
Abstract
Introduction
Furthermore, the major challenges in the purification of ctDNA include the fragile nature of the relatively short fragmented DNA, in addition to the intrinsic rarity of tumor-derived DNA in the peripheral blood. However, the lack of efficient and automated cfDNA purification methods that can be readily applied in small community clinics has resulted in a significant bottleneck for broader adoption of the technology. Thus, to maximize the potential use of cfDNA for personalized cancer therapy and early cancer diagnosis, it is important to perform cfDNA purification in local hospitals once the blood samples are collected.
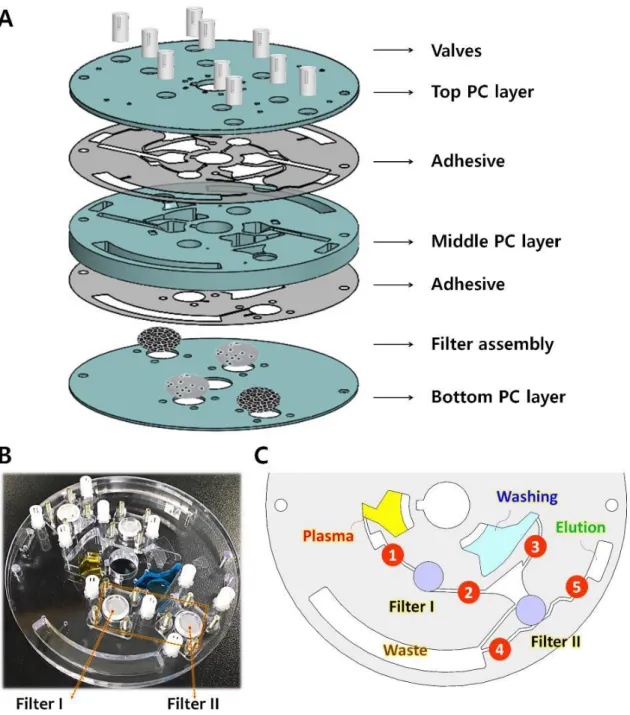
Several previous studies have reported microfluidic-based DNA purification.120-121 For example, solid-phase extraction on a microfluidic chip has been developed to purify DNA from whole blood using silica-coated magnetic beads. .122-124 In addition, microfluidic centrifugal devices for DNA isolation from bacteria and viruses were developed integrating protocols based on magnetic beads.124-126 However, although these methods offer a level of benefit in terms of simple steps of operation, cost and time, they are not directly applicable for ctDNA purification due to the lack of fully integrated plasma separation steps and the ability to process large volumes (>3 mL) of peripheral blood, which is essential due to the extreme rarity of ctDNA. Current isolation protocols require complex, time-consuming and manual procedures, including blood sample collection, delivery to central laboratories, plasma separation by centrifugation, and cfDNA purification, as schematically illustrated in Figure 2.1. The newly developed, reversible, electromagnetically actuated diaphragm valves integrated into a disc enable the entire cfDNA purification process including separation of plasma from peripheral blood, lysis of residual proteins, DNA binding, and elution using a custom designed centrifuge system. .
As a proof-of-concept study, we demonstrated the utility of the proposed lab-on-disk for EGFR mutation monitoring from isolated cfDNA during the course of EGFR-TKI therapy of NSCLC patients.
A fully automated cfDNA isolation on a disc
- Experimental details
- Result and discussion
- Conclusion
The surface of the PC board activated by oxygen plasma was treated with 1% APTES solution for 20 minutes. Valve #3 is opened and the binding buffer stored in chamber 4 is added to the lysate to increase the absorption power of the nucleic acids on the surface of the silica beads. We conclude that the reversible nature of ID valves is essential to achieve efficient binding of cfDNA from a large volume (3.7 ml) plasma lysate solution.
Solid yellow arrows indicate fluid flow and dashed yellow arrows indicate disk mixing mode. It is noteworthy that the procedure time used for the disc was shorter and the same amount of reagents were used for the disc and manual operation. The level of EGFR L858R in laboratory-purified cfDNA on a 3 mL whole blood disk correlated well with disease progression from the initial blood test point to the third sample collection on day 76 (D+76).
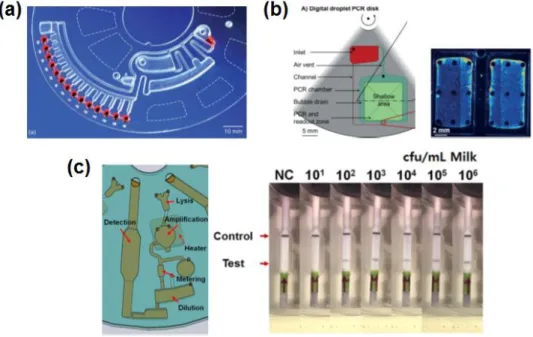
One disc is used per patient due to blood sample volume limitation.. dimensional histograms of the ddPCR assay.
Fully automated, label-free isolation of EVs from whole blood on a disc
Abstract
Introduction
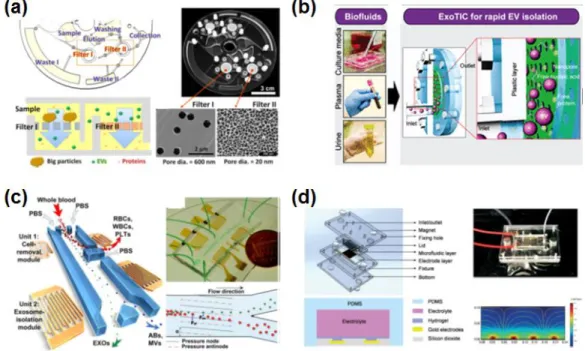
Furthermore, the rather high viscosity and density of plasma samples tend to hinder otherwise rapid and efficient isolation protocols. Therefore, there is a critical and urgent need for a reliable method to isolate EVs from blood samples. To achieve robust and efficient isolation of intact EVs, a fully integrated centrifugal microfluidic device, called Exodisc, has previously been shown to enrich EVs from urine and cell culture supernatant within 30 minutes.28 Exodisc can isolate EVs at relatively low g-force values (less than 500 g) through sequential and tangential flow filtration processes using two nanofilters with pore diameters of 600 nm and 20 nm, positioned so that the flow direction through the membrane remains perpendicular to that of the centrifugal pump force.
The results of subsequent quantitative analyses, including nanoparticle tracking assay (NTA), enzyme-linked immunosorbent assay (ELISA), and real-time polymerase chain reaction (PCR) of mRNA, confirmed that a process involving the Exodisc results in the concentration and isolated purity. EVs that are significantly higher than those of the UC process.28. In this study, Exodisc-B and Exodisc-P were developed - which are optimized for EV isolation from blood and plasma samples, respectively. This study shows that the use of Exodisc-B/P can result in unbiased, intact EVs with higher yield and purity and higher mRNA and protein content than the UC process.
The proof of concept from a longitudinal study conducted on small live animals clearly demonstrated the unique and important advantage of using Exodisc-B/P, which can robustly isolate EVs directly from a small volume of blood/plasma.
A fully automated plasma-driven EV isolation on a disc
- Experimental details
- Result and discussion
- Conclusion
Nanoparticle Tracking Analysis (NTA) . EV concentration and size distribution were measured using a Nanosight NS500 NTA system; Malvern Instruments, Malvern, UK). A fully automated procedure for the isolation of EVs from whole blood samples using the Exodisc-B and a bench-top spinner is shown in Figure 3.5, together with the working principle of sequential, tangential flow filtration on a spinning disc. After filtration, the EVs are washed (Figure 3.3D) and the solution at the bottom of the membrane is removed (Figure 3.3E).
The EVs were then isolated using Exodiscs (D20 and D100) and the UC process compared in terms of particle number, capture efficiency, protein content, purity, process time and particle size distribution (Figure 3.7A and 3.7C). The SEM images of 20 nm and 100 nm filters after plasma filtration at D20 and D100, respectively (Figure 3.7A) clearly show that a significant amount of plasma protein contaminants remained on the 20 nm filter along with the EVs. Interestingly, the nanoparticle tracking analysis (NTA) data showed no significant difference in the particle size distributions of the EVs enriched by two different conditions of Exodisc operation (Figure 3.7C and Figure 3.10).
When the diluted plasma samples were used, the capture efficiency did not change, while the filtration time was increased (Figure 3.13). The temporal changes in the expression levels of CD9 and CD81 along with those of other cancer markers, including PSMA, EGFR1 and HSP90, were analyzed from the lysates of the EVs isolated from individual tumor-bearing and control mice via direct ELISA (Figure 3.14 E-I). No correlation was observed between the EV marker expression levels and the preoperative plasma PSA levels of PCa patients (Figure 3.19).
General conclusions and plans
General conclusions
Second, a fully automated, label-free EV isolation on a disk from whole blood was demonstrated in a point-of-care manner. This proposed device, also called 'Exodisc-B', provides simple, fast and efficient EV isolation to obtain intact EVs with high reproducibility even from small amounts of whole blood (<30 µL). We confirmed that Exodisc-B/P showed significantly better ability for EV isolation than the gold standard method (UC) evaluated by measuring the number of particles (NTA), exosomal protein expression (ELISA) and mRNA expression (RT-qPCR).
As a proof of concept study, the temporal changes of tumor-associated protein markers in EVs, as well as exosomal markers, isolated from living tumor-bearing mice could be monitored over a period of 13 weeks due to the fact that the proposed EV isolation system can isolate EVs from minimal amounts of plasma samples with the least painful protocol. The results describe a significant increase in cancer-associated markers (PSMA, EGFR and HSP90) two weeks after inoculation of tumor cells in the mouse model. These results clearly demonstrate that the proposed EV isolation system is able to isolate EVs with high yields and purity, which facilitates the analysis of cancer-associated markers in EVs isolated from the blood of cancer patients.
In conclusion, we believe that the developed cfDNA and EV isolation system in this thesis, with their proof-of-concept clinical studies, provide a useful tool for liquid biopsy in clinical practice.
Plans for the future
- Fully automated lab-on-a-disc for label-free enrichment of highly pure platelets from whole
- Pure extracellular vesicle preparation on a disc
- Simultaneous isolation of CTCs and EVs from whole blood on a disc
L.; Gibbs, P., et al., Detection of circulating tumor DNA in early and late stage human malignancies. D.; Murphy, D.; Parpart-Li, S.; Riley, D.; Nesselbush, M.; Sengamalay, N., et al., Direct detection of early-stage cancer using circulating tumor DNA. S.; Sandler, A.; Ballinger, M., et al., Blood-based tumor mutational burden as a predictor of clinical benefit in atezolizumab-treated non-small cell lung cancer patients.
D.; Sherman, S.; House, M.; Korc, M., A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. H.; Danesi, R., The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in patients with metastatic prostate cancer. Demirci, U.; Wang, S., An integrated dual-filtration microfluidic device for isolation, enrichment and quantification of extracellular urinary vesicles for bladder cancer detection. 100) Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A., Integrating liquid biopsies into cancer treatment. D.; Murphy, D.; Parpart-Li, S.; Riley, D.; Nesselbush, M.; Sengamalay, N., et al., Direct detection of early-stage cancers using circulating tumor DNA.
S.; Ratcliffe, M.; Reck, M., A guide to detecting epidermal growth factor receptor (EGFR) mutations in the ctDNA of patients with advanced non-small cell lung cancer. M.; Carracedo, A., Vesicle-MaNiA: extracellular vesicles in liquid biopsy and cancer.