In relation to the increase in energy demand and environmental concerns, the development of renewable energy with low greenhouse gas emissions has led. Effective and technological developments to the practical application of renewable energy are derived from a deep understanding of reactions related to renewable energy and environment. In this thesis we have described the theoretical research on the reaction mechanism related to renewable energy system.
In Chapter 1, we briefly introduce the renewable energy systems and the mechanistic studies for an in-depth understanding of chemical engineering. In Chapter 4, we theoretically proposed the feasibility of a universal synthesis of the metal sulfide electrolyte that can improve the performance of all solid-state batteries developed for effective renewable energy storage and a smaller environmental footprint.
Introduction
General introduction to renewable energy and the environment
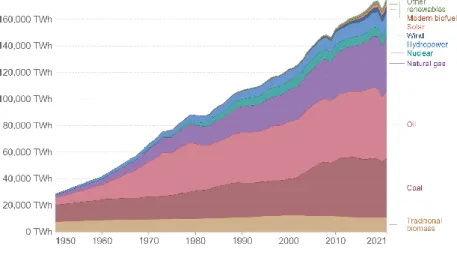
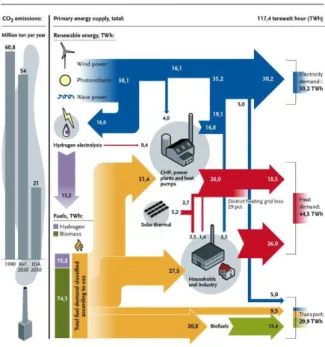
Renewable energy sources have significant potential to provide energy with almost zero greenhouse gas emissions, diversify the energy supply and reduce dependence on fossil fuels (Figure 1.2).5,6 Many problems, such as high financial risks, legal regulation, low availability of renewable resources, and low power quality, persists even though significant research is being done to develop renewable technologies, such as technologies for producing desired products while reducing carbon footprints, methods of synthesizing industrial chemicals while minimizing CO2 formation using biomass , and technologies for carbon capture and utilization (Figure 1.3).7−10 To overcome these challenges, in-depth knowledge of environmental and renewable energy-related reactions is required, and new materials or strategies for increasing efficiency should be based on this knowledge is designed.
Mechanistic study: an in-depth understanding of chemical reactions
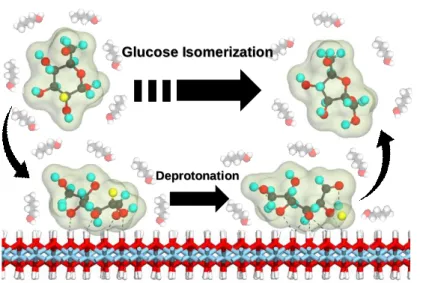
- Mechanistic study on effective biomass conversion
- Mechanistic study on effective rechargeable lithium-ion batteries
- Mechanistic study on the effective mineral trapping of CO 2
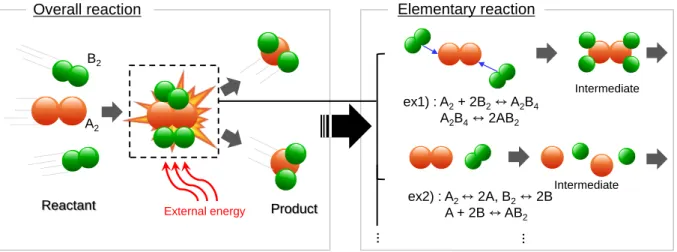
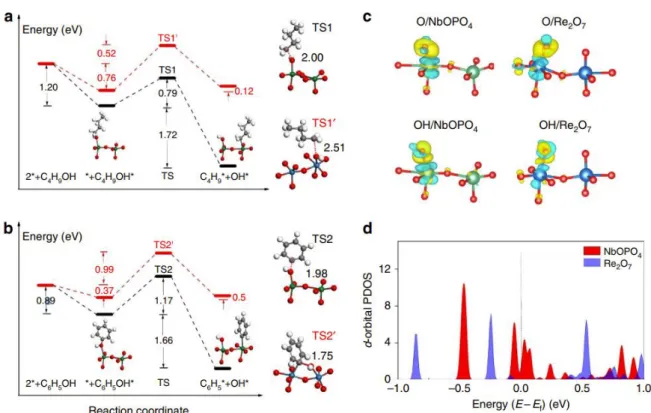
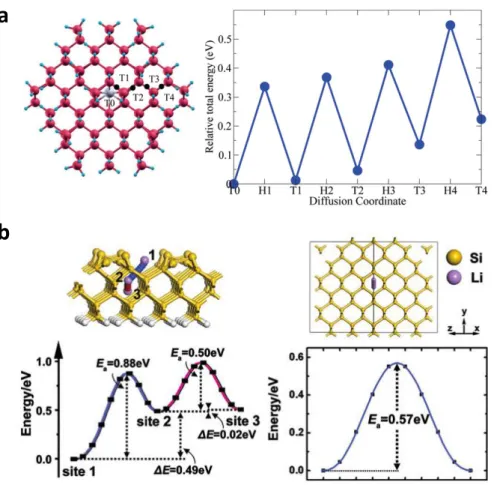
The determination of the reaction rate and activation energy by this theoretical approach is supported by the molecular simulation method, which can also identify the exact reaction path and feasibility through this knowledge. It was easy to use molecular simulations to identify the RDS and to evaluate the performance of the heterogeneous catalysts. The black dots in the left figure indicate the hexagonal locations, which are saddle points of the diffusion path from T0 to T4.
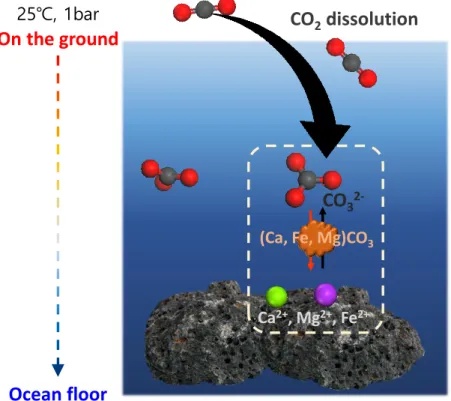
Close packing of G quartets in the vertical direction results in the formation of one-dimensional central channels that allow direct Li+ conduction pathways to the Li+ -centered G quadruple. Considering that the dissolution of metal cations from the surface of silicate minerals has been found to be the rate-limiting step in the mineralization reaction with wet scCO2,38 the adsorption characteristics of water and CO2 molecules on the mineral surface have been addressed.39 Deep understanding of the mineral carbonation reaction mechanism will provide a renewable energy source and a solution to the air pollution problem because it will result in the rapid annihilation of CO2.
Highly Effective and Selective Catalytic System for the Biomass Production
- Highly Efficient Hydrotalcite/1-Butanol Catalytic System for the Production of the High-
- Introduction
- Computational Models and Methods
- Results and Discussion
- Conclusion
- References
- A Robust and Highly Selective Catalytic System of Copper–Silica Nanocomposite and 1-
- Introduction
- Computational Models and Methods
- Result and Discussion
- Conclusion
- References
- Introduction
- Computational Models and Methods
- Result and Discussion
- Conclusion
- References
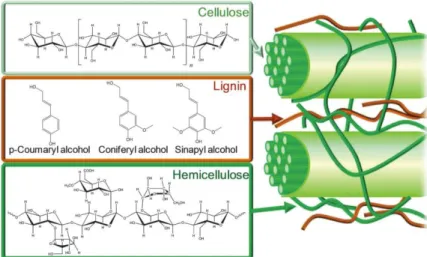
For the fructose molecule, the carbon, hydrogen, and oxygen atoms are colored in gray, white, and red, respectively. For a clear view, the atoms that make up the lignin bond of the dilignol molecule are colored green with notations to indicate the bonding atoms. For a clear view of the reaction mechanism, the reactant molecules in each hot break are represented using a ball-and-stick model, while the others are shown by a stick model.
Empty arrows indicate sequential procedures, and filled arrows indicate the reactant and product of the reaction. The atoms containing lignin bond of the dilignol molecule are colored green and transferring H atoms (or OH groups) are colored blue.
Universal Solution Synthesis of Sulfide Solid Electrolytes Using Alkahest for All-
- Introduction
- Computational Models and Methods
- Result and Discussion
- Conclusion
- Reference
The dissolved three Ge atoms are colored pink and the remaining Ge, C, S, N and H atoms are colored green, gray, yellow, blue and white, respectively. For clear viewing, the dissolved three Ge atoms are colored pink, and the remaining Ge, C, S, N, and H atoms are colored green, gray, yellow, blue, and white, respectively. For a clear picture, thiolate molecules and dissolved Li atom are represented using the ball-and-stick model, and atoms in the Li2S surface are represented by the stick model.
The dissolved Li atom is colored in blue, the remaining Li, C, S, and H atoms are colored in red, gray, yellow, and white, respectively. The dissolved Na atom is colored blue, the remaining Na, C, S, and H atoms are colored purple, gray, yellow, and white, respectively.
Universal Solution Synthesis of Sulfide Solid Electrolytes Using Alkahest for All-
Introduction
Computational Models and Methods
Result and Discussion
Conclusion
Summary and Future Perspectives
Summary
A deep understanding of chemical reactions is important in various research topics related to renewable energy and the environment. This dissertation proposes theoretical studies of the reaction mechanism in renewable energy and environmental applications using multiscale molecular simulations. In Chapter 1, a brief overview of the importance of chemical reactions in renewable energy and the environment was discussed.
We provided the in-depth understanding of chemical reaction and revealed the driving force of the chemical phenomena by investigating the reaction mechanism. In the mechanism, the formic acid provided hydrogen to facilitate the dissociation of the C−O bond of lignin. In short, the degradations of C-C and C-O bonds of lignin under the ethanol-FA mixing environment were more favorable than the degradations of the thermal and ethanol-assisted degradation reactions.
Thus, this result will provide new opportunities for the synthesis of chemistry-directed soft conductors for ASSBs that reduce the carbon footprint. We theoretically demonstrated the effect of intercalated water on the carbonation reaction of calcium hydroxide. In particular, the intercalated water molecules can lower the heat of reaction and the activation energy of the carbonization reaction, thus causing spontaneous carbonization.
This approach improves the fundamental understanding of the role of water in the carbonation reaction and provides a new strategy for CO2 storage using cementitious materials. Overall, we explored the mechanism of chemical reaction for renewable energy and environmental applications, which could propose the new protocol for biomass products or develop the materials that reduce the environmental footprint. From these studies we can provide the fundamental knowledge of the highly effective chemical reaction from a molecular point of view and propose a new strategy for designing new material or process that goes beyond our molecular understanding of the reaction.
Future Perspectives
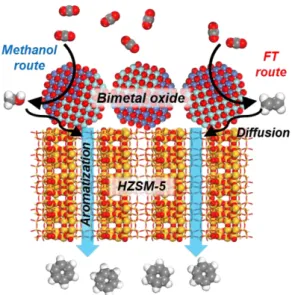
In order to solve the environmental and sustainable energy problems, the current trend is studying the conversion of CO2 through various strategies, such as photocatalytic, chemo-biocatalytic, chemoenzymatic and catalytic reaction.4−7 Of these methods we plan to convert CO2 through the most efficient and practical catalytic reaction. To design the optimal catalyst, it is first necessary to investigate which high-value chemical materials can be produced from CO2, and the reaction mechanisms for these products must be investigated. Jaramillo and colleague discovered a total of 16 different reaction products: several of them were new products identified and quantified for the first time based on a Cu metal catalyst surface.
Of the 16 reaction products detected, 12 of them were C2 or C3 species, consisting of a broad mixture of oxygenated species, including hydrocarbons, ketones, aldehydes, carboxylic acids, and alcohols.8 They also presented the mechanistic view of each product formed from CO2 . However, since the reaction of CO2 conversion is quite complex, it is difficult to obtain the desired products with simple heterogeneous catalyst. Tan's research team performed the catalytic conversion of CO2 to high-value-added hydrocarbons using a tandem catalyst composed of different types of catalytic sites.9 Using this strategy, we will also investigate the reaction mechanism and descriptors of tandem catalyst composed of zeolite and metal oxide.
Through these descriptors we want to develop the most optimal catalyst by screening different zeolite models and metal types. This future research direction for the development of a new optimal tandem catalyst is expected to be one of the most promising strategies in the conversion of CO2 into sustainable fuels and renewable energy.
જિન ચુલ કિમ†, જેઓંગ હ્યોન લી†, સેઉંગ હાક ઓહ, ક્યોંગમીન બેક, જોંગ-સાન ચાંગ*, અને સાંગ ક્યૂ ક્વાક*, "γ-એલ્યુમિના પર ટેટ્રાફ્લોરોમેથેનના હાઇડ્રોલિસિસનો મિકેનિસ્ટિક અભ્યાસ," સબમિટ કર્યું. વૂ ચેઓલ જીઓન†, જીઓંગ હ્યોન લી†, જિન ચુલ કિમ†, સાંગ-હ્યુન જંગ, સૂ ગ્યોંગ ચો અને સાંગ ક્યૂ ક્વાક*, "નેનોકોન્ટેનર્સ અને બાહ્ય આંચકામાં ફેરફાર કરીને નેનોબોમ્બનું નિયંત્રણક્ષમ વિસ્ફોટ". જેઓંગ હ્યોન લી†, જિન ચુલ કિમ†, વૂ ચેઓલ જીઓન†, સૂ ગ્યોંગ ચો* અને સાંગ ક્યૂ ક્વાક*.
Jeena†, Seongeon Jin†, Keunsoo Jeong, Yuri Cho, Jin Chul Kim, Jeong Hyeon Lee, Seokyoung Lee, Suk-Won Hwang, Sang Kyu Kwak*, Sehoon Kim* og Ja-Hyoung Ryu*,. Kyung Ho Cho, Ji Woong Yoon, Jeong Hyeon Lee, Jin Chul Kim, Kiwoong Kim, Jong-San Chang*, Sang Kyu Kwak*, U-Hwang Lee*, "Separation af ethan/ethylengasblanding ved ethan-selektiv CAU- 3-NDCA adsorbent”. Oh†, Sung-Ha Park†, Jeong Hyeon Lee, Jin Chul Kim, Jong Bum Lee, Hyeong Ju Eun, Yun-Sang Lee, Bo Eun Seo, Woojin Yoon, Hoseop Yun, Sang Kyu Kwak*, O-Pil Kwon* og Jong H.
Ju Hyun Park, Sun Ha Kim, Jin Chul Kim, Byoung-Young Choi, Sang Kyu Kwak, O.H. Xiaobo Shang†, Inho Song†, Jeong Hyeon Lee, Wanuk Choi, Jaeyong Ahn, Hiroyoshi Ohtsu, Jin Chul Kim, Jin Young Koo, Sang Kyu Kwak* in Joon Hak Oh*, »Surface-Doped Quasi-2D Chiral Organic Single Crystals za kiroptično zaznavanje. Kyung Ho Cho, Ji Woong Yoon, Jeong Hyeon Lee, Jin Chul Kim, Kiwoon Kim, U-Hwang Lee, Sang Kyu Kwak* in Jong-San Chang*, »Učinek togosti ogrodja v kovinsko-organskih ogrodjih za adsorptivno ločevanje etan/ethylene «.
Xiaobo Shang†, Inho Song†, Jeong Hyeon Lee, Myeonggeun Han, Jin Chul Kim, Hiroyoshi Ohtsu, Jaeyong Ahn, Sang Kyu Kwak* and Joon Hak Oh*, “Tuning of supramolecular chirality and optoelectronic performance of chiral perylene diimide nanowires via N -substituted side chain technique”. Asim Riaz, Deepak Verma, Hassan Zeb, Jeong Hyeon Lee, Jin Chul Kim, Sang Kyu Kwak* and Jaehoon Kim*, “Solfothermal liquefaction of alkali lignin to obtain high yield of aromatic monomers while suppressing solvent consumption” . Soochan Lee, Jeong Hyeon Lee, Jin Chul Kim, Sungmin Lee, Sang Kyu Kwak* and Wonyoung Choe*, “Porous Zr6L3 Metallocation with Synergistic Binding Centers for CO2”.