In particular, carbon fiber reinforced composites are considered as one of the excellent multifunctional materials that can offer structural and electrochemical energy storage functions. Therefore, tin oxide nanorods were synthesized on woven carbon fiber (WCF) surface using two-step hydrothermal methods as a kind of whiskerization treatment. Therefore, the mechanical and electrochemical performances of tin oxide nanostructured carbon fiber composites were investigated.
In this thesis, the mechanical and electrochemical energy storage performances of tin oxide nanorods on carbon fiber surface were characterized. The growth of SnO2 was improved mechanical and electrochemical energy storage performances than conventional carbon fiber composites.
Carbon fiber reinforced composites
Tin oxide nanomaterials as secondary reinforcement
Structural composite capacitor as multifunctional material
Synthesizing tin oxide nanostructures on the carbon fiber surface would be suitable candidate to increase the surface area and energy storage capability (high theoretical capacitance). In addition, the tin oxide nanostructures are evaluated as secondary interphase reinforcements in CFRP composite by characterizing the structural and energy storage performances. In the work reported here, tin oxide nanorods grown carbon fiber composite has been systematically investigated.
Similarly, tin oxide can be synthesized for whiskers on the carbon fiber surface via the hydrothermal method. Tin oxide nanostructures were synthesized on the surface of carbon fibers as whiskers, in addition, this material is often used in energy storage applications. SnO OHSn OOH (3.4) Sodium stannate trihydrate, sodium hydroxide and ethanol (Samcheon Pure Chemical) were.
For sample 1, there was no growth of tin oxide nanorods due to insufficient heating temperature and time. Mechanical properties of tin oxide nanorod-WCF composites as a function of SnO2 molar concentration [95]. The electrochemical performance of SnO2 NRs on carbon fibers was demonstrated at a scan rate of 0.1 V/s over a potential range of 0 to 1 V in terms of tin oxide growth concentration, including bare carbon fibers (Figure 4-3).
The obtained current densities were the highest in bare carbon fiber (0 mM) composite due to the light weight of the two electrodes. Even the content of tin oxide on carbon fiber surface, electrochemical performance of structural capacitors is not increased. The weight of the tin oxide samples was measured and the density of resin, carbon fiber and SnO2 was used for the calculation of volume fractions.
From equation (2.2), the energy storage performances are mainly influenced by the specific electrode surface area and the distance between two electrodes. The internal resistance of the capacitors would be increased due to the resistance of the grown tin oxide. In this thesis, tin oxide nanorods on carbon fiber surfaces were focused and investigated to highlight mechanical and electrochemical energy storage properties.
Both improved mechanical properties and energy storage performances were achieved by tin oxide nanorods on CF surfaces.

Research motivations and objectives
Outline of thesis work
In addition, a multifunctional structural composite was fabricated with SnO2-CF as electrodes and demonstrated electrochemical energy storage capability by CV, GV and EIS measurements. In addition, the principles of the energy storage mechanisms of capacitors are explained with electrical double layer models. The surface of the SnO2-CF electrodes is analyzed by the BET method to observe the improvement of the carbon fiber electrode surface.
The electrochemical energy storage performance of structural capacitors is demonstrated through CV, GV and EIS testing. Furthermore, the energy storage performance is compared with other reported results of carbon-based structural capacitors in the Ragone plot.
Carbon fiber reinforced composites
In addition, the overall fiber properties are not fully realized due to the presence of debonding at the fiber-matrix interface, as shown in Figure 2-3 [61]. The surface of the fiber may not burn evenly, resulting in a highly reactive surface area. Chemical reactions increase the surface roughness and wettability of the fibers, therefore the interlaminar shear strength of the composite and the mechanical interlocking at the fiber-matrix interface are improved.
Although some improvements are achieved by oxidation, it also damages the surface of the fiber and weakens the tensile strength of the carbon fiber [62]. The low temperature does not affect the elastic properties of the fibers and creates a strong interface.
Multifunctional materials
The synthesized ZnO nanowire showed a greatly increased surface area and showed a 320% increase in interfacial shear strength than the carbon fiber/epoxy interface. CuO nanowires on the surface of carbon fibers strengthened the interfacial interactions and thus improved the surface and mechanical properties of the composite. The synthesis of tin oxide nanorods by the hydrothermal method has been reported [21, 75], but only a few studies have been reported on the type of whiskers on the surface of carbon fibers for composite reinforcements.
The structural capacitors using carbon-fiber/epoxy-matrix composite were fabricated with 1.2 μF/m2 capacitance at 2MHz [27]. In this research, the structural capacitor is focused by lightening carbon fiber reinforced polymer composite.
Working principles of capacitors
The Helmholtz, Gouy-Chapman and Stern models are shown in Figure 2-5, where Ψ is the potential, Ψ0 is the electrode potential, IHP and OHP are the inner and outer Helmholtz planes in the Stern model [83]. The concept of a double layer was first described by von Helmholtz, which is the simplest theory for demonstrating the spatial charge distribution at the interface. The charge of the electrode is balanced by opposing ions at a distance (d) from the surface to the center of the ions.
This model fails for a highly charged double layer whose charged ions are closely spaced on the electrode surface [85]. This model showed that the double layer capacitance is also influenced by the surface properties of the electrode [86]. Two different types of energy storage mechanisms can be classified into electrochemical double layer capacitance (EDLC) and pseudocapacitance.
When an external voltage is applied between electrodes, in order to maintain a neutral system, ions in the electrolyte are absorbed on the surface of the oppositely charged electrode, which is why the double layer structure appears at both electrodes. E+ is the positive electrode, A- is the anion and // is the interface between electrode and electrolyte. In the double layer region, the capacitance and resistance are held at the same voltage, so that the circuit presents a parallel relationship between Cdl and Rp.
The redox reaction is slower than the EDLC process due to the impedance of the reaction [92]. Cφ is the pseudocapacitance, RF is the electrode-to-electrolyte resistance, and RD is the Faradic resistance that can act during discharge when the ions desorb. The pseudocapacitance is larger than the double layer capacitance at certain potentials and the parallel combination of this pseudocapacitance helps the capacitance development [94].
![Figure 2-5. Electric double layer models, (a) Helmholtz model, (b) Gouy–Chapman model, and (c) Stern model [83]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/31.892.136.762.121.620/figure-electric-double-layer-models-helmholtz-chapman-stern.webp)
Materials and reagents
Experiments
Preparation of SnO 2 NRs on WCF
Fabrication of SnO 2 -WCF composites
80 mm x 80 mm square size of CFs were immersed in ethanol for 1 min and dried in oven at 100 ℃ for 10 min to remove the impurities from the surface of carbon fiber. These clean CFs are dipped in the prepared seed solution for 10 minutes and then annealed in an oven (Thermo Fisher Scientific Inc., Massachusetts, USA) to improve adhesion between carbon fiber surface and tin oxide nanostructures. Next, the seeded CFs were dipped in prepared growth solution and kept in a stainless steel teflon autoclave heated at 220℃ for 24 hours in oven.
After the hydrothermal process, the SnO2-CF sample was cleaned with distilled water to stop the further growth of SnO2. Samples of different molar growth concentrations were prepared to observe the effects of SnO2 NRs on the surface morphology and mechanical properties.
![Figure 3-1. Schematics of SnO 2 nanorods grown woven carbon fiber composites [95]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/38.892.137.780.123.535/figure-schematics-nanorods-grown-woven-carbon-fiber-composites.webp)
Experimental results and discussions
- Morphology analysis
- X-ray diffraction and XPS studies
- Tensile tests
- Impact tests
SnO2 NRs were considered as secondary reinforcements at interphase area which is between matrix and carbon fiber surface. The bare carbon fiber composite (SnO2 = 0 mM) shows the lowest strength and modulus values, namely, it has the lowest interactions between matrix and fiber surface. In Figure 3-6 (a) the % increments of the ultimate tensile strength and elastic modulus are calculated compared to the bare carbon fiber composite.
In other words, the as-grown tin oxide nanorods enhanced the interphase interactions between matrix and fiber surface as secondary reinforcements. SnO2 nanorods on this work improved the in-plane shear strength of the hybrid SnO2-CF composite. The SnO2 nanorods network facilitated the charge transfer through strong interactions of ionic and polar behavior of tin oxide nanorods with surface functional groups (hydroxyl, carboxyl and carbonyl).
For the characterization of the impact energy absorption of the SnO2-CF polymer composite, an instrumented drop-down impact tester was used. The measured impact energy absorption results for bare carbon fiber and SnO2-CF resin composite are shown in Figures 3-7 [95]. The increased energy absorption was shown to increase the content of SnO2 NRs on the carbon fiber surface due to the enhanced interphase interactions in the composite.
The strong ionic bonds of tin oxide nanostructures with surface functional groups of carbon materials have been reported. Similarly, carbon fiber functional groups are carboxyl, hydroxyl and carbonyl, and therefore the Sn4+ ions in the SnO2 surface interact with the hydroxyl and carboxyl functional groups on the carbon fiber surface. Thus, the combined effects of these different parameters improved the overall energy absorption of the composite.
![Table 3-1. Experimental conditions and results for tin oxide nanorods [95]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/41.892.112.787.158.497/table-experimental-conditions-results-tin-oxide-nanorods-95.webp)
Materials and reagents
Experiments
Solid-state capacitor assembly
The prepared resin was injected into the system through the inlet pipe and then the chamber was kept under vacuum for 48 hours in order to ensure sufficient curing time. Functionalized resin, in other words, solid electrolyte was prepared by mixing ionic liquid (20 %), lithium salt (10 %) and polymer resin (70). The lithium salt was added to the ionic liquid and stirred continuously until completely dissolved.
This mixture of lithium salt and ionic liquid was thoroughly mixed with the polymer resin until a homogeneous matrix was established. After arranging the uniformly mixed matrix, it was injected into the VARTM system and acted as a solid electrolyte. Ionic liquids are widely used in conventional supercapacitors due to their large operating voltage window, high conductivity, and superior thermal stability [109].
The presence of lithium salt in the ionic liquid prevents the phase separation of solid electrolyte and provides homogeneous monolithic matrix. The electrochemical performance of capacitor is improved as the content of the ionic liquid increases; however, the mechanical properties of composite were reduced [110]. Therefore, the amount of polymer resin was controlled at 70% to minimize the deterioration of structural role.

Electrochemical characterization
Experimental results and discussions
BET and morphology analysis
Cyclic voltammetry for SnO 2 -WCF electrodes (three-electrode system)
Y et al., Tensile properties of polypropylene composites reinforced with short glass fibers and short carbon fibers, Composites, Part A.
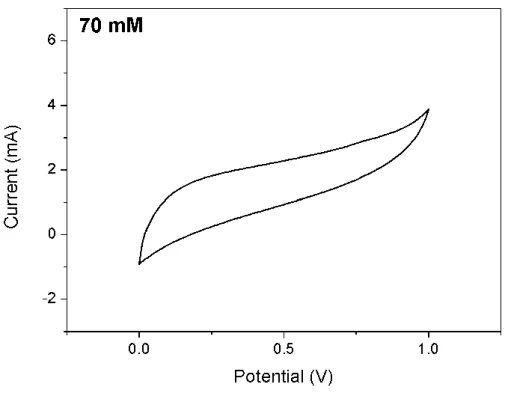
Electrochemical characterization for SnO 2 -WCF composite (two-electrode system)
![Figure 2-2. Basic weave types [4]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/21.892.129.763.152.614/figure-2-2-basic-weave-types-4.webp)
![Figure 2-3. Debonding of the fibers from the matrix showing the low adhesion of fibers to matrix that reduces the strength of the composite [61]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/23.892.130.767.128.635/figure-debonding-fibers-showing-adhesion-reduces-strength-composite.webp)
![Figure 2-4. Change of carbon fiber tensile strength after synthesis of CNTs through CVD [50]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/26.892.129.717.145.575/figure-change-carbon-fiber-tensile-strength-synthesis-cnts.webp)
![Figure 2-6. Ragone plot for various electrochemical energy storage systems [87]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/33.892.130.769.127.552/figure-ragone-plot-various-electrochemical-energy-storage-systems.webp)
![Figure 2-7. Equivalent electrical circuit of an EDLC cell [89]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/34.892.342.550.972.1056/figure-2-equivalent-electrical-circuit-edlc-cell-89.webp)
![Figure 2-8. Equivalent electrical circuit of a Pseudocapacitor [94]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10529367.0/35.892.316.578.946.1044/figure-2-equivalent-electrical-circuit-of-pseudocapacitor-94.webp)