In-situ Raman spectra were collected for zirconia and it represents that the zirconia phase could be different due to the DH concentration. However, the management of zirconium fuel cladding corrosion is also a key issue to guarantee the safety of nuclear power plants.
Introduction
Background
Therefore, it is important to determine the types of oxides (i.e. oxide phases) in the oxide layer formed on the zirconium alloy during the in situ oxidation process. With in situ Raman spectroscopy, it is possible to characterize the oxide phase and the oxide film on the metal surface, providing the oxidation mechanisms.
Goal and approach
Because it does not require removing the sample from the oxidation environment, which may affect the structure and oxide phase of the sample. Therefore, Raman spectroscopy and X-ray reflectivity (XRR) measurement methods were used in this thesis for in-situ zirconium oxide analysis, which can be used in high-pressure and high-temperature water environments.
![Figure 1-1 Schematic illustration of pressurized water reactors and nuclear fuel assembly [1]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10517165.0/19.892.128.751.397.800/figure-schematic-illustration-pressurized-water-reactors-nuclear-assembly.webp)
Basic theory and literature study
Characteristic of zirconium alloys and zirconium oxide
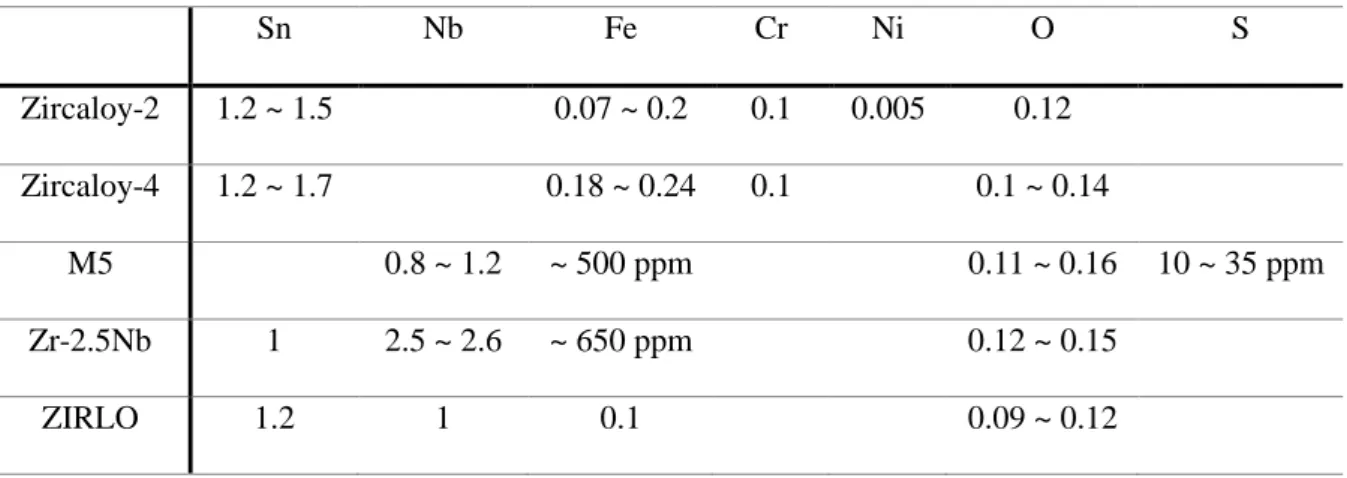
- Characteristic of zirconium alloys for nuclear industry
- Corrosion kinetics of zirconium oxide
The phase diagrams show that the tetragonal zirconia can be a stable phase for temperatures above 1150. However, the tetragonal zirconia can exist at low temperatures in the presence of high compressive stresses.
Primary water chemistry of pressurized water reactors
Raman spectroscopy and electrochemical impedance spectroscopy
The impedance response consists of the real part of impedance and imaginary part of impedance, Z = Zr + jZj where Zr represents the real part of impedance, and Zj is the imaginary part of impedance. From the EIS measurement, the thickness of oxide can be calculated using permittivity of oxide materials, and the capacitance of the oxide from Nyquist plot.
Scanning transmission X-ray microscopy
High resolution X-ray reflectivity
As a result, the quantity of structure factor CTR can be divided into two parts, Fsurf. The structure of the water/oxide interface on the subÅngström scale can be investigated by XRR measurement.
![Figure 2-10 The Bragg scattering geometry (A) X-ray path length for two parralel layers, (B) described by momentum transfer vector, Q [47]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10517165.0/44.892.173.725.287.946/figure-bragg-scattering-geometry-parralel-described-momentum-transfer.webp)
Methods
In-situ oxide investigation of zirconium alloy using Raman spectroscopy and EIS
- Introduction
- Materials
- Experimental
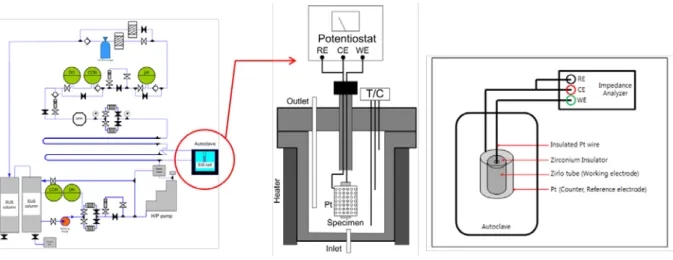
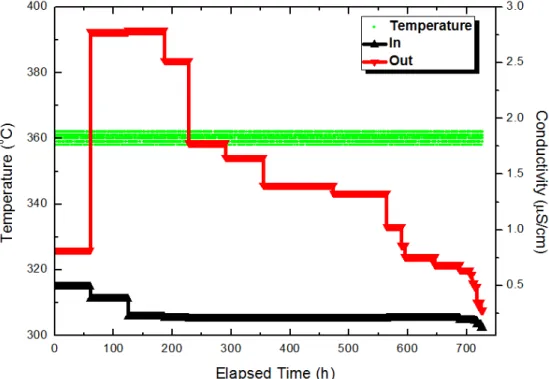
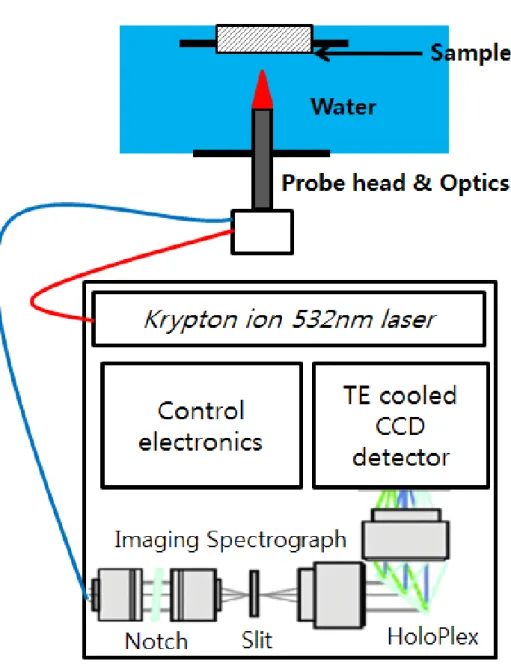
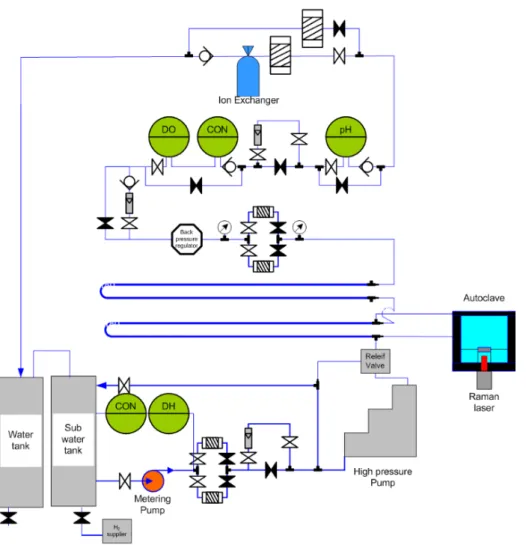
In this study, in situ Raman spectroscopy measurements were performed using a RamanRXNTM, manufactured by Kaiser Optical Systems, Inc., which uses a 532 nm wavelength krypton ion laser with a peak power of 100 mW, and the area of radiation on the specimen was 100 μm2. Detailed descriptions of the in situ Raman system are presented in the authors' previous studies [9,50]. In situ Raman spectroscopic measurements with corrosion tests were performed for the Zr-Nb-Sn alloy in a primary water of pressurized water reactors using an autoclave system, which was specially built for this study.
For each measurement, in situ Raman spectra were collected for 30 m and repeated twice to increase the signal-to-noise ratio. After each in situ Raman spectroscopy measurement, the Zr-Nb-Sn alloy specimen was removed from the autoclave for ex situ measurements. The ex situ investigation was carried out to characterize the oxide layer of the sample using JEOL JEM-2100F TEM coupled with energy dispersive X-ray spectroscopy.
In situ Raman spectroscopy and the TEM analysis results were compared for a comprehensive understanding of the effects of dissolved hydrogen on the zirconium oxide properties.
Investigation of electronic structure of zirconium oxide with scanning transmission X-ray
- Introduction
- Materials
- Experimental
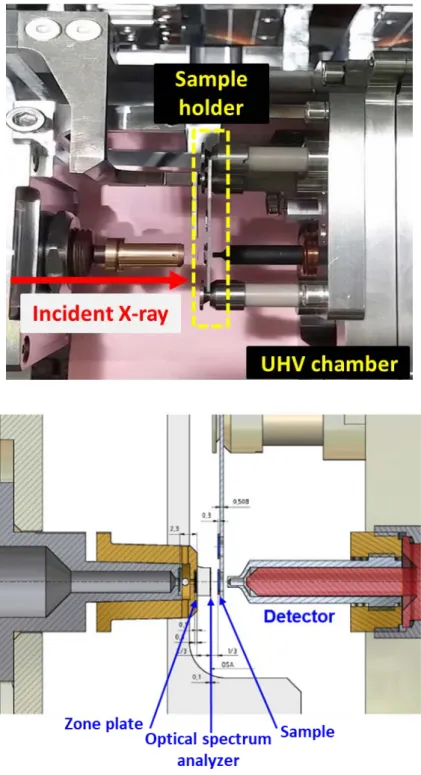
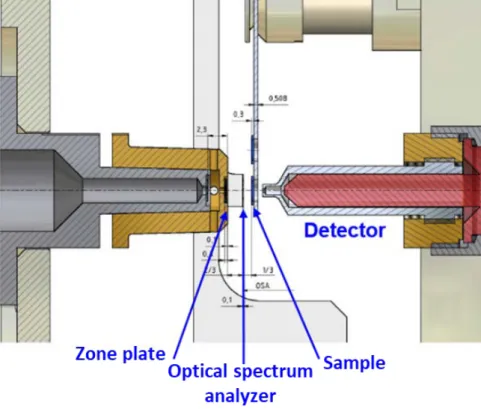
The local electronic structure of the oxidized zirconium samples was investigated using X-ray absorption fine structure spectroscopy at the OK edge, and the energy range of the OK edge is set to 520 to 560 eV. The monochromatic X-ray of the beamline passes through the output sludge and its size is from 10 to 30 μm. After the exit slit, the X-rays penetrate through the zone plate and the zone plate acts as a focusing lens for X-rays.
The sample is scanned by the incident X-ray, and the change in intensity of penetrated X-ray is detected by photon counter (detector). The resolution of STXM analysis is about 20 nm, and the X-ray scan is performed at the 100 kPa helium condition and the temperature is room temperature.
In-situ X-ray reflectivity study of interfacial structure between high temperature water and
- Introduction
- Materials and sample preparation for thin film cell measurement
- Experimental procedure
The sample was kept in distilled water immediately after purification until the sample was mounted on the cell for in-situ XRR analysis. At the 33BM-C beamline from the Advanced photon source (APS), the full beam size at the sample was 0.1 mm (for the low 2θ angles to prevent beam spillage) and 0.5 mm in the vertical and 0.8 mm in the horizontal direction. The beamline is equipped with a four-circle [47] diffractometer for surface diffraction experiment and a Pilatus 100K area detector is used as a standard detector for measuring X-ray reflectivity.
The sample was mounted in a thin-film cell geometry on the goniometer center of the four-circle diffractometer as shown in Fig. The sample was covered with 8 μm thin Kapton film, which holds the thin water film (~10-30 μm thick). on the surface of the single crystal substrate by the capillary force and serves as the transparent X-ray window. To study the hydrothermal process of zirconium oxide in high pressure and temperature condition, which simulates the operating condition of PWRs, the high resolution X-ray reflectivity hydrothermal cell was used.
The cell body is made of Inconel 625 alloys, and the X-ray window and optical window is CVD polycrystalline diamond, and the photograph and schematic representations of the component are in Fig.
Results
In-situ Raman spectroscopy and EIS results
- In-situ Raman spectroscopy results at normal DH condition
- In-situ Raman spectroscopy results at high DH condition
- In-situ EIS measurement results at two different DH conditions
- Ex-situ characteristic of zirconium oxide after high temperature water corrosion
Table 4-2 also shows the positions of the zirconium oxide peaks at 4.15 mg/kg DH. The in situ Raman spectroscopy result shows that the DH concentration can affect the zirconia phase in the primary coolant state. Using the Z-view program, parameter values can be derived after optimizing the experimental impedance data for zirconia as shown in the table.
4-4, the Nyquist plots and the fitted EIS in situ plots for the oxidized Zr-Nb-Sn 4.15 mg/kg specimen and the parameter values after optimization in the experimental resistance data for the zirconium oxide of 4.15 mg/kg conditions are presented in the table. Microstructural characterization of the zirconium oxide layer was performed via TEM, EDS and Fast Fourier transform (FFT) analysis after removing the oxidized Zr-Nb-Sn bond from the autoclave. Also, the zirconium oxide and chemical compositions of the second phase particles are investigated via EDS.
These results are in good agreement with the in situ Raman spectroscopy results, which reveal that the zirconia phase can vary due to the position of the phase in the oxide layer.
![Figure 4-1 In situ Raman spectroscopy results of oxidized Zr-Nb-Sn alloy after different oxidation time at 2.49 mg/kg DH [61]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10517165.0/68.892.161.685.354.755/figure-raman-spectroscopy-results-oxidized-alloy-different-oxidation.webp)
Scanning transmission X-ray microscopy results
- Scanning transmission X-ray microscopy results at normal DH condition
- Scanning transmission X-ray microscopy results at high DH condition
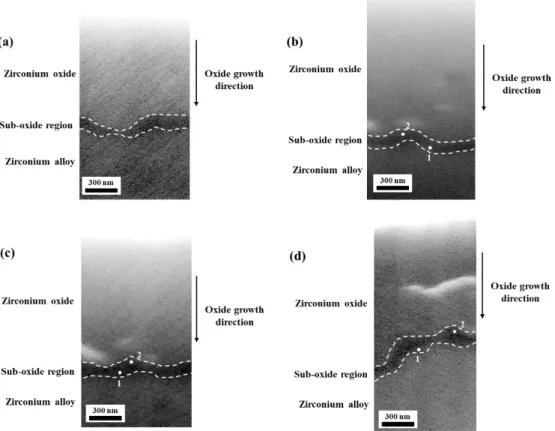
The STXM results of oxidized zirconium alloy with different oxidation time are illustrated in Fig. So similar to the previous case, all STXM images show the dark-contrast region that existed just above the O/M interface. 4-14, the suboxide layer is observed just above the zirconia matrix layer, and the suboxide layer is dark area in the 534 eV image.
Similar to the previous case, all STXM images show that the thickness of the suboxide layer is below 100 nm, and the obvious twin peaks are observed in the XAS spectrum of zirconium oxide. O The twin-peak structure of the K-edge means that t2g and e.g. the oxygen orbital is shown in the XAS images. In the suboxide region, the top position of e.g. the same as the bulk zirconium oxide region, 532 eV, however, the t2g peak position changes to 536.2 eV.
These results show that peak separation between two peaks, e.g. and t2g, decreases from the suboxide region to the bulk zirconia.
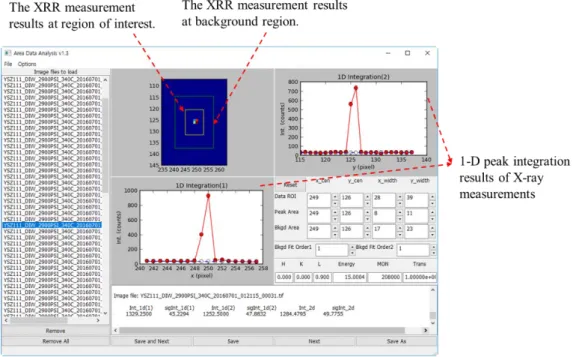
X-ray reflectivity results
- X-ray reflectivity results at ambient condition (room temperature, atmospheric
- X-ray reflectivity results at high temperature water condition
The four layers for the top layers of YSZ (111) are set as the relaxed layers in this fitting model. Finally, the semi-infinite unit cell of YSZ (111) is used for the substrate layer with specific unit cell lattice constant. The reduced χ2 value for the nonlinear quadratic fit is 1.430 with 19 variables and 77 data points.
On the other hand, the lattice constant variations of the unit cells below the upper unit cell are negligible. Also, the assembly parameters are calculated from the assembly model for YSZ (111) and water interface at 340℃ state with the same model in the previous case. This result reproduces the experimental data well, and the reduced χ2 value for the nonlinear quadratic fit is 1.883 with 19 variables and 77 data points.
On the other hand, the changes in the lattice constant of the unit cells below the upper unit cell are also negligible.
Discussion
Characteristic of O/M interface and phase transformation of zirconium oxide
In general, the corrosion rate in the oxidation kinetics in zirconium alloy before the transition can be described by an empirical law of the form. In the case of zirconium oxidation, the value of n in Equation 5-1 is typically below 0.5, and the corrosion rate kinetics are subparabolic. It reveals that the corrosion kinetics of zirconium oxide is affected by the level of DH.
Previous studies revealed that the tetragonal oxide near the O/M interface is important for zirconium alloy's corrosion characteristic, and the main reason of transformation is commonly known as the stress in the tetragonal zirconium oxide [26,27,50]. The tetragonal phase to monoclinic phase oxide transformation can be represented in the following equation (5-2) using the Gibbs free energy equation, for the two phases ΔGt→m. In addition, the water molecules can be combined in the tetragonal zirconium oxide lattice during the oxidation process in high-temperature water, and this leads to the expansion of lattice parameters and the acceleration of the transformation [75].
It indicates that the properties of oxide change are related to the water chemistry changes and the charged species transport in the oxide.
![Figure 5-1 The thickness of zirconium oxide with different DH conditions [68,77,78]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10517165.0/114.892.197.743.369.792/figure-thickness-zirconium-oxide-different-dh-conditions-68.webp)
Atomistic structure of interfacial layer between zirconium oxide and high temperature
Role of DH on corrosion characteristic of zirconium alloy in high temperature water
Conclusion
Water species diffuse much more easily at the O/M interface in the high DH state, then the oxide grows faster at the O/M interface in the high DH state during the pre-transition state of zircon oxidation. The DH level for nuclear power plant operation should be considered with a comprehensive understanding of zirconium fuel cladding materials and structure materials, and it is inappropriate to set the upper limit of the DH range without further work. Saario, Influence of water chemistry on the corrosion mechanism of a zirconium-niobium alloy under simulated light water reactor coolant conditions, Corros.
Preuss, The microstructure and microtexture of zirconium oxide films studied by transmission electron backscatter diffraction and automatic crystal orientation mapping with transmission electron microscopy, Acta Mater. Kim, Influence of dissolved hydrogen on the early stage corrosion behavior of zirconium alloys in simulated light water reactor coolant. Maroto, Analysis of zirconium oxide formed during oxidation at 623 K on Zr-2.5Nb and Zircaloy-4, Mater.
Livigni, In-situ electrochemical impedance spectroscopic measurements of the conductivity of zirconium alloy oxide: Relation to hydrogen uptake, Corros.
![Figure 2-4 In-situ Raman spectra of zirconium alloy heated to 407 ℃ in aqueous solution [2]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10517165.0/34.892.209.692.316.894/figure-raman-spectra-zirconium-alloy-heated-aqueous-solution.webp)
![Figure 2-5 In-situ Raman spectra of zirconium oxide with different temperature [2]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10517165.0/35.892.204.691.350.855/figure-situ-raman-spectra-zirconium-oxide-different-temperature.webp)
![Figure 2-11 Scattered X-ray intensity as different number of atomic layer, N [47]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10517165.0/45.892.221.674.388.730/figure-scattered-ray-intensity-different-number-atomic-layer.webp)