During my degree, I investigated the impact of TonEBP in myeloid cells on neuroinflammation and obesity-induced insulin resistance. Transcription factors NF-κB and AP-1 are key mediators of inflammation associated with many inflammatory diseases, including AD. Here, we show that myeloid cell-specific TonEBP depletion reduced inflammation and insulin resistance in mice with high-fat diet-induced obesity, but did not affect obesity.
This phenotype was associated with a reduced accumulation and a reduced ratio of M1/M2-like macrophages; decreased expression of inflammatory factors associated with insulin resistance; and increased insulin sensitivity in epididymal white adipose tissue and liver.
Background
Alzheimer’s disease (AD)
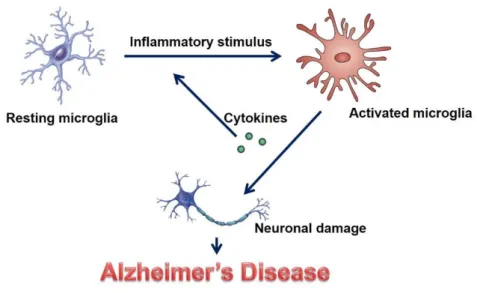
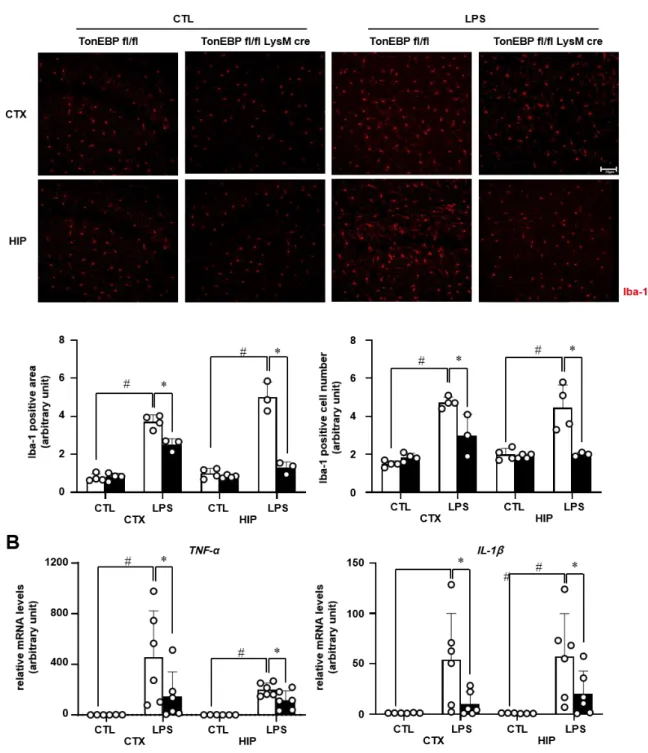
Alzheimer's disease (AD) is a neurodegenerative disease associated with aging that shows memory loss and cognitive deficits. However, there is still no effective treatment to stop, prevent or reverse AD even with diagnostic tools. The exact cause of Alzheimer's disease is unknown, but neuroinflammation has recently emerged as an important component in the pathology of Alzheimer's disease (11-13).
In AD, excess neurotoxic factors such as NO, TNF-α, and IL-1β cause neuronal damage and ultimately neuronal death ( 11 – 13 ).
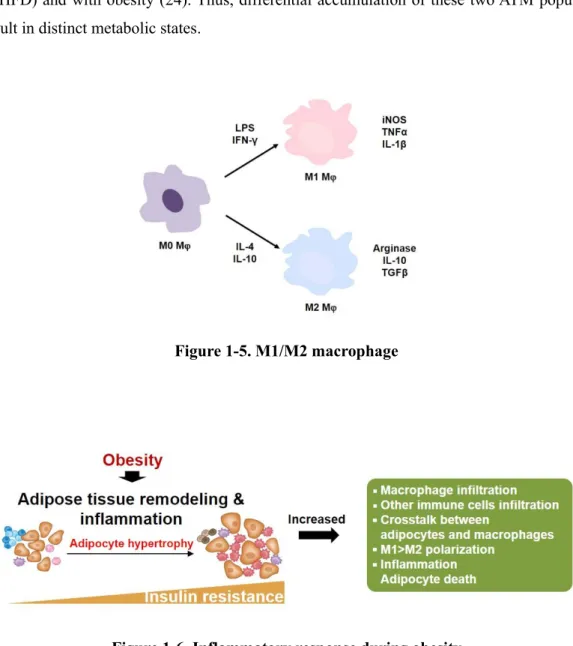
Adipose tissue macrophage (ATM)
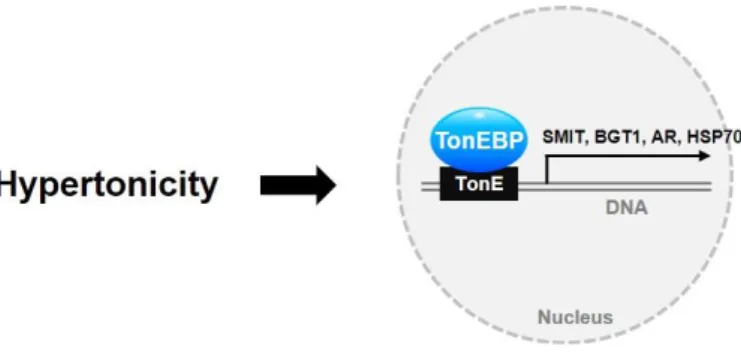
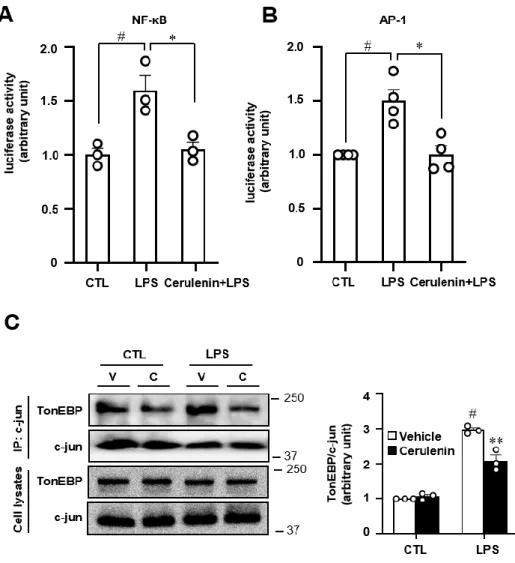
Microglial TonEBP mediates LPS-induced inflammation and memory loss as
Introduction
Methods
Results
Discussion
Conclusions
Supplementary Figures
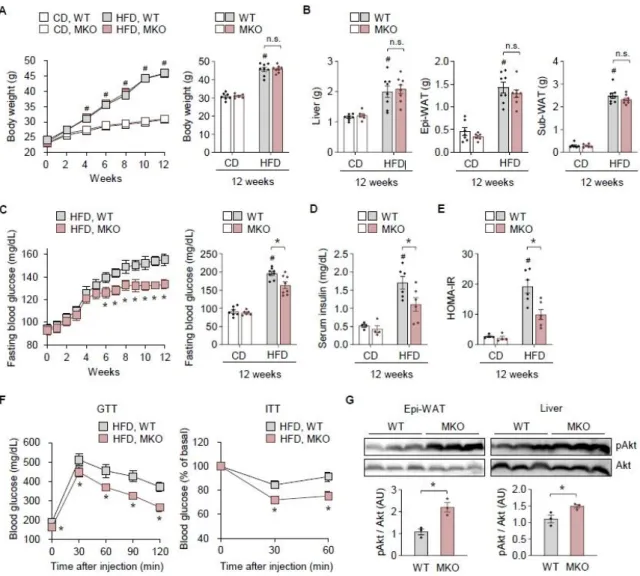
TonEBP in myeloid cells promotes obesity-induced insulin resistance and
Introduction
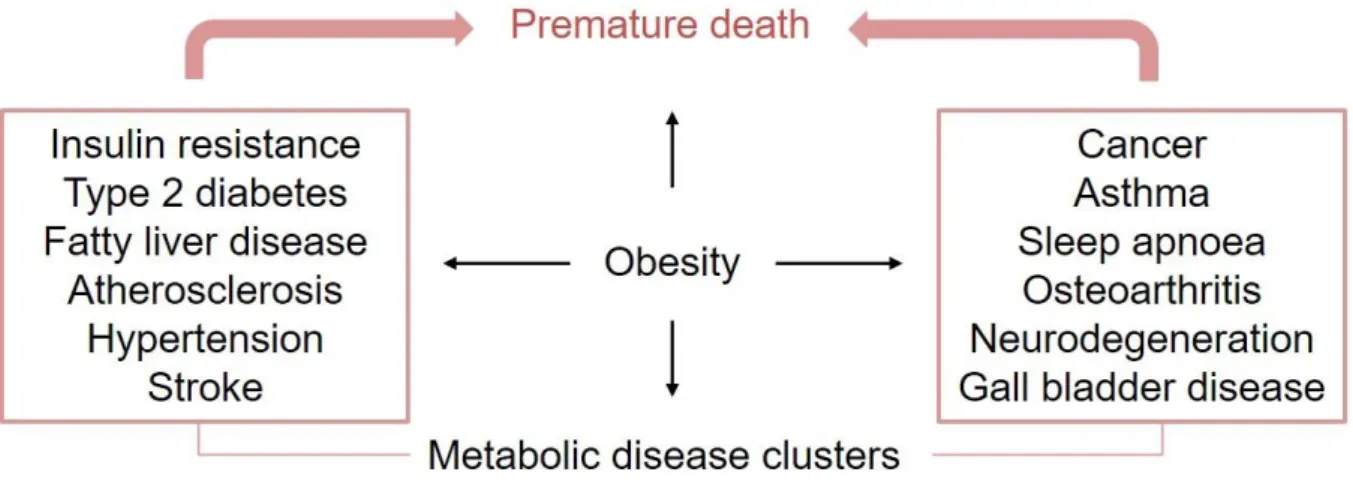
Adipose tissue inflammation is considered to be a major driving force for the development of insulin resistance and type 2 diabetes (T2D) in obese individuals [2]. As a key cell type contributing to inflammatory responses, macrophages accumulate in adipose tissue of humans and rodents with increasing body weight [3] and adipose tissue macrophages (ATMs) are key effector cells orchestrating adipose and systemic inflammation in obesity [4 ] . Traditionally, ATMs are considered to accumulate during obesity due to recruitment of circulating monocytes and subsequent differentiation into macrophages [ 7 , 8 ].
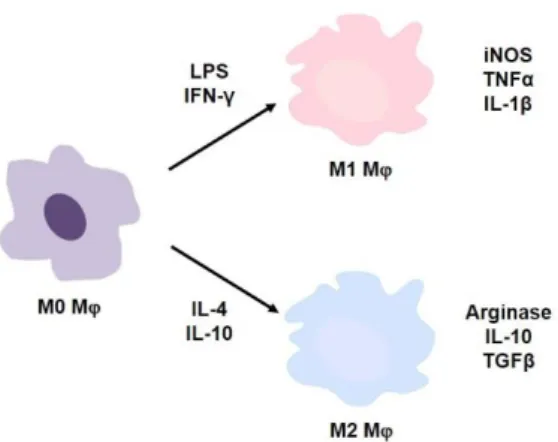
In addition to the increased number of ATMs, the phenotype of these cells changes dramatically during obesity. ATMs in the lean state typically have an anti-inflammatory phenotype that helps maintain tissue homeostasis. In contrast, during obesity, ATMs respond to metabolic cues such as excess free fatty acids (FFA), glucose, and gut-derived endotoxin and adopt a metabolically activated (MMe) phenotype distinct from M1 or M2 activation [ 9 , 10 ]. .
MMe ATMs exert both deleterious and beneficial functions through independent pro-inflammatory and anti-inflammatory pathways during obesity [ 9 , 10 ]. Although the role of TonEBP in macrophages is well studied, relatively little is known about its intrinsic role in the identity and function of ATMs during obesity. Therefore, this study examined the impact of macrophage TonEBP in the context of obesity-related inflammation and insulin resistance.
We found that TonEBP expression was increased in the ATMs of HFD-induced obese mice, and this promoted the development of obesity-associated inflammation and insulin resistance. We thus identified TonEBP as a crucial factor in ATM activation states and in the control of metabolic dysfunction during obesity.
Research design and Methods
PMA-differentiated THP-1 macrophages and RAW264.7 cells were transfected with scrambled (Scr) siRNA or target gene-specific siRNAs for 48 h using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA). For overexpression, RAW264.7 cells were infected with empty vector control virus (Ad-EV) or adenovirus carrying the human TonEBP gene (Ad-TonEBP) at a multiplicity of infection of 50 for 24 h. Cell surface markers were identified using primary antibodies purchased from BD Biosciences (Franklin Lakes, NJ, USA; Supplementary Table 1).
Flow cytometry data were acquired on a BD LSR Fortessa instrument (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, Oregon, USA). After reverse transcription, real-time PCR was performed using the CFX384 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Measured cycle thresholds were normalized using the reference gene cyclophilin A or GAPDH and expressed as fold changes relative to control samples.
Transfected cells were treated as indicated in the figure legends and lysed in passive lysis buffer. The luciferase assay was performed with a dual luciferase reporter system (Promega, Madison, WI, USA). After washing, cells were sonicated and immunoprecipitated with anti-IgG, anti-Sp1 IgG (ab231778, Abcam), and anti-C/EBPβ IgG (3082S, Cell signaling) antibodies at 4°C overnight.
After elution and reverse cross-linking of the antibody/DNA complexes, DNA was purified with DNA purification kit (Qiagen, Redwood, CA) and subjected to real-time PCR using appropriate primers. A one-way analysis of variance followed by Tukey's post-hoc test was used to compare multiple conditions.
Results
Consistently, HFD-fed MKO mice had lower serum levels of CCL2, TNFα, and IL-1β (Figure 2F). Consistent with the decreased F4/80 mRNA level, the livers of HFD-fed MKO mice contained fewer F4/80+ macrophages ( Fig. 2J ). Notably, TonEBP mRNA expression was elevated in the SVF of epi-WAT from HFD-fed mice (Figure 3D).
As expected given the decrease in epi-WAT ( Fig. 2B and C ), F4/80 mRNA expression was lower in SVFs of epi-WAT from HFD-fed MKO mice ( Fig. 3D ). Consistent with increased macrophage content, metabolically activated pro-inflammatory and anti-inflammatory gene expression was increased in SVFs of epi-WAT from HFD-fed WT mice (Supplementary Fig. 2C). Notably, the mRNA expression of both PPARγ1 and PPARγ2 was higher in BM-Mo and BMDM from MKO mice than in the corresponding cells from WT mice ( Fig. 3H ).
Furthermore, TonEBP knockdown stimulated induction of PPARγ1 by the M2 inducer IL-4 (20 ng/ml) [24] (Supplementary Fig. 3D). Moreover, mRNA levels of these genes were higher in SVFs of epi-WAT from HFD-fed MKO mice (Fig. 4D). Notably, mRNA expression of TNF, IL-1 and Ccl2 was lower in TonEBP-deficient cells, while mRNA expression of IL-10 was higher (Fig. 4F).
Furthermore, there was a significant correlation between TonEBP and TNF mRNA levels (Supplementary Figure 6B). Importantly, TonEBP mRNA expression was higher in PBMCs from diabetic patients than from non-diabetic subjects, and was positively correlated with fasting blood glucose levels (Fig. 6C and Supplementary Table 3).
Discussion
Here, we show that TonEBP depletion in human and mouse macrophages decreases induction of pro-inflammatory gene expression and cytokine secretion, but promotes expression of genes related to anti-inflammatory macrophages in response to PA, thereby improving insulin signaling and glucose uptake in adipocytes . This is interesting in light of the previous finding that Sp1 and TonEBP play different roles in the expression of pro- and anti-inflammatory genes. First, TonEBP and Sp1 have opposing roles in transcriptional regulation of IL-10, a potent anti-inflammatory and immunosuppressive molecule in macrophages.
Sp1 is a key transcription factor involved in LPS-mediated induction of IL-10 gene expression, and is recruited to a putative binding site in the promoter region in mouse and human macrophages [ 55 ]. Interestingly, TonEBP represses transcription of the IL-10 gene by reducing chromatin accessibility and thus the recruitment of Sp1 to its promoter [23]. The current study and these previous findings suggest that a previously unrecognized bidirectional regulatory loop exists between Sp1 and TonEBP in the context of LPS-mediated IL-10 expression.
TonEBP, however, negatively regulates the recruitment of Sp1 to the IL-10 promoter, thereby abrogating the ability of Sp1 to stimulate IL-10 transcription. Thus, the interactions between Sp1 and TonEBP are an important aspect of the regulation of IL-10 expression. This regulatory relationship between Sp1 and TonEBP has functional implications for preventing the anti-inflammatory role of Sp1 and promoting the pro-inflammatory role of TonEBP and Sp1, thereby promoting pro-inflammatory macrophage activation.
These results indicate a mechanism that may help to explain the differential regulation of pro- and anti-inflammatory genes. TonEBP depletion profoundly alters the pro-inflammatory-anti-inflammatory MMe macrophage ratio and reduces macrophage accumulation in adipose tissue and liver.
Supplementary Tables & Figures
The p-values were determined by a one-way ANOVA. related to Fig. 3) (A) THP-1-derived macrophages were transfected with Scr siRNA or two siRNAs (#1 or #2) targeting different regions of human TonEBP mRNA for 48 h. The p-values were determined by an ANOVA with Tukey's post-hoc test. related to Fig. 5) (A) THP-1-derived macrophages were transfected with the human TonEBP promoter-luciferase construct containing the -4591 to +409 region or the pGL3 basic vector. n represents the number of independent experiments with triplicates.
Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. Free fatty acids increase basal glucose production in the liver and induce insulin resistance in various places in the liver. TonEBP/NFAT5 promotes obesity and insulin resistance through epigenetic suppression of white adipose tissue.
나는 대식세포의 TonEBP가 비만과 관련된 인슐린 저항성과 포도당 항상성 파괴에 기여한다는 것을 조사했습니다. 이러한 ATM의 M1-M2 불균형은 지방 조직과 전신 염증뿐만 아니라 인슐린 저항성을 촉진합니다. 제가 학위과정을 성공적으로 마칠 수 있도록 도와주신 많은 분들께 감사의 말씀을 전하고 싶습니다.
먼저, 아무것도 모르는 저에게 연구자로서의 길을 닦아주신 권혁무 교수님께 감사의 말씀을 전하고 싶습니다. 제가 학생이 아닌 연구자로서 자립할 수 있도록 성장할 수 있도록 도와주신 교수님께 감사 인사를 전하고 싶습니다. 아울러, 공부하는 동안 많은 도움을 주신 이화선 교수님을 비롯해 생명과학과 모든 교수님들께 감사의 말씀을 전하고 싶습니다.
또한 박사과정 동안 많은 가르침과 도움을 주신 최수연 교수님께도 깊은 감사의 말씀을 전하고 싶습니다. 그리고 저에게 가장 큰 힘이 되어준 가족들에게도 감사하다는 말씀을 전하고 싶습니다.