Project Dissertation submitted to Universiti Teknologi PETRONAS Chemical Engineering Program has partially fulfilled the requirement for. I hereby certify that I am responsible for the work submitted in this project, that the original work is mine except as noted in the references and acknowledgments, and that the original work contained herein has not been taken or performed by unspecified sources or persons. In this study, we will test Ni-Al, Zn-Al and Ni-Zn-Al catalyst obtained from hydrotalcite.
First of all, the author would like to express his utmost gratitude to Almighty God for showering his blessings in completing this Final Year Project within the time frame. His advice and encouragement have been a great impetus for the author to complete this project in the desired manner. Asna Mohd Zain for organizing the invaluable workshop on improving the overall research methodology and guidelines for the writing process.
Finally, the author would like to thank his parents, grandmother and sister for their kind input for their great support and encouragement to motivate me to complete this final year. In addition, a big thank you goes to his friends who helped the author in every way to successfully complete this final project.
INTRODUCTION
Background
Problem statement
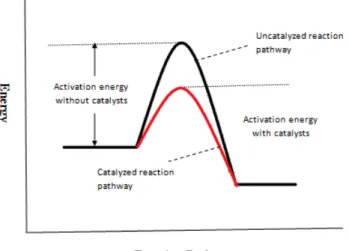
Furthermore, it is also important to conduct a comprehensive study on the catalysis mechanism and catalytic activity, which is important for synthesizing a new catalyst for the conversion of target gas to methanol.
Objective
LITERATURE REVIEW
- Fossil fuel depletion
- Fuel synthesis from syngas
- Methanol production
- Hydrotalcite derived catalyst
- Selection of transition metal for methanol production from syngas
Synthesis of synthesis gas processes in methanol production is a very common and commercially used in the petrochemical industry. Based on the energy of the reactions, to stimulate a high yield of methanol production requires high pressure and high temperature (Dagle et al., 2014). Normally, the syngas that would be obtained from a specific gasifier would be further used for methanol production.
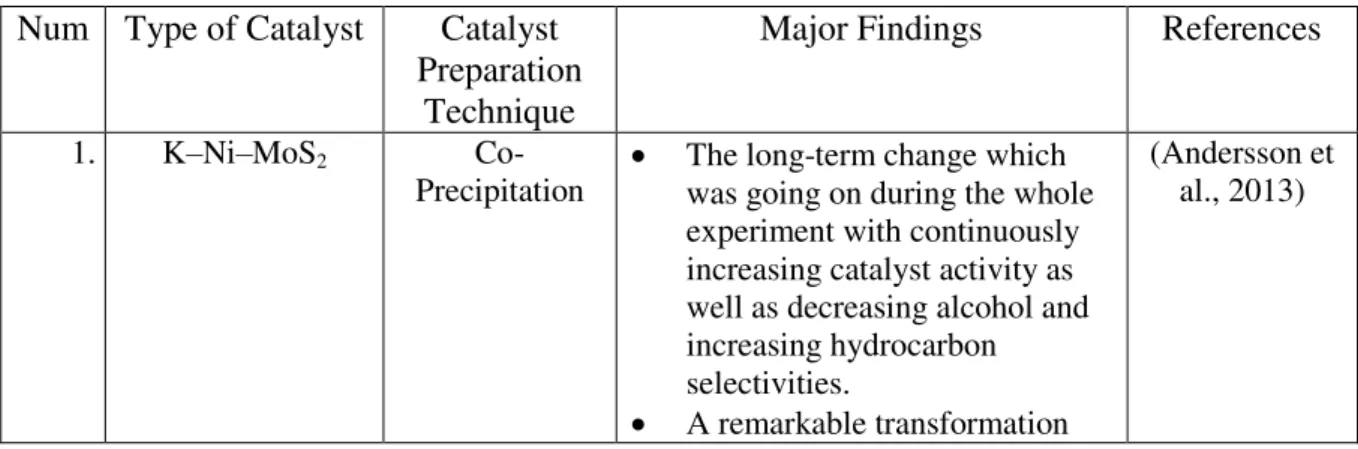
Precipitation The long-term change which was ongoing throughout the experiment with continuously increasing catalyst activity as well as decreasing alcohol and increasing hydrocarbon selectivities. Precipitation The main reaction products over the K-promoted Cu-Zn-Al catalysts were alcohols, with methanol being the main carbon-oxygenated product along with CO2 and small concentrations of hydrocarbons. This study will be good to further improve and validate that Zn catalyst can be generous in methanol production even at a lower temperature.
In addition, this study will also study the trimetallic combination of Ni and Zn with the support of alumina in the production of methanol production. This study of this combination is very limited as it is a back combination and methanol production prefers high temperature and pressure conditions for a reasonable yield.
METHODOLOGY
- Research methodology
- Raw materials and chemicals needed
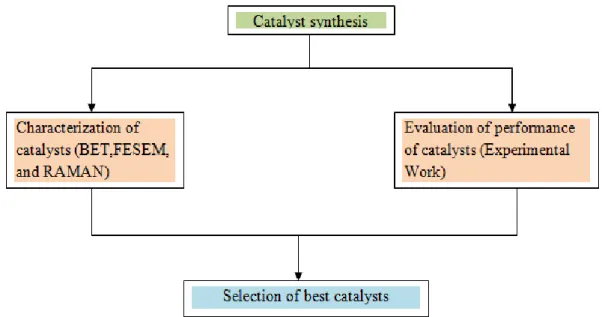
- Catalyst synthesis
- Characterization of catalysts
- Evaluation of catalyst performance
- Key milestone
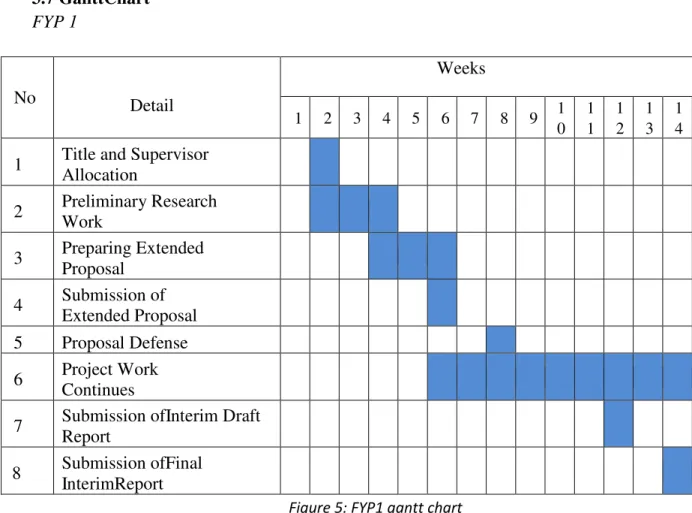
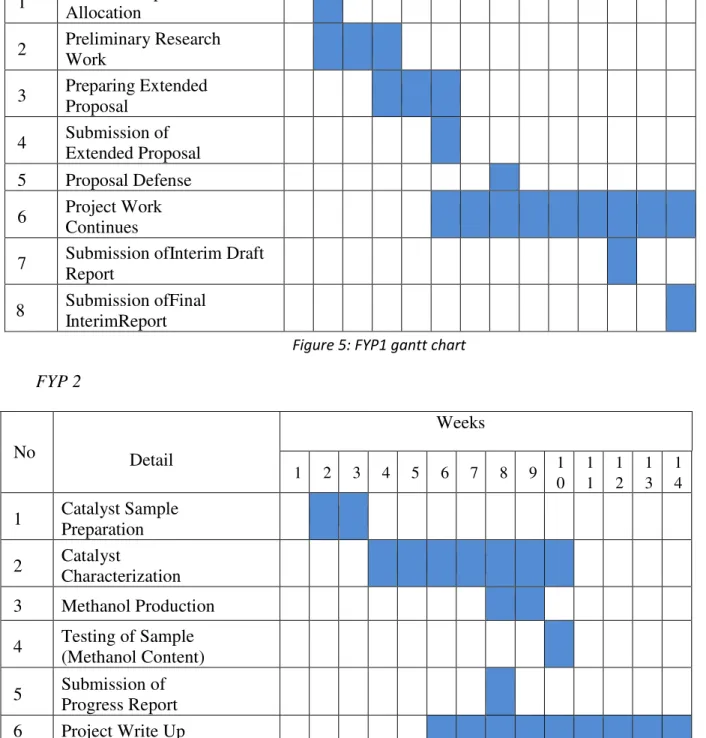
- Gantt Chart
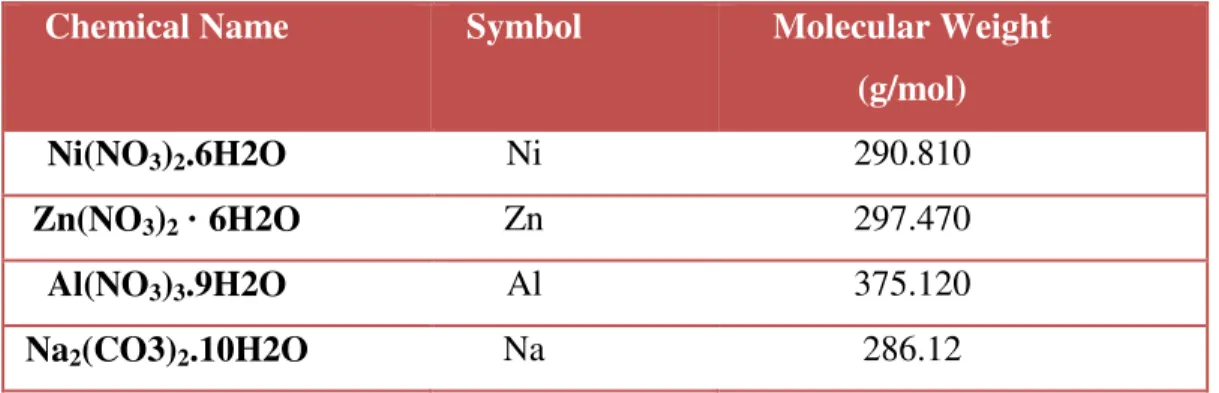
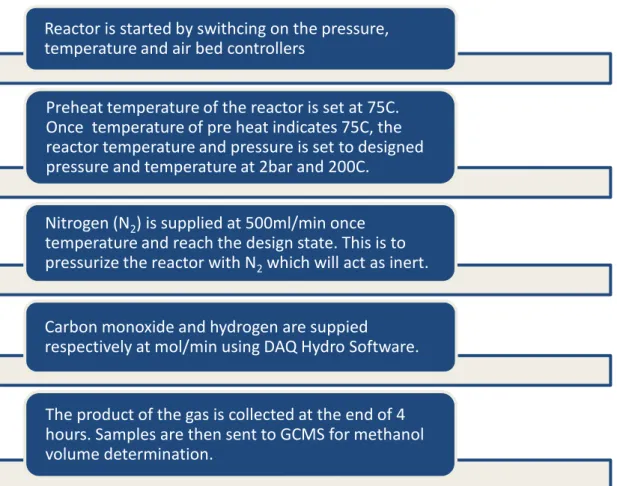
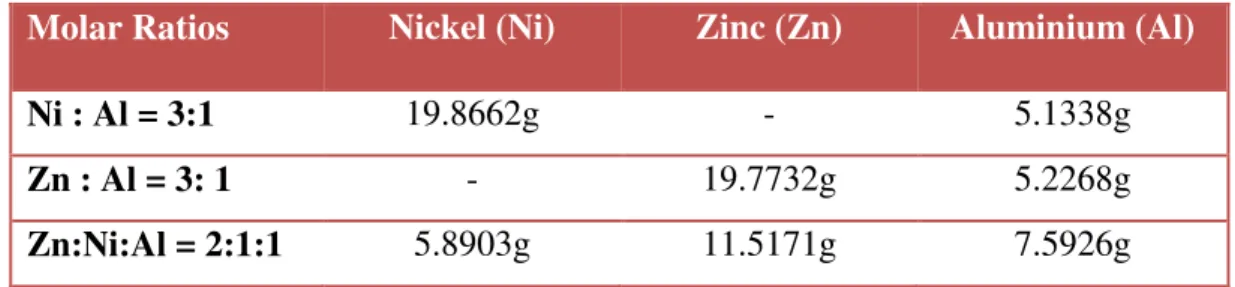
This research work requires different raw materials and chemicals to ensure the success of the project. Three catalyst samples of Ni-Al, Zn-Al and Zn-Ni-Al with a molar ratio of 3:1, 3:1 and 2:1:1 were prepared by the co-precipitation method, respectively. Ni and Zn act as metal precursors, while aluminum nitrate nonahydrate (Al) acts as a support for the catalyst.
Performance test Review of findings Catalyst characterization. metal precursors, supports and also precipitating agent. The solution of metal precursors is then mixed with the supporting solution before being titrated into sodium carbonate decahydrate solution. Then, the filtered samples were dried in an oven for 12 h at 110°C to produce dried hydrotalcite-derived catalyst.
Mixed oxide conversion of the hydrotalcite-derived catalyst was achieved via calcination at 500°C for 6 hours. The following analysis is intended to be performed for the characterization of the catalysts. i) RAMAN spectroscopy analysis. RAMAN spectroscopy analysis provided detailed information about the molecular vibrations on the respective catalyst samples.
After the preheat temperature shows 75C, the reactor temperature and pressure are set to the design pressure and temperature at 2bar and 200C. The samples are then sent to GCMS for determination of methanol volume. ii) Brunauer-Emmet-Teller (BET) surface area analysis. BET surface area analysis aimed to provide accurate estimation of the specific surface area of catalysts by multilayer nitrogen adsorption measured as a function of relative pressure using a fully automated analyzer.
This technique includes external area and pore area evaluations to determine the total specific surface area to study the effects of surface porosity and particle size in influencing methanol conversion from syngas. iii) Field emission scanning electron microscopy (FESEM) analysis. All the catalyst will be tested with the syngas presence in a hydrocarbon cracked tube reactor for the conversion to methanol. The time it takes, temperature, pressure and other operating conditions will be manipulated accordingly to select the best catalyst for converting syngas to methanol.
RESULTS AND DISCUSSIONS
Catalyst preparation
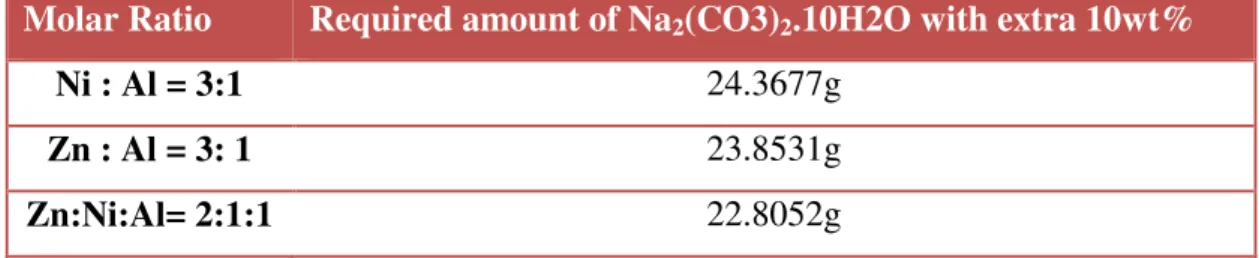
A sample chemical mass calculation based on molar ratios is made below for a Ni:Al = 3:1 sample. Appropriate other metal combinations used the same calculation steps to determine the weight of chemicals required for the co-precipitation method. The following is the calculation of the required total amount of Na2(CO3)2.10H2O for each catalyst sample.
Sample calculation for the required amount of required amount of Na2(CO3)2.10H2O based on the molar ratios is performed below for the sample Ni : Al = 3:1.
Catalyst Characterization
- RAMAN spectroscopy analysis
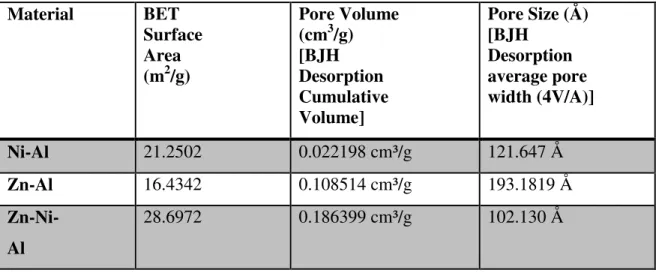
- Brunauer–Emmett–Teller (BET) Surface Area Analysis
- Field Emission Scanning Electron Miscroscopy (FESEM) Analysis
Ni-Al Adsorption Ni-Al Desorption Zn-Al Adsorption Zn-Al Desorption Zn-Ni-Al Adsorption Zn-Ni-Al Desoprtion. FESEM images above indicate that the Ni-Al catalysts exhibit cubic shape, while the Zn-Al catalysts showed a thin layer of square surface. In addition, it can be observed that smaller nickel and zinc particles were dispersed on the surface of Al2O3 support for the Ni-Al, Zn-Al and Zn-Ni-Al catalyst structures.
In addition, Zn-Ni-Al has a better homogeneous morphology, comparable to the structure of Ni-Al and Zn-Al. From this we can conclude that the Ni-Al catalyst needs a higher temperature to be activated, as the retention time curve is longer. The third highest component contained in this Ni-Al catalyst conversion is also hexadecanoic acid at a retention time of 4,300 with a volume of 7.29 cm3.
This proves that the Ni-Al catalyst is not able to convert synthesis gas up to the methanol range as it could only achieve methane production. The retention time to form products is much shorter for Zn-Al catalyst, similar to Ni-Al catalyst, from which it can be deduced that the temperature used is quite suitable, as it rapidly produces a product in a very early time frame larger volume formed, but tends to decrease rapidly in the next section. The third highest component present in this conversion over a Ni-Al catalyst is N-cyclohexylbenzyl imine with a retention time of 1.541 with a coverage of 7.31 cm3.
Retention time to form products is much more moderate and very suitable compared to Ni-Al and Zn-Al catalyst comparable to Ni-Al catalyst where it can be deduced that the temperature used is reasonably appropriate as the rapidly formed product in larger volume at a very intermediate time frame. The following component which in this conversion using Zn-Ni-Al catalyst comprises methane at retention time 2.983 with coverage of 9.59 cm3. Besides that, it would also increase the higher production of methanol yield as mentioned by (Liu, Murata, Inaba, & Takahara, 2013). Therefore, it also proves that this trimetallic catalyst is the most suitable for the production of synthesis gas for methanol compared to Ni-Al and Zn-Al.
Based on the results above, it can be concluded that Zn-Ni-Al is the most suitable catalyst compared to Ni-Al and Zn-Al for the conversion of syngas to methanol. It was also proved based on the RAMAN results that the molecular vibrations are different and higher for Zn-Ni-Al catalyst compared to Ni-Al and Zn-Al catalyst. The presence of both Zn and Ni in the combination of the metal catalyst has enabled an improvised chemical reaction for methanol production from synthesis gas. Besides that, the characterization of Zn-Ni-Al catalyst also supports these findings where it has the smallest pore which produces a larger surface area for catalytic reaction to take place.
The complete combination and uniform dispersion of nickel and zinc metal based on EDX mapping proves that the Zn-Ni-Al catalyst will have a favorable catalytic reaction in producing the highest yield of methanol. Based on the results obtained from the entire project, Zn-Ni-Al is the most suitable hydrotalcite-derived catalyst to support the conversion of syngas to methanol compared to Ni-Al and Zn-Al catalyst, which produced the highest amount of methanol. 19.49 cm3.